Protective mechanism of Coprinus comatus polysaccharide on acute alcoholic liver injury in mice,the metabolomics and gut microbiota investigation
Jinyn Yu,Jingung Sun,Min Sun,Weidong Li,Dongmei Qi,Yongqing Zhng,Chunho Hn,*
a School of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan 250355, China
b Hepatology Department of Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250355, China
c Experimental center, Shandong University of Traditional Chinese Medicine, Jinan 250355, China
Keywords:Coprinus comatus Polysaccharide Alcoholic liver disease Metabolomics Gut microbiota
ABSTRACT Coprinus comatus polysaccharide (CCP) has significant hepatoprotective effect.To explore hepatoprotective mechanism of CCP,the study analyzed preventive effect of CCP on acute alcoholic liver injury in mice by histopathological examination and biochemical analysis. Simultaneously,hepatoprotective mechanism was also analyzed in conjunction with metabolomics and proliferation of gut microbiota.The results showed that CCP significantly decreased alanine aminotransferase (ALT),aspartate aminotransferase (AST) and triglyceride (TG) levels in serum of alcoholic liver disease (ALD) mice.Histopathological examination showed that CCP can significantly improve liver damage.Metabolomics results showed that there were significant differences in the level of metabolites in liver tissue of control group,ALD group and CCP group,including taurine,xanthosine,fumaric acid and arachidonic acid,among others.Metabolites pathways analysis showed that hepatoprotective effect of CCP was related to energy metabolism,biosynthesis of unsaturated fatty acids,amino acids metabolism and lipid metabolism.Additionally,CCP inhibited an increase in the number of Clostridium perfringens,Enterobacteriaceae and Enterococcus,and a decrease in the number of Lactobacillus and Bifidobacterium in the gut of ALD mice.All these findings suggested that CCP treatment reversed the phenotype of ethanol-induced liver injury and the associated metabolites pathways.
1. Introduction
Alcoholic Liver Disease (ALD) means damage to the liver caused by acute or chronic alcohol abuse[1]. Alcohol has become the second largest cause of liver damage after viruses.ALD,mainly including fatty liver,hepatitis,cirrhosis,hepatocyte necrosis and even liver failure,is usually caused by excessive alcohol consumption and has become an ever-growing health issue worldwide.It is estimated that ALD incidence will continue to increase in the coming decades,inextricably be linked to psychosocial issues that our society is facing[2].The available options for the treatment of ALD are limited,and most possess some adverse side effects[3-4].Therefore,there is of great significance to excavate natural products with strong antioxidant effects from food resources for prevention of alcoholic liver injury.Natural polysaccharide widely exists in plants,animals and microorganisms.In particular,fungal polysaccharide displays various biological activities,such as antioxidant,immunomodulatory and hepatoprotective activity,which have attracted increasing attention[5-7].Based on the research,Ganodermalingzhipolysaccharide showed strong antioxidant activity and can inhibit ethanol-induced steatohepatitis in mice[8].Treatment withCoriolusversicolormycelia polysaccharide exerted hepatoprotective effects against alcoholinduced liver injury in mice by reducing oxidative stress and modulating immunity[9].
Coprinuscomatusis a wild edible mushroom,which is rich in nutritional value[10].It contains many nutrients,including polysaccharides,fatty acids,tocopherols,organic acids,phenolic acids,ascorbic acid,and carotenoids[11].C.comatuspolysaccharide(CCP),as a kind of fungal polysaccharide,has been confirmed by studies to have a significant hepatoprotective effect[12-14].At present,researchers have explored the structure,prebiotic-like effects and antioxidant capacity of CCP[13,15].However,the metabolic profiling of CCP in liver tissue has not been understand.The systemic measurement of metabolites that originate in the liver can provide clear signposts to liver wellbeing or disease such as ALD.Metabolomics has offered an opportunity to highlight the potential biomarkers which have pointed most directly to ALD mechanisms.On the other hand,studies have shown that polysaccharide have an important impact on the composition and function of the gut microbiota[16].For example,the prebiotic xylooligosaccharide(XOS) promotes the growth of certain beneficial bacteria includingBifidobacteriumandLactobacillus[17].There is a gut-liver axis between the liver and the gut[18],and the gut microbiota plays an important role in the study of liver damage mechanism.Taking the gut microbiota as a potential target for the treatment of ALD,possible mechanisms of CCP in preventing alcoholic liver injury can be explored,which can provide experimental evidence for further development of hepatoprotective drugs.
In this study,ALD mouse models were constructed.The protective effects of CCP on acute alcoholic liver disease in mice were explored through histopathological examination and biochemical analysis.Simultaneously,the effects of CCP on the gut microbiota and endogenous metabolites of ALD mice were explored.The possible liver-protecting mechanism provides a theoretical basis for exploring natural hepatoprotective drugs for treatment of ALD.
2. Materials and methods
2.1 Biological materials
C.comatusfruit-body was bought from Xuzhou Edible Fungus Base (Jiangsu Province,China) and identified by Professor Xu Lingchuan of Shandong University of Traditional Chinese Medicine.Kunming (KM) mice were purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China).Bifendate was purchased from Beijing Union Pharmaceutical Factory(Beijing,China).
2.2 Polysaccharide preparation
C.comatusfruit body was extracted by water (1:30) at 90 °C for 3 times.The filtrates were precipitated with 80% ethanol for 10 h,and then the precipitate was washed 3 times with anhydrous ethanol.After redissolution,the solution was treated with Sevage reagent for deproteinization.Then,the solution was removed from the organic solvent.Polysaccharide solution with protein impurities removed was placed in a dialysis bag and dialyzed with running water for 2 days to remove small molecule impurities from the polysaccharide.The dialyzed polysaccharide solution was lyophilized to obtain CCP[15].Polysaccharide purity of CCP measured by phenol sulfuric acid was 59.08% (m/m).The monosaccharide contents in CCP were as follows: glucose (26.36%),galactose (15.21%),mannose (0.75%),rhamnose (0.57%) and glucuronic acid (0.55%).The molecular weight distribution of CCP was determined,the results were as follows: values of CCP were 7.843 × 105 Da and 3.740 × 106 Da in terms ofmnandmw,the Mz of CCP was 2.215 × 107 Da.The molecular weight of CCP was principally distributed in (0.4–0.7) ×103kDa and (0.7–3.5) × 103kDa,accounting for 53.4% and 30.2%respectively.Moreover,the results of the infrared spectrum were that the absorption peak at 2 923 cm−1was the stretching vibration of C-H in CCP.Meanwhile,the absorption roughly at 1238 cm−1which was resulted from the stretching vibration of C-O-C in the sugar ring was very weak in CCP. CCP exhibited a wide absorption peak at 3 387 cm−1,which was the stretching vibration of -OH.Simultaneously,it implicated that there were intermolecular hydrogen bonds.The strong absorption peak at 1 648 cm−1represented the stretching vibration of C=O,which corresponded to the carboxyl functional group.The absorption peak at 1 414 cm−1was attributed to C-H variable angular vibration.Characteristic absorption peak ofα-pyranose was observed at 1 077 cm−1[15].
2.3 Animal experiments
All of mice were male,4-5 weeks old,and weighing (20 ± 2) g.The mice adapted the environment for seven days before formal experiment.The states of the living environment are listed as follows:the living environment was specific pathogen free (SPF) grade.The mice room temperature was maintained at 18−20 °C with a relative humidity of 40% to 60%.Standards for Animal Experimentation of Shandong University of Traditional Chinese Medicine were used in all experiments,which were in conformity to the rules of the Animal Ethics Committee of the institution (Shandong,China).
All mice were divided into four groups as follows: control group,ALD group,CCP group (300 mg/kg CCP) and bifendate group(200 mg/kg bifendate),six mice in each group[13].Equal amounts of distilled water were administered to control group and ALD group by gavage at regular intervals every day.The mice in CCP group and bifendate group were gavaged with CCP (300 mg/kg bw) and bifendate (200 mg/kg bw) daily.
The treatment was continued for two weeks and normal feeding and drinking were maintained throughout the experiment.After the last gavage for 4 h,others except the control group were gavaged with 50% ethanol (10 mL/kg bw),control group was treated with the same volume of distilled water.The mice were fasted for 12 h after modeling without water.After 24 hours of acute alcohol modeling,the fecal samples of mice were collected by the sterile EP tube.Blood samples were collected and centrifuged at 10 000 r/min for 5 min to separate serum for further analysis.The liver sample of mice were collected by the EP tube and weighted.
2.4 Hematoxylin and eosin (H&E) staining
The liver samples were stored in 4% formaldehyde solution and embedded in paraffin.Then,the paraffin sections (4 µm)were dewaxed and rehydrated in differential alcohol gradients for subsequent H&E staining to observe histopathological changes by standard light microscopy.
2.5 Measurement of biochemical parameters in serum
Serum alanine aminotransferase (ALT),aspartate aminotransferase (AST) and triglyceride (TG) (microplate method)were analyzed using commercial kits according to the manufacturer’s protocols (Nanjing Jiancheng Co.,Ltd.)
2.6 Metabolomics analysis
2.6.1 MetaboliteExtraction
The liver (100 mg) was individually grounded with liquid nitrogen and the homogenate was resuspended in prechilled 80% methanol and 0.1% formic acid using a vortex.The samples were then incubated on ice for 5 min and then were centrifuged at 15 000 ×g,4 °C for 20 min.Some of the supernatants were diluted to the final concentration containing 53% methanol using liquid chromatographymass spectrometry (LC-MS) grade water.The liver tissue was subsequently transferred to fresh ep tubes and centrifuged at 15 000 ×g,4 °C for 20 min.Finally,the supernatant was injected into the LC-MS/MS system analysis[19].
2.6.2 UHPLC-MS/MSAnalysis
Ultrahigh performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) analyses were performed using a Vanquish UHPLC system (Thermo Fisher,Germany) coupled with an Orbitrap Q Exactive TMHF-X mass spectrometer (Thermo Fisher,Germany) in Gene Denovo Co.,Ltd.(Guangzhou,China).Samples were injected onto a Hypersil Gold column (100 mm × 2.1 mm,1.9 μm) using a 17 min linear gradient at a flow rate of 0.2 mL/min.The eluents for positive polarity mode were eluents A (0.1% FA in water) and B (methanol).The eluents for negative polarity mode were eluents A (5 mmol/L ammonium acetate,pH 9.0) and B (methanol).The solvent gradient was set as follows: 2% B,1.5 min;2%-100% B,12.0 min;100% B,14.0 min;100%-2% B,14.1 min;2% B,17 min.Q-Exactive TM HF-X mass spectrometer was operated in positive/negative polarity mode with a spray voltage of 3.2 kV,capillary temperature of 320 °C,sheath gas flow rate of 40 arb and auxiliary gas flow rate of 10 arb.
2.7 Gut microbiota analysis
Aseptically take 0.1 g of mouse feces and serially dilute 10-fold.Pipette 200 μL of appropriately diluted fecal diluent and spread on the specific medium.After culturing for a period of time under suitable conditions,growth ofClostridiumperfringens,Enterobacteriaceae,Enterococcus,LactobacillusandBifidobacteriumwere observed and counted[20-22].
2.8 Statistical analysis
All data are expressed as the mean ± standard deviations(SD).SPSS 22.0 software was used for statistical analysis.Peak identification,peak filtering,and peak alignment were performed on the raw data to obtain results,including mass-to-charge ratio(m/z),retention time,and peak area information.Principal component analysis (PCA) was performed using R package ‘gmodels’.PLSDA was performed using R language ‘ropls’ package.To verify the reliability of the PLS-DA model,model cross-validation and permutation tests were performed.Differential metabolites clustering heatmap normalized (Z-score) the differential metabolites between the comparison groups for clustering analysis,and R language heatmap function was used to draw a heatmap which can intuitively display the accumulation differences of differential metabolites in the comparison group.
3. Results
3.1 Effect of CCP on weight and organ index
In general,after reaching a certain dose of drugs,it is easy to produce toxic or even lethal effects on mice.In the present study,the mice had shiny fur,normal urine and stool,and changes in body weight are shown in Fig.1.There were no significant differences in body weight between different treatment groups,and there were no deaths,indicating that the dose of CCP and bifendate used had no toxic effect on mice.
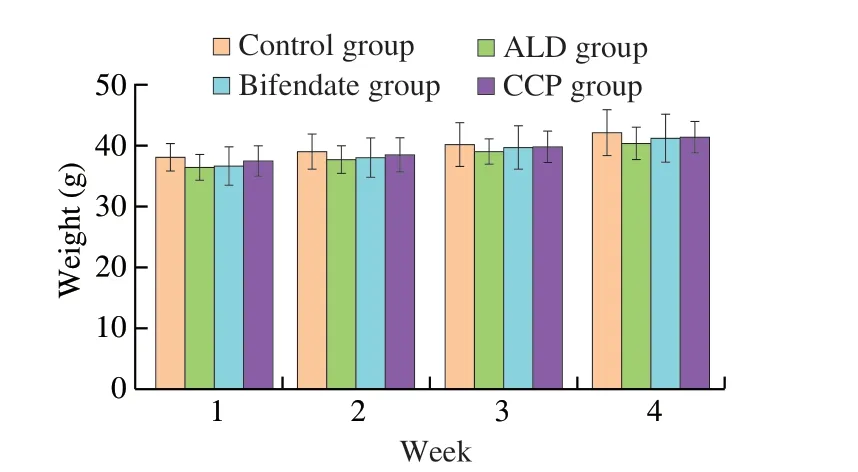
Fig.1 Effects of CCP on body weight of mice with ALD.Values are expressed as mean ± SD.Mice number is 6 per group.
The liver index reflects degree of hepatomegaly,which is a common clinical symptom of ALD[23].As shown in Fig.2,liver index of the mice in ALD group increased by 10.86% compared with control group (P<0.05),indicating an enlarged liver due to excessive alcohol intake.Meanwhile,compared with ALD group,liver index of mice in CCP group decreased by 9.23% (P<0.05),indicating that CCP could effectively alleviate liver enlargement caused by excessive alcohol.

Fig.2 Effect of CCP on liver index in mice with ALD.a P <0.05,indicating comparison with the control group;b P <0.05,indicating comparison with the model group.Values are expressed as mean ± SD.(n=6)
3.2 Morphology of liver tissues
The liver tissue of mice in each group was macroscopically observed.Histological evaluation could provide the most intuitive evidence for ALD.During the experiment,histopathological results manifested that the liver cells of control group showed typical normal liver cell morphology with uniform cytoplasm and clear nuclei(Fig.3A).However,the mice of ALD group showed disorganized arrangement of perivascular hepatocytes,shrunken hepatic sinusoids and cytoplasmic loosing of liver surface cells after administration of alcohol (Fig.3B).
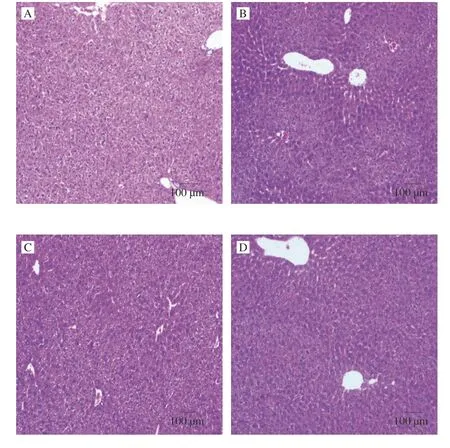
Fig.3 Effects of CCP on pathological changes in mice liver tissue.A: Control group;B: ALD group C: Bifendate group D: CCP
Based on histopathological results of bifendate group and CCP group,the size of liver sinusoids was more normal than ALD group,and the liver cells were arranged regularly (Fig.3C,D).It was proved that after the mice were administrated CCP,the liver damage caused by alcohol was relieved to a certain extent.
3.3 Biochemical indicators of liver injury
Analyzing the levels of ALT,AST,and TG in serum is a common method to determine whether liver is functioning normally.To evaluate the therapeutic effects of CCP,ALT,AST and TG levels in serum were measured and compared with control group.The results are shown in Fig.4.
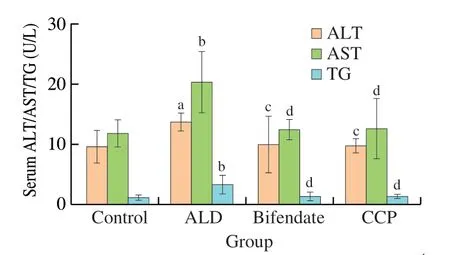
Fig.4 Effects of CCP on serum metabolites.a P <0.05 and b P <0.01,indicating comparison with the control group;c P <0.05 and d P <0.01,indicat
Compared with control group,ALT,AST and TG levels in ALD group increased by 41.98%,71.80% and 181.94%,respectively,suggesting that alcohol can significantly increase ALT,AST and TG levels in mice serum (P<0.05).Whereas bifendate and CCP can effectively reduce the abnormal increase in ALT,AST and TG levels caused by excessive alcohol intake.Compared with ALD group,ALT,AST and TG levels of CCP group decreased by 39.94%,60.19% and 139.63%,respectively,indicating that CCP has a certain mitigating effect on ALD (P<0.05).
3.4 Metabolomics analysis
Metabolomics is a study of the metabolic response of biological systems after external stimuli.It focuses on small molecule compounds,and metabolites pathways and changes in endogenous metabolites of the whole organism,tissue or organs[24].Therefore,metabolites may be small,but the influence of metabolites in human disease and human physiology is truly profound.In this study,metabolomics has offered an opportunity to highlight potential biomarkers which have pointed most directly to ALD mechanisms.To elucidate the mechanism of CCP in treatment with ALD,liver tissue of three groups,including that from control group (CK),ALD group (Model),and CCP group (CCFPS),will be used for metabolic analysis by metabolomics.
3.5 Principal component analysis (PCA)
PCA provides an overall response to overall metabolic differences between groups of samples and the magnitude of variability between samples within groups.Metabolic differences were assessed by PCA among control,ALD,CCP groups.PCA results showed that the clear separation of control and ALD groups,indicating that significant metabolic changes occurred in ALD group (Fig.5A).Fig.5B showed that CCP group was completely separated from ALD group,which proved that CCP changed the metabolism of ALD mice to a certain extent.
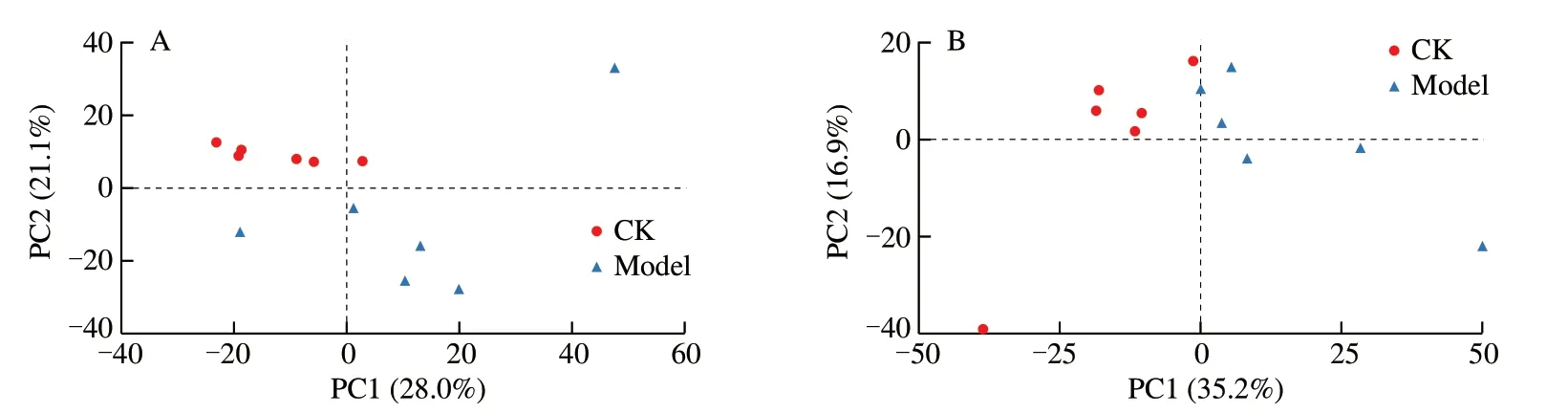
Fig.5 Principal component analysis (PCA) score plot of Control group vs ALD group and ALD group vs CCP group.(A) Control group vs ALD group;(B)ALD group vs CCP group.CK-control group;Model-ALD group;CCFPS-CCP group.
3.6 Partial Least-Squares Discriminant Analysis (PLS-DA)
PLS-DA is a supervised algorithm that combines feature extraction and discriminant analysis into one algorithm[25].PLS-DA can maximize the discrimination between groups,which is conducive to finding different metabolites.The results showed that the samples of control group,ALD group,and CCP group were distributed in different areas and could be separated completely,but samples of the same groups had the tendency to aggregate (Fig.6).It showed that repeatability of the measured metabolism data group was good,and difference among different groups was obvious.The data of metabolome group was highly reliable.
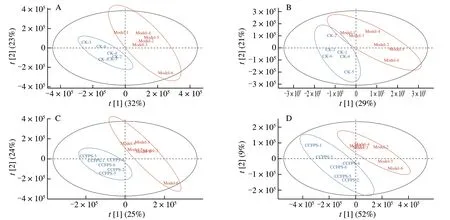
Fig.6 Partial least-squares discriminant analysis of Control group vs ALD group and ALD group vs CCP group.(A) Control group vs ALD group in POS mode;(B) Control group vs ALD group in NEG mode;(C) ALD group vs CCP group in POS mode;(D) ALD group vs CCP group in NEG mode.CK-control group;Model-ALD group;CCFPS-CCP group.
3.7 Hierarchical cluster analysis
The differential metabolites acquired through above-mentioned analysis often have functional similarity or complementarity in biology,or have similar or opposite expression characteristics in different experimental groups due to positive or negative regulation of the same metabolite pathways[26].Hierarchical clustering analysis of such features helps us classify metabolites with the same characteristics into one class and finds the characteristics of metabolites among experimental groups[27].Fig.7 shows variation for each biomarker,illustrating a relative increase or decrease in the biomarker value comparisons among mice in each group.To further evaluate the reversed condition of potential biomarkers via administration of CCP,we analyzed the changes in potential metabolites in different groups.In control vs ALD groups,a total of 25 metabolites contents were up-regulated and 33 metabolites contents were down-regulated under POS and NEG,includingL-norleucine,nicotinamide,inosine,taurocholic acid,valine,adrenic acid,lithocholic acid,L-phenylalanine,valylproline,guanine,phosphocholine,corticosterone,cortisone,proline,taurine,hypoxanthine,DL-malic acid,xanthosine,uridine,whereas in ALD vs CCP groups,a total of 8 metabolites contents were up-regulated and 45 metabolites contents were down-regulated under POS and NEG,including choline,L-ergothioneine,DL-lysine,adenine,pyridoxamine,andrographolide,itaconic acid,fumaric acid,purine.Hierarchical cluster analysis plots (Fig.7A,B,C,D) showed that the different metabolites had good classification results.

Fig.7 Heat map for the identified metabolite in mice.(A) Control group vs ALD group in POS mode;(B) Control group vs ALD group in NEG mode;(C) ALD group vs CCP group in POS mode;(D) ALD group vs CCP group in NEG mode.The redder the color,the higher the abundance of metabolites,and the bluer the color,the lower the abundance of metabolites.
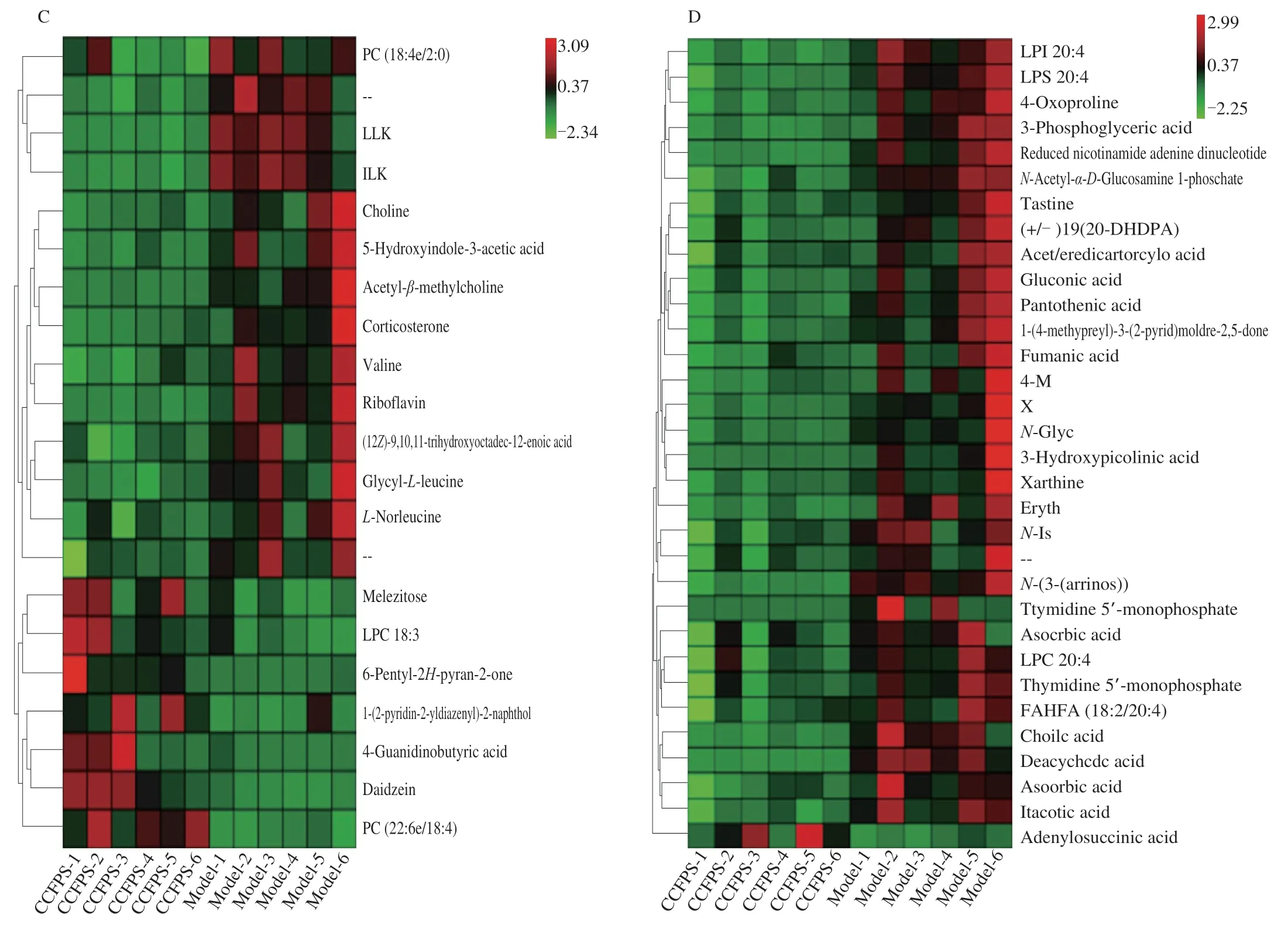
Fig.7(Continued)
3.8 Differential metabolites screening
A variable importance in projection (VIP) score of PLS model is applied to rank the metabolites that the best distinguished between two groups.The threshold of VIP is set to 1.In addition,t-test is also used as a univariate analysis for screening differential metabolites.Those with aPvalue ofttest<0.05 and VIP ≥ 1 are considered differential metabolites between two groups.There were 8 key metabolites that were screened in ALD vs control groups,mainly including fatty acyls,organooxygen compounds carboxylic acids and derivatives and purine nucleosides.At the same time,a total of 12 potential biomarkers were screened in comparison of differential metabolites between ALD group and CCP group,including fatty acyls,carboxylic acids and derivatives,lipids and lipid-like molecules and steroids and steroid derivatives.The results are shown in Table 1 and Table 2.
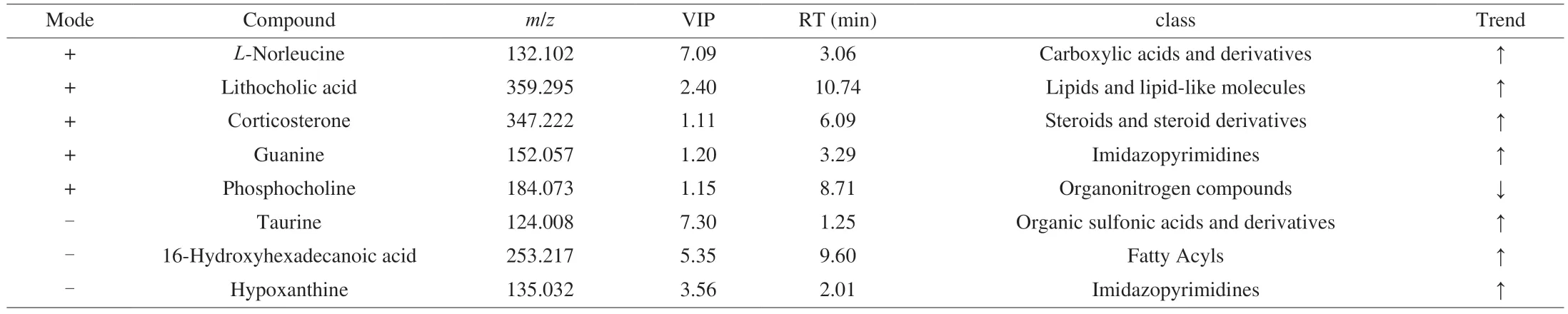
Table 1 List of screened differential metabolites in control vs ALD group.

Table 2 List of screened differential metabolites in ALD vs CCP group.
3.9 Analysis of partial differential metabolites pathways
After finding the metabolites,perform metabolites pathways enrichment analysis on the differential metabolites through KEGG,which is used to compare the most important biochemical metabolites pathways and signal transduction pathways involved in differential metabolites among groups.Metabolites were mapped to KEGG metabolites pathways for annotation and enrichment analysis.Pathway enrichment analysis identified significantly enriched metabolic pathways or signal transduction pathway or signal differential metabolites comparing with the whole background.The selected differential metabolites pathways related to ALD and the changed metabolites pathways after CCP treatment are shown in Table 3 and Table 4.The enrichment degree of each differential metabolite pathway is shown in Fig.8.

Fig.8 KEGG enrichment bubble plot.(A) Control group vs ALD group (B) ALD group vs CCP group.
3.10 Trend analysis
Trend analysis is a method of clustering the expression patterns of metabolites based on the characteristics of multiple continuous samples.Through trend analysis,it is possible to find and visualize the changing trend of metabolites abundance in the continuously changing group.As shown in Fig.9,the expression of some genes in CCP group had a significant upward or downwlog trend compared with ALD group,and gradually approached to control group,indicating that physical metabolism was disturbed by alcohol and this disturbance was alleviated via CCP treatment.
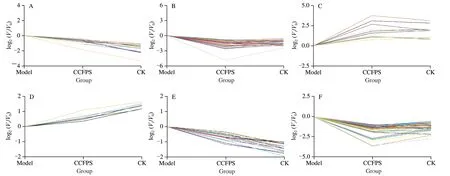
Fig.9 Pictures of gene expression trends in the control group,ALD group,and CCP group.(A-D) positive ion mode;(E-H) negative ion mode.CK-control group;Model-ALD group;CCFPS-CCP group.

Fig.9 (Continued)
3.11 The effect of CCP on the number of gut microbiota in mice
There is evidence that gut microbiota plays a role in the pathophysiology of ALD.Drinking alcohol can cause dysbiosis,largely altering the quality and quantity of gut microbiota[28].Compared with control group,the number ofClostridiumperfringens,Enterobacteriaceae,andEnterococcusin the intestinal tract of ALD group mice increased,whereas the number ofLactobacillusandBifidobacteriumdecreased.Compared with ALD group,the number ofClostridiumperfringens,Enterobacteriaceae andEnterococcusdecreased in CCP group,whereas the number ofLactobacillusandBifidobacteriumincreased in Fig.10.CCP can cause a decrease in the proportion of harmful bacteria and an increase in the proportion of beneficial bacteria in the intestinal tract of ALD mice.The above results proved that CCP can ameliorate alcoholic liver injury in mice by modulating gut microbiota.
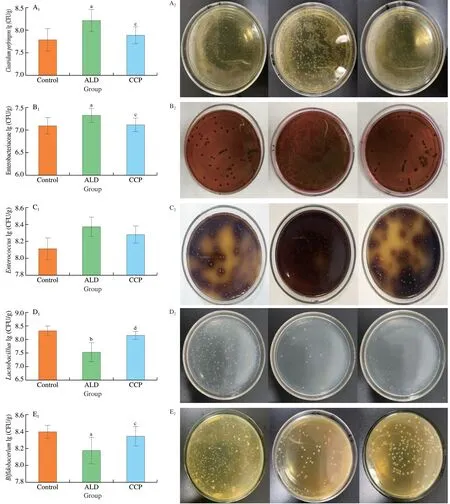
Fig.10 Effects of CCP on the number of gut microbiota in mice with alcoholic liver injury.A: Clostridium perfringens;B: Enterobacteriaceae;C: Enterococcus;D: Lactobacillus;E: Bifidobacterium;1: control group;2: ALD group;3: CCP group.a P <0.05 and b P <0.01,indicating comparison with the control group;c P <0.05 and d P <0.01,indicating comparison with the ALD group.Values are expressed as mean ± SD.(n=6)
4. Discussion
The pathogenesis of ALD is complicated.Excessive alcohol consumption increases the metabolic load of liver.The harmful metabolites cannot be decomposed and eliminated from body in time.Therefore,it will cause disorder of the relevant metabolite pathways in cells,leading to liver cell damage[29,30].Damaged liver cells can lead to increasing levels of substances such as ALT and AST in serum[31,32].
ALT and AST are presented in the cytoplasm and mitochondria of hepatocytes,respectively.The levels of ALT and AST in serum are extremely low under physiological conditions.When the hepatocyte membrane is damaged,ALT and AST are leaked into serum,resulting in a sharp increase in serum ALT and AST levels[33].The levels of ALT and AST are sensitive markers for monitoring the impairment of liver function.In addition,the inability of liver to metabolize alcohol in a timely manner causes a disorder in lipid metabolism resulting in elevated TG levels in serum eventually[34].The levels of ALT,AST and TG in serum can indirectly reflect the degree of liver injury.Compared with control group,levels of ALT,AST and TG in ALD group increased by 41.98%,71.80% and 181.94% respectively in present study.Both CCP and bifendate can reduce the levels of ALT,AST and TG effectively.Particularly,ALT,AST and TG levels of CCP group decreased by 39.94%,60.19% and 139.63% compared with ALD group,respectively.Consistent with biochemical analysis results,H&E staining showed an amelioration of injured liver tissue via CCP treatment.Additionally,during the period when the mice were taking CCP,weight of the mice did not change significantly compared with the mice in control group,and various physiological activities were normal,which proved that CCP did not have any toxic and side effects.The liver index results also showed that mice in CCP group decreased by 9.23% compared with ALD group,indicating CCP effectively relieved liver enlargement caused by excessive alcohol intake.
Metabolomics,a systematic analysis of all metabolites and metabolites pathways in a given biological system,has been increasingly recognized in the study of drug mechanisms on metabolic diseases[35].Liver is a key metabolic organ of living systems mediating the expression levels of large numbers of metabolites[36,37].Liver tissue metabolomics were performed to gain clues to ALD mechanism during the experiment.It has potential to reflect the physiological changes behind ALD.
Herein,PCA and PLS-DA scores showed a significant difference in metabolic characteristics among control group,ALD group,and CCP group.The PCA score plots (Fig.5) showed relative aggregation within three treatment groups,with significant separation among groups.Compared with control group,ALD group showed an obvious separation trend,which proved that ALD model was successfully constructed and excessive alcohol intake had a significant effect on liver metabolism.These results demonstrated that alcohol treatment perturbed the metabolic profile of liver and also suggested changes in endogenous small molecule metabolites.Compared with ALD group,CCP group also showed an obvious separation trend,which proved that CCP did change the liver metabolism profile disturbed by alcohol to a certain extent.Simultaneously,each point represents a sample in PLS-DA plots,and control group,ALD group and CCP group showed good aggregation in both positive and negative ion modes,and three groups could be clearly distinguished in Fig.6.Among them,ALD group could be significantly separated from control group,indicating that the metabolic profile of mouse livers in ALD group changed after alcohol induction.However,the metabolic profile of drug-administered group was reversed after CCP intervention.These results were consistent with detected biochemical markers and pathological changes,suggesting that liver metabolic profile could reflect protective effect of CCP against alcohol-induced liver injury.A total of 16 different metabolites related to ALD were screened in this experiment.In ALD group,the liver was damaged and levels of fumaric acid and pantothenic acid in liver tissue were increased in case of excessive alcohol intake[38].It is well known that energy required for physical functions mainly comes from glycolysis and the TCA cycle[39].The increase in level of fumaric acid,an intermediate in the TCA cycle,indicating that glycolysis pathway and ATP metabolism pathways were inhibited,and aerobic metabolism was promoted.CCP can effectively reduce level of fumaric acid in ALD group to achieve hepatoprotective effects.
Pantothenic acid can reduce fatty degeneration,cytoplasmic vacuolization and necrosis in mice with ALD[40].During the experiment,level of pantothenic acid in the liver tissue was abnormally increased.Whereas level of pantothenic acid in CCP group was reduced,which proved the protective effect of CCP on alcoholic liver injury in mice.
The liver is a main organ for amino acid metabolism and nucleotide synthesis[41].In ALD group,abnormal amino acid metabolism and nucleotide synthesis occurred.This was consistent with an increase in hypoxanthine,adenosine 5’-monophosphate andL-norleucine levels in ALD group,butL-norleucine and hypoxanthine levels were decreased in CCP group.
Hepatocyte damage caused by alcohol-induced oxidative stress is manifested in an increase of choline and valine levels in liver tissue of ALD mice.Oxidative stress can cause lipid peroxidation of liver cell membranes.Choline is an important component of cell membranes and lipoprotein phospholipids,and plays an important role in maintaining cell membrane integrity and lipid metabolism.A higher level of choline brings about damage of cell membranes structure[42,43].CCP effectively reduced the elevated levels of choline and valine in ALD group to protect the liver.
It is worth noting that CCP can significantly up-regulate or down-regulate the elevated levels ofL-norleucine,corticosterone,and taurine in ALD group to restore them to normal levels.It can be concluded that CCP can alleviate ALD by regulating the metabolism of amino acid and organic compounds in the liver of mice.
Based on the results of comparison metabolites pathways among three groups,the following conclusion can be made that the same metabolites pathways includes purine metabolism,pyrimidine metabolism,linoleic acid metabolism,beta-Alanine metabolism,taurine and hypotaurine metabolism,glycerophospholipid metabolism,pantothenate and CoA biosynthesis,amino sugar and nucleotide sugar metabolism,biosynthesis of unsaturated fatty acids,arachidonic acid metabolism and ABC transporters.Analyzing the differential metabolites pathways,these interactions among them are shown in Fig.11.It can be seen that CCP may affect the above pathways to play a hepatoprotective effect.These findings suggested that physical metabolism was disturbed by alcohol and this disturbance was reversed via CCP treatment.
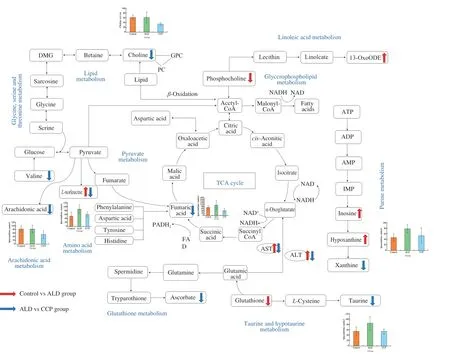
Fig.11 Schematic diagram of metabolic pathway associated with detected biochemical compounds in LC-MS spectrometry.
Gut microbiota participates in different metabolic processes of body,such as affecting the ‘enterohepatic circulation’ by participating in the metabolic process of bile acid.Therefore,the liver is closely related to gut,and the pathogenesis of ALD is closely related to gut microbiota dysbiosis[44,45].Excessive drinking can lead to gut microbiota dysbiosis,which in turn causes a large amount of endotoxin secretion,induces endotoxemia,leading to liver damage eventually[46,47].Studies have confirmed that ALD patients have an increase in the number of harmful bacteria such asClostridium perfringens,Enterobacteriaceae andEnterococcus,and a decrease in the number of beneficial bacteria such asBifidobacteriumandLactobacillus[48,49].In this experiment,the same results appeared in ALD group of mice,which indicated that gut microbiota dysbiosis occurred in the ALD mice.However,CCP can alleviate intestinal dysbiosis to a certain extent.CCP can promote the proliferation of beneficial bacteria and inhibit the proliferation of harmful bacteria in the gut of ALD mice (Fig.10).LactobacillusandBifidobacteriumare main probiotics in gut,which can produce short-chain fatty acids(SCFAs),and effectively alleviate metabolic diseases.SCFAs play an important role in lipid homeostasis and reducing inflammation[50-53].In this study,CCP significantly increased the number ofLactobacillusandBifidobacteriain the gut of ALD mice,indicating that CCP can alleviate ALD in mice by regulating the structure of gut microbiota,increasing the number of beneficial bacteria,and affecting lipid metabolism.Studies have shown that ursolic acid and vitamin C have a regulatory effect on the gut microbiota of mice with ALD.Compared with model group,the contents of Enterobacteriaceae andEnterococcusdecreased by ursolic acid and vitamin C intervention,whereas the contents ofBifidobacteriumandLactobacillusincreased.This is consistent with the changing trend of gut microbiota observed in this experiment[54,55].The above results suggest that CCP can regulate gut microbiota to a certain extent,improve intestinal dysbiosis,and then play a role in improving alcoholic liver injury.
Currently,Ganodermalingzhipolysaccharide showed strong antioxidant activity and can inhibit ethanol-induced steatohepatitis in mice[8].Treatment withCoriolusversicolormycelia polysaccharide exerted hepatoprotective effects against alcohol-induced liver injury in mice by reducing oxidative stress and modulating immunity[9].Echinacea purpurea polysaccharide can prevent alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways[56].These are consistent with the result that CCP can alleviate alcoholic liver injury in mice,but its mechanism for treating ALD is slightly different.CCP can improve liver metabolism in ALD mice by regulating gut microbiota,then alleviating alcoholic liver injury.
5. Conclusions
In conclusion,CCP can significantly reduce AST,ALT and TG activities in serum,suggesting that CCP has a protective effect on alcohol-induced liver injury.Simultaneously,this study reveals that the mechanism of CCP in alleviating ALD had the characteristics of multi-metabolic pathway,which are mainly involved in amino acid metabolism,purine metabolism,lipid metabolism,arachidonic acid metabolism and linoleic acid metabolism.Additionally,CCP can promote the proliferation of beneficial bacteria and inhibit the proliferation of harmful bacteria.CCP can affect liver metabolism by changing the structure of gut microbiota,thereby alleviating alcoholic liver damage.These findings can provide a basis for the in-depth study of CCP to improve ALD.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The current project is funded by Shandong Provincial Natural Science Foundation,China (ZR2020MH370),Major Science and Technology Innovation in Shandong Province (2017CXGC1307) and Ji’nan Science and Technology Project (201303055).
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

