Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
Wenjun Xue,Wenzhu Zho,Siji Wu,Zhipeng Yu,*
a School of Food Science and Engineering,Hainan University,Haikou 570228,China
b College of Food Science and Engineering,Bohai University,Jinzhou 121013,China
c Lab of Nutrition and Functional Food,Jilin University,Changchun 130062,China
Keywords:ACE inhibitory peptide Kidney Mechanism Metabolomics Spontaneously hypertensive rats
ABSTRACT The angiotensin-converting enzyme (ACE) inhibitory peptide NCW derived from Mizuhopecten yessoensis has been demonstrated to have significant in vivo anti-hypertensive effects,however,its anti-hypertensive mechanism is still not fully clarified.This study established a UPLC-Q-TRAP-MS/MS-based widely targeted kidney metabolomics approach to explore the changes of kidney metabolic profiles and to clarify the antihypertensive mechanism of peptide NCW in spontaneously hypertensive rats (SHRs).Multivariate statistical analysis indicated that the kidney metabolic profiles were clearly separated between the SHR-NCW and SHRUntreated groups.A total of 85 metabolites were differentially regulated,and 16 metabolites were identified as potential kidney biomarkers,e.g.,3-hydroxybutyrate,malonic acid,deoxycytidine,and L-aspartic acid.The peptide NCW might regulate kidney metabolic disorder of SHRs to alleviate hypertension by suppressing inflammation and improving nitric oxide production under the regulation of linoleic acid metabolism,folate related pathways,synthesis and degradation of ketone bodies,pyrimidine metabolism,β-alanine metabolism,and retinal metabolism.
1. Introduction
Food-derived bioactive peptides play vital physiological roles in human health and disease prevention for their safety,high activity,good absorption,and strong targeting ability[1,2]. Especially,the application of angiotensin-converting enzyme (ACE) inhibitory peptides as dietary and nutritional supplements to prevent and improve hypertension have recently attracted great attention due to the rising prevalence and mortality of hypertension[3].
ACE inhibitory peptides withinvivoanti-hypertensive effect have been identified from multiple food sources by using spontaneously hypertensive rats (SHRs),including peptides QIGLF,TNGIIR from egg white[4,5],peptide LRW from pea[6],peptide WGAP from rabbit meat[7],and peptide LVLPGE from broccoli[8]. However,theinvivoanti-hypertensive mechanism of most ACE inhibitory peptides has not been fully clarified,thereby greatly limiting their development and application[9,10].Therefore,it is necessary to further investigate thoroughly and provide a more comprehensive understanding of theinvivoanti-hypertensive mechanism of ACE inhibitory peptides for better preventing and improving hypertension.
Small molecule metabolites as the final products of gene expression are the basis for biological phenotypes,and metabolic dysfunction is a fundamental core mechanism of hypertension[11].Therefore,metabolomics appears to be a promising and effective approach to reveal the underlying anti-hypertensive mechanism of ACE inhibitory peptides,which can detect the changes of a great number of metabolites,especially those metabolites that cannot be detected by traditional technologies and can achieve the qualification and quantification of the metabolites simultaneously[12].However,very limited research has been reported on the application of metabolomics in investigating the mechanism of ACE inhibitory peptides.The untargeted serum metabolomics analysis carried out by Yu et al.demonstrated that a total of 8 potential serum biomarkers were identified in SHRs after intervention with peptide QIGLF and peptide QIGLF might lower blood pressure by improving endothelial dysfunction[13].Manoharan et al.found that the levels of serum metabolites associated with the renin-angiotensin system were significantly changed in SHRs after administration with peptide GVR[14].It should be noted that the kidney is a key target organ for blood pressure regulation,hypertension,and anti-hypertensive treatment,which can provide highly selective metabolite information[15,16].Additionally,ACE inhibitory peptide can regulate kidney metabolic disorder to exert anti-hypertensive effects[4,17].
Our previously published study has found that ACE inhibitory peptide Asn-Cys-Trp (NCW,IC50value=35.5 μmol/L) derived from myosin ofMizuhopecten yessoensishas a significant antihypertensive effect in SHRs after three weeks of oral administration[18],which could significantly reduce systolic and diastolic blood pressure of SHRs by (48.08 ± 3.84) mmHg and (48.92 ± 5.77) mmHg,respectively.However,theinvivoanti-hypertensive mechanism of peptide NCW has not been fully clarified,and how peptide NCW lowered blood pressure by improving kidney metabolic disorder also needs an in-depth investigation.Therefore,the primary objectives of this present study were to explore the influence of peptide NCW on kidney metabolic profiles of SHRs,to identify the potential kidney biomarkers and corresponding metabolic pathways associated with the anti-hypertensive effect,and to clarify the underlying anti-hypertensive mechanism of peptide NCW by using a widely targeted kidney metabolomics approach (a new technology that integrates the advantages of untargeted and targeted metabolomics)combined with multivariate statistical analysis and bioinformatics analysis.The findings of this study will provide a novel insight into the development and application of ACE inhibitory peptides as dietary and nutritional supplements in the prevention and improvement of hypertension.
2. Materials and methods
2.1 Materials and reagents
Methanol and acetonitrile (UPLC grade) were provided by Merck (Darmstadt,Hesse,Germany).Formic acid (UPLC grade) was obtained from Aladdin (Shanghai,China).Standards of metabolites were provided by Sigma-Aldrich (Saint Louis,MO,USA) and BioBioPha Co.,Ltd (Kunming,Yunnan,China).The peptide NCW was synthesized by Nanjing YuanPeptide Biotechnology Co.,Ltd (Nanjing,Jiangsu,China) and was dissolved in 0.9% saline obtained from Shangdong Kelun Pharmaceutical Co.,Ltd (Binzhou,Shangdong,China) before being administered to SHRs.Rodent feed(Catalog number: LAD 1000 M) for SHRs was supplied by Trophic Animal Feed High-Technology Co.,Ltd (Nantong,Jiangsu,China).
2.2 In vivo animal experiments
Twelve SPF-grade male SHRs (SCXK 2016-0006,tail SBP >180 mmHg,10 weeks,approximately 255 g) were provided by Beijing Vital River Laboratory Animal Technology Co.,Ltd (Beijing,China).All SHRs were kept at standard conditions with a 12 h light/ dark cycle,(22 ± 3) °C,(60 ± 5)% relative humidity,and were fed with feed and waterad libitum.After a week of adaption,the SHRs were divided into two groups at random (n=6 in each group): SHR-NCW group (peptide NCW at 80 mg/kg body weight) and SHR-Untreated group (saline alone).All SHRs were carefully administered with the corresponding sample solution (5 mL/kg) at 8: 00 am for consecutive 21 days.After the last administration,the SHRs were fasted but fed with water for 24 h,and then were euthanized with ether.After confirming that the SHRs were euthanized,the abdominal cavities of the SHRs were opened along the midline of the lower abdomen.The kidney tissues were immediately separated on ice from SHRs within 5 min,frozen with liquid nitrogen,and stored at −80 °C until further analysis.All effort was taken to minimize the pain of SHRs.All animal experimental procedures were recognized by the Animal Research Ethics Committee of Jilin University (Approval Number:201702003) and were strictly performed following the regulations set by the care and use of laboratory animals.
2.3 Extraction of kidney metabolites and preparation of quality control samples
Frozen kidney tissues were thawed on ice.Each kidney tissue was taken for 50 mg,and then homogenized 4 times (30 Hz,0.5 min each time) using a MM400 high-speed vibrating ball mill (Retsch GmbH,Hanna,Germany).One mL cold methanol (70%) containing internal standard was added to the homogenized kidney tissue.Subsequently,the mixture was vigorously whirled for 5 min,placed on ice for 15 min,and centrifuged for 10 min (16 000 ×g,4 °C).The collected supernatant was placed at -20 °C for 12 h,and then centrifuged for 3 min (16 000 ×g,4 °C),so as to obtain high-purity kidney metabolites and prevent blocking the chromatographic column in the subsequent experiment.Finally,the supernatant (400 μL) was taken for ultrahigh pressure liquid chromatography triple-quadrupole linear ion-trap tandem mass spectrometry (UPLC-Q-TRAP-MS/MS).
Moreover,each kidney sample of the same volume (10 μL) from the SHR-NCW and SHR-Untreated groups was mixed and prepared as a quality control (QC) sample.Before kidney sample analysis,three QC samples were run to stabilize the instrument,and then a QC sample was run once after every 10 kidney samples during the whole run to monitor the stability and repeatability of the instrument.
2.4 UPLC-Q-TRAP-MS/MS analysis
Kidney metabolites were separated by a ExionLCTMAD UPLC system (AB SCIEX,Framingham,MA,USA).Two μL kidney sample was injected a Waters ACQUITY UPLC HSS T3C18column(1.8 μm,2.1 mm × 100 mm) at a column temperature of 40 °C.The solvent system consisted of water containing 0.1% formic acid(mobile phase A) and acetonitrile containing 0.1% formic acid (mobile phase B).The detailed gradient elution parameters were presented in Table S1.The separated metabolites were alternatively connected to a Triple QuadTM6500+Mass Spectrometer equipped with an electrospray ionization (ESI) source (AB SCIEX).The specific ESI source operation conditions were as follows: ion spray voltage,5 500 V (positive)/ −4 500 V (negative);ESI source temperature,500 °C;ion source gas I,55 psi;ion source gas II,60 psi;curtain gas,25 psi;collision-activated dissociation,high.Moreover,10 and 100 μmol/L polypropylene glycol were used for instrument tuning and mass calibration in triple quadruple (QQQ) and linear ion trap (LIT)modes,respectively.
2.5 Qualitative and quantitative analysis of kidney metabolites
Qualitative analysis of kidney metabolites was achieved by searching the internal database MWDB (Metware Biotechnology Co.,Ltd.Wuhan,Hubei,China) and public databases (e.g.,HMDB,MassBank,and METLIN) based on primary and secondary mass spectrometry data.Quantitative analysis of kidney metabolites was carried out by the multiple reaction monitoring (MRM) mode of the mass spectrometer.The mass spectrum peak of the same metabolite from different samples was integrated and corrected by MultiQuant software (version 3.0.3).The area of each mass spectrum peak represented the relative content of the corresponding metabolite.
2.6 Data processing and statistical analysis
MS data acquisition and analysis were performed using Analyst 1.6.3 software (AB SCIEX,version 1.6.3).And the data was log2-transformed for statistical analysis to improve normality and was normalized.The normalized metabolic data was further processed by univariate statistical analysis [fold change (FC) analysis and student’st-test] and multivariate statistical analysis [principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA)].Unsupervised PCA was performed by R package (http://www.r-project.org,version 3.5.0) to clarify the overall metabolic difference and variation degree between the kidney samples.Supervised OPLS-DA model was generated using MetaboAnalystR (version 1.0.1) and was validated using a permutation test (200 times).The variable importance projection (VIP)value from the OPLS-DA model was calculated for investigating the contribution of a kidney metabolite to the separation between the groups.And the VIP values were visualized in the form of the S plot.
2.7 Screening of differential kidney metabolites and bioinformatics analysis
The metabolite with VIP value >1,P-value <0.05,and FC value >1.5 (up-regulated) or <0.67 (down-regulated) was screened as a differential kidney metabolite,which were visualized in a volcano plot.The cluster heatmap analysis of differential kidney metabolites was performed using ComplexHeatmap software (version 2.2.0).The Kyoto Encyclopedia of Genes and Genomes (KEGG)database (https://www.genome.jp/kegg) was used for annotation and enrichment analysis of the differential kidney metabolites to obtain specific metabolic pathways information.A differential kidney metabolite with metabolic pathways and closely related to the antihypertensive effect of peptide NCW was identified as a potential kidney biomarker.Finally,a metabolic mechanism network of key potential kidney biomarkers and related metabolic pathways was constructed to clarify the anti-hypertensive mechanism of peptide NCW.
3. Results and discussion
3.1 Data quality assessment by quality control analysis
To obtain reliable and repeatable data,the total ion current(TIC) curves of three QC samples were overlapped and analyzed in the positive and negative ion modes,respectively.As shown in Fig.S1,the TIC curves of the QC samples were basically completely overlapped in the peak intensity and retention time,indicating that the mass spectrometer had high stability when detecting the same sample at different times.This excellent stability of the mass spectrometer provides an important guarantee for the reliability and repeatability of data[19].
3.2 Multivariate statistical analysis of kidney metabolic profiles
A total amount of 613 kidney metabolites were detected in this study,and multivariate statistical analyses including PCA and OPLS-DA were performed based on these metabolites.PCA was utilized to explore the changes in the overall metabolic profiles by visualizing clusters and reducing the dimension of complex data[13].The PCA score plot (3D) showed a distinct separation between the SHR-NCW and SHR-Untreated groups(Fig.1A),which indicated that peptide NCW greatly altered the kidney metabolic profiles of SHRs.
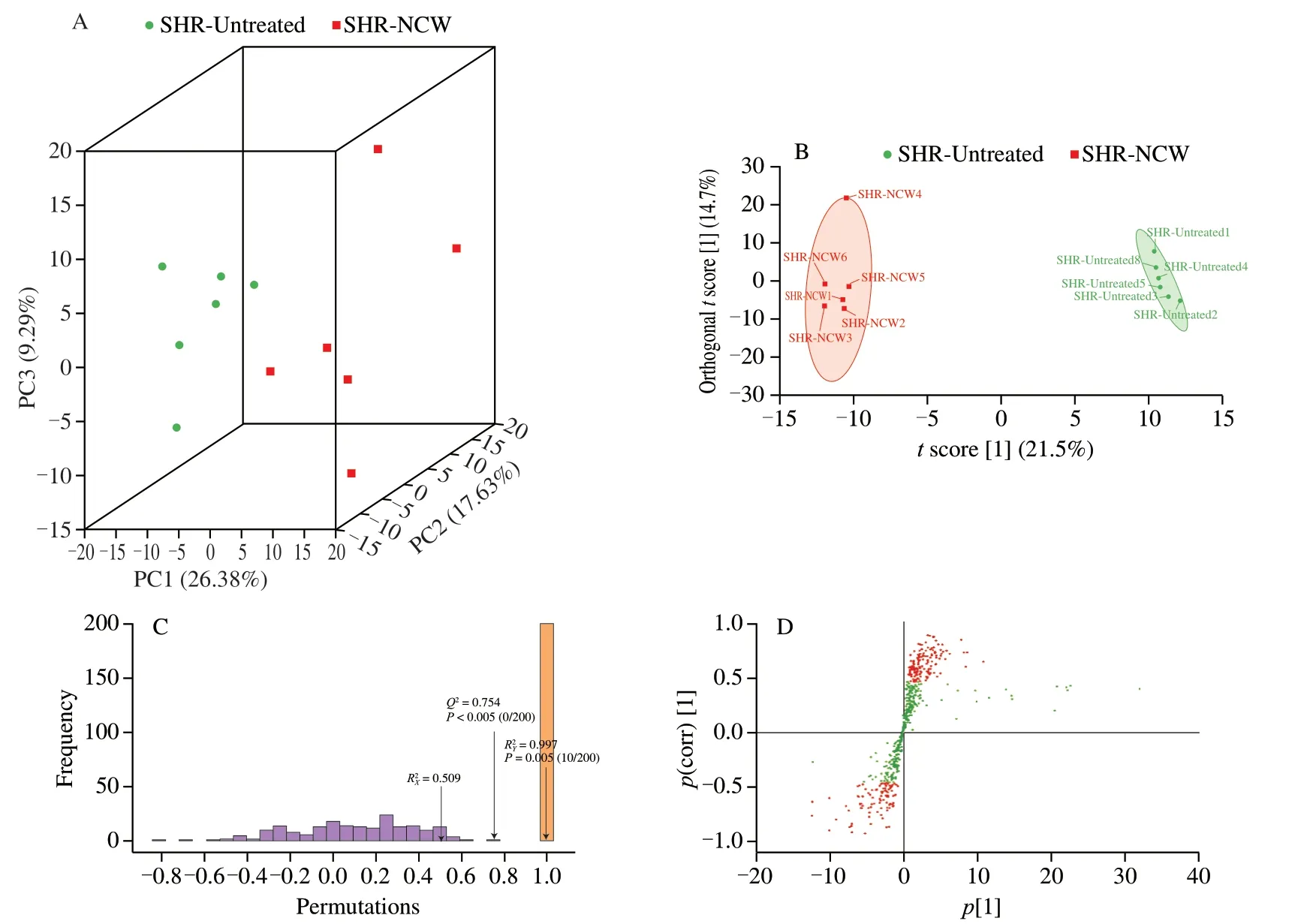
Fig.1 Multivariate statistical analysis of kidney samples after intervention with peptide NCW between the SHR-NCW and SHR-Untreated groups,(A) PCA (3D) score plot;(B) OPLS-DA score plot;(C) permutation test plot of the OPLS-DA model;(D) S plot of the OPLS-DA model.
Subsequently,an OPLS-DA model was conducted for improving the classification performance and obtaining the differential kidney metabolites.Similarly,the SHR-NCW group was clearly separated from the SHR-Untreated group in the OPLS-DA score plot (Fig.1B),indicating the kidney metabolites were significantly affected after treatment with peptide NCW for 3 weeks.andQ2values as the important parameters of the OPLS-DA model could measure the goodness of interpretation rate and prediction ability,respectively.The closer the two parameters are to 1,the more reliable and stable the OPLS-DA model would be[20].TheandQ2values of the OPLSDA model were higher than 0.7,suggesting this reliable model was effectively established,and had outstanding interpretation rate and prediction ability.Moreover,the result of the permutation test (200 times) showed that theP-value ofwas less than 0.005 and theP-value ofQ2was less than 0.05 (Fig.1C),indicating the establishing OPLS-DA model was optimal and not overfitting in the current study.
To investigate the contribution of kidney metabolites to intergroup separation,S plot analysis was carried out based on the VIP values calculated by the OPLS-DA model[21].In the S plot (Fig.1D),red dots represented the metabolites with VIP values >1,while green dots represented the metabolites with VIP values <1.There were 259 metabolites with VIP values >1,which were likely to be differential metabolites for anti-hypertensive effect of peptide NCW.
3.3 Identification and classification analysis of differential kidney metabolites
Combined with univariate statistical analysis,a total number of 85 metabolites were identified as differential kidney metabolites (Table S2).The difference of the expression levels of these differential kidney metabolites between the SHR-NCW and SHR-Untreated groups could be visualized by a volcano plot[22].In the volcano plot(Fig.2A),40 differential kidney metabolites represented by red dots were up-regulated (FC >1.5),and 45 differential kidney metabolites represented by green dots were down-regulated (FC <0.67) after treatment with peptide NCW in SHRs.In addition,the differential kidney metabolites were roughly divided into 10 categories(Fig.2B),main concentrating on amino acid and its metabolites(16.47%),organic acid and its derivatives (16.47%),fatty acyls(15.29%),and nucleotide and its metabolites (14.12%),indicating which played important roles for anti-hypertensive effect of peptide NCW.The change trends and number of differential kidney metabolites contained in each type of metabolite were shown in Fig.2C.Moreover,the association and change law of differential metabolites could be quickly obtained by cluster heatmap analysis[23].The cluster heatmap showed that the SHR-NCW and SHR-Untreated groups were clearly divided into two different bands,and most differential kidney metabolites were well clustered (Fig.2D).
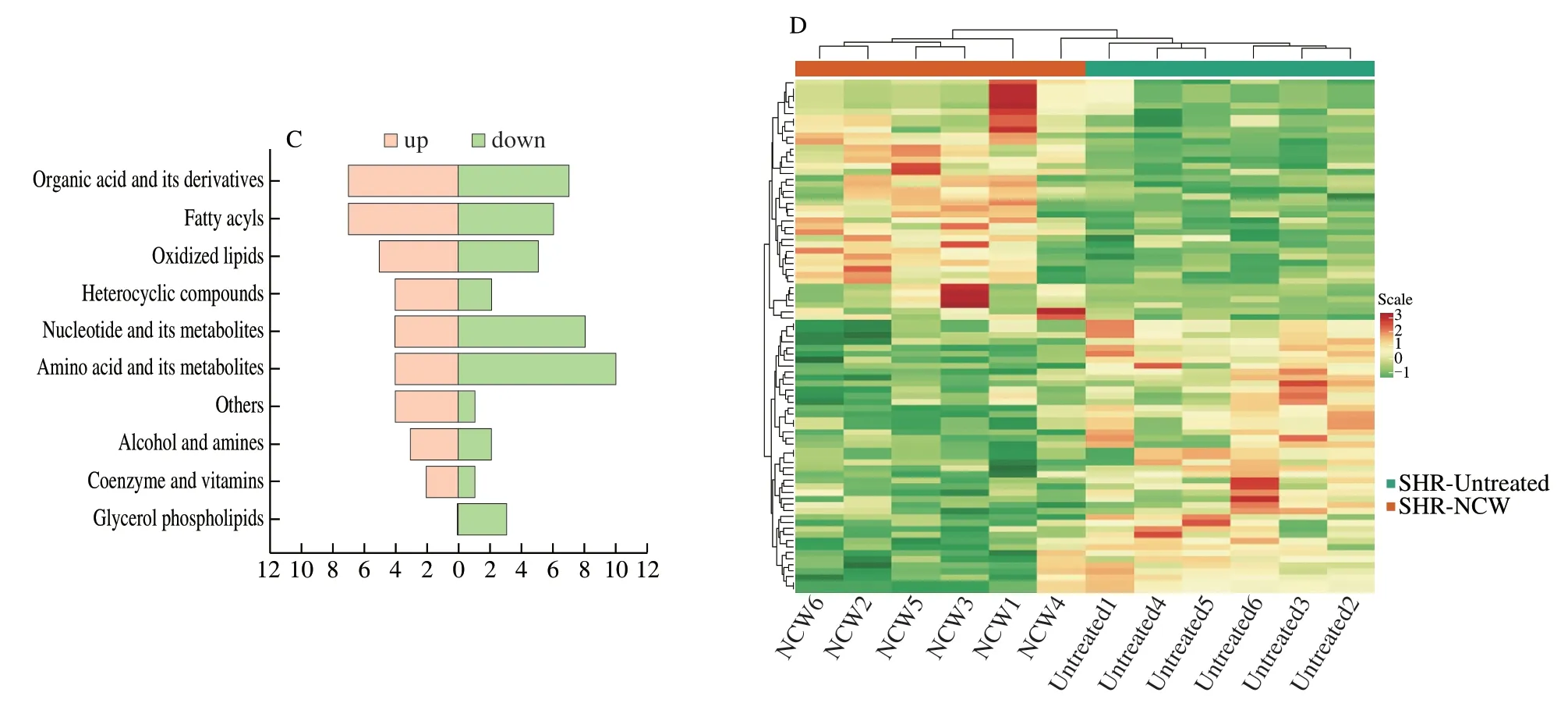
Fig.2 (Continued)
3.4 KEGG pathway annotation and enrichment analysis of differential kidney metabolites
To better understand how the changes of differential kidney metabolites caused by peptide NCW intervention posed antihypertensive effect in the SHRs,the KEGG pathway annotation and enrichment analysis was performed based on the KEGG database.A total of 41 differential kidney metabolites were annotated and distributed in 79 metabolic pathways.The top 20 KEGG metabolic pathways with the most significant enrichment were displayed in Fig.3.Ultimately,comprehensively analysis of theP-values and rich factors of metabolic pathways,and the biological functions,VIP values,and FC values of the metabolites contained in these metabolic pathways,8 metabolic pathways (linoleic acid metabolism,folate biosynthesis,one carbon pool by folate,antifolate resistance,synthesis and degradation of ketone bodies,pyrimidine metabolism,β-alanine metabolism,and retinal metabolism) were chosen as the important pathways closely associated with the anti-hypertensive effect of peptide NCW,and 16 differential kidney metabolites were identified as potential kidney biomarkers based on the results of KEGG pathway enrichment analysis and related literature (Table 1).Additionally,the expression intensities of these potential kidney biomarkers were visualized in the form of violin plots (Fig.4).Compared with the SHR-Untreated group,the levels of eicosapentaenoic acid (EPA),15-oxo-eicosatetraenoic acid (15-oxo-ETE),7,8-dihydrobiopterin(BH2),3-hydroxybutyrate,malonic acid,barbituric acid,deoxycytidine,thymine,4-aceylaminobutyric acid,and 11-cisretinol in the SHR-NCW group were significantly increased,whereas those of 9,10-dihydroxy-12Z-octadecenoic acid (9,10-DiHOME),9-hydroperoxyoctadecadienoic acid (9-HpODE),10-formyltetrahydrofolate (10-formyl-THF),uridine-5-monophosphate (UMP),L-aspartate,and spermidine were significantly decreased.The above potential kidney biomarkers alterations induced related metabolic pathways changes,thus improving metabolism disorders and lowering blood pressure in SHRs.
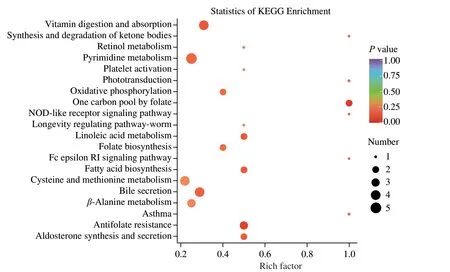
Fig.3 The top 20 KEGG metabolic pathways with the most significant enrichment.The size of the dot represents the number of differential kidney metabolites contained in a metabolic pathway.The color of the dot represents the P-value of a metabolic pathway,and the redder a dot is,the more the metabolic pathway is significant.
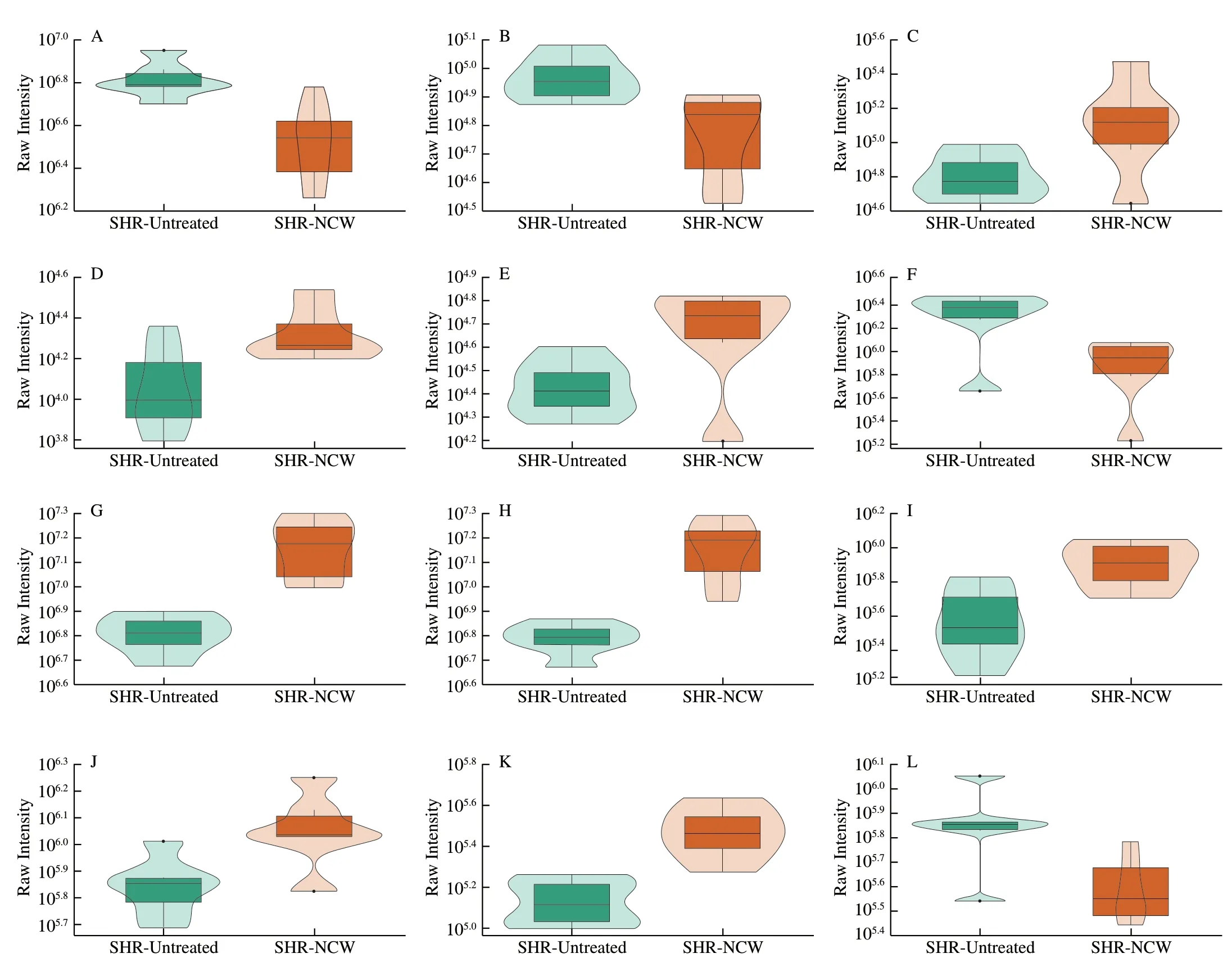
Fig.4 Violin plots of the potential kidney biomarkers.The abscissa represents the grouping,and the ordinate represents the expression intensities (that is original peak areas) of potential kidney biomarkers.The box in the middle represents the four-digit score range,and the thin black line extending from it represents the 95%confidence interval.The black horizontal line in the middle represents the median of the data,and the external shape represents the distribution density of the data.
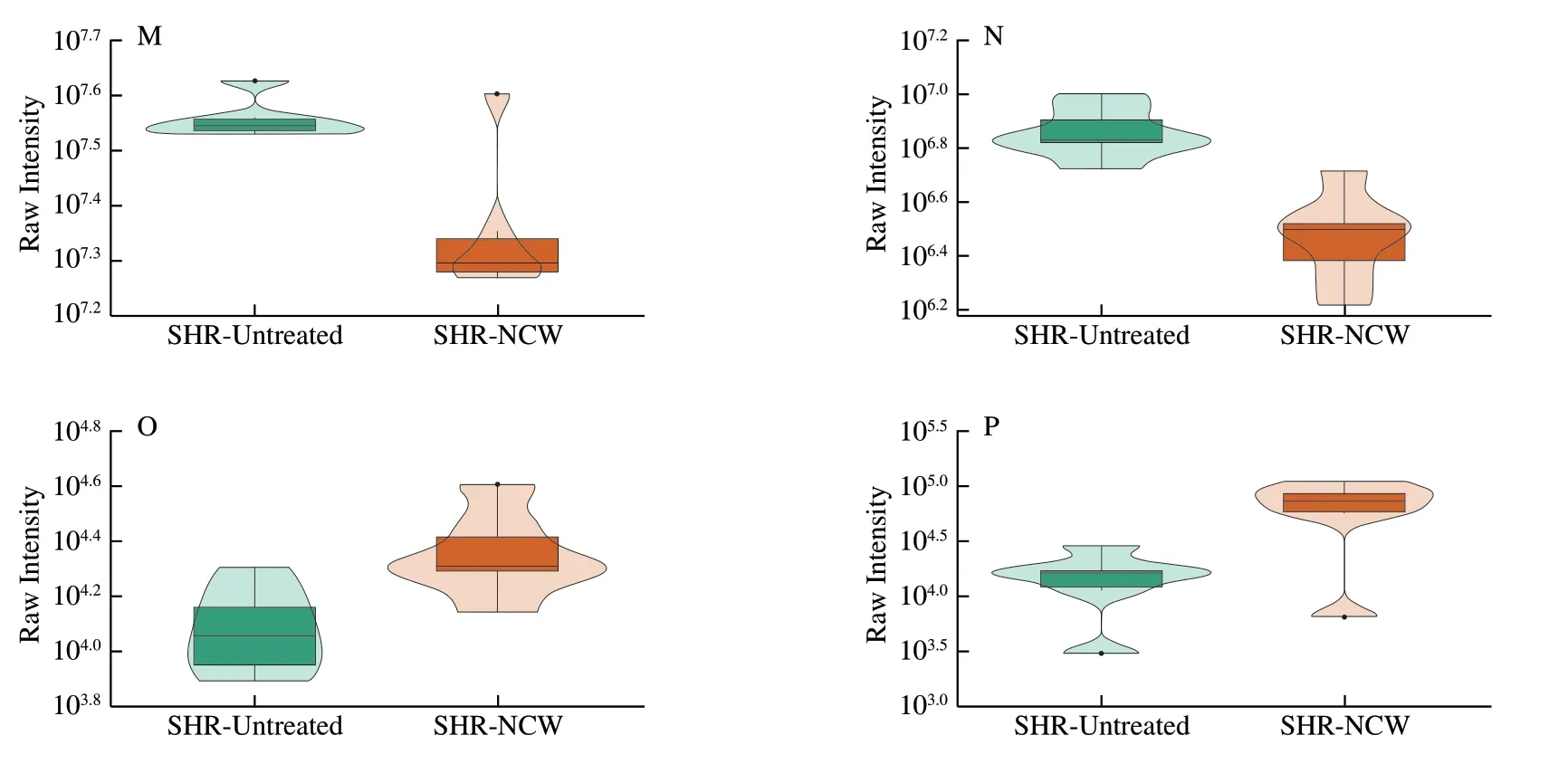
Fig.4 (Continued)
3.5 Construction and analysis of the metabolic mechanism network of potential kidney biomarkers and related metabolic pathways
To intuitively understand the anti-hypertensive mechanism of peptide NCW,a metabolic mechanism network was constructed based on these potential kidney biomarkers and related metabolic pathways(Fig.5).

Fig.5 Metabolic mechanism network of the potential kidney biomarkers and related metabolic pathways associated with the anti-hypertensive effect of peptide NCW.Red dashed box: up-regulated potential kidney biomarker;green dashed box: down-regulated potential kidney biomarker.Solid arrow: direct reaction;dashed arrow: indirect reaction;bidirectional arrow: reversible reaction.
As aω-6 polyunsaturated fatty acid (PUFA),linoleic acid has an important effect on cell signal transduction and gene expression regulation,and its oxidation products are widely used as important homeostatic regulators of inflammation,vasodilation,and other physiological processes[24].9,10-DiHOME and 9-HpODE,which were involved in linoleic acid metabolism,were decreased by 0.541-fold and 0.66-fold in the SHR-NCW group,respectively.9,10-DiHOME is a leukotoxin derivative of linoleic acid diol produced by inflammatory leukocytes,and has cytotoxicity and tissue toxicity.Mitochondrial dysfunction,increased oxidative stress,and apoptosis are the main toxicity characteristics of 9,10-DiHOME[25].It has been reported that high concentrations of 9,10-DiHOME and 9,10-EpOME can activate AP-1 and NF-κB transcription factors,both of which mediate inflammation[26].However,9-HpODE is extremely unstable and further decomposed to form 9-hydroxyoctadecaenoic acid (9-HODE)invivo[27].9-HODE has pro-inflammatory properties and can promote the apoptosis of monocytes and macrophages[28].However,EPA and 15-oxo-ETE as the important related metabolites of linoleic acid metabolism,were significantly increased by 2.208-fold and 1.767-fold after intervention with peptide NCW.EPA is an importantω-3 PUFA produced fromα-linolenic acid by desaturase and elongation enzyme.EPA is believed to improve impaired endothelial function and increase endothelium-dependent vasodilation,mainly by activating endothelial nitric oxide synthase (eNOS) and promoting nitric oxide (NO,a endothelial derived relaxing factor) production in endothelial cells[29].In addition,15-oxo-ETE is formed from 15-hydroxyprostaglandin dehydrogenase-mediated oxidation of 15-HETE.And 15-oxo-ETE is a vital anti-inflammatory mediator,which can activate anti-inflammatory Nrf2 signaling and downregulate pro-inflammatory cytokine[30].Combining of all the above analysis,peptide NCW might regulate the related metabolites of linoleic acid metabolism in the kidney of SHRs to inhibit inflammation and apoptosis,and improve NO production.
The role of folate related pathways in maintaining homeostasis,material metabolism,and energy metabolism has been gradually underscored.Compared with the SHR-Untreated group,the level of BH2in folate biosynthesis was increased by 1.801-fold in the SHR-NCW group.BH2is reduced to tetrahydrobiopterin (BH4) by dihydrofolate reductase,and BH4is an important NOS cofactor[31].BH4can stabilize and couple the dimer structure of eNOS,and catalyze the conversion ofL-arginine toL-guanidine and NO.BH4deficiency can lead to eNOS uncoupling,resulting in reduced NO synthesis,increased peroxide synthesis,and vascular endothelial damage[32].In this study,the change of BH2might increase the production of BH4to attenuate hypertension by mediating the eNOS uncoupling and improving the production of NO in the kidney of SHRs.In addition,one carbon metabolism is necessary for the biosynthesis required for cell proliferation and vital for redox balance[33].10-Formyl-THF as a pivotal precursor of purine and N-formylmethionine synthesis,was decreased by 0.38-fold in the pathways of one carbon pool by folate and antifolate resistance after treatment with peptide NCW.Reportedly,if an accumulation of 10-formyl-THF occurs in endothelial cells,this accumulation might influence the adjacent pathways of folate metabolism,and then affect the activity of eNOS[34].
3-Hydroxybutyrate,which was involved in synthesis and degradation of ketone bodies,was increased by 2.28-fold in the SHR-NCW group.As is well known,3-hydroxybutyrate belongs to the ketone body family produced mainly by the liver,and has the abilities of anti-inflammatory,anti-oxidative,and preventing oxidative stress[35].And 3-hydroxybutyrate is a vital inhibitor of the over activation of the Nlrp3 inflammasome,which has a vital role in kidney injury and contributes to aggravating inflammation[36].Nutritional supplement with the 3-hydroxybutyrate precursor 1,3-butanediol can attenuate hypertension and suppress kidney injury by inhibiting the renal Nlrp3 inflammasome formation in salt-sensitive hypertension[37].Moreover,a recent study showed that 3-hydroxybutyrate was an autophagy-dependent vasodilator[38].The above analysis suggested that peptide NCW might attenuate hypertension by increasing the level of 3-hydroxybutyrate to inhibit inflammation in the kidney of SHRs.
Pyrimidines are the basic constituents of DNA and RNA,and pyrimidine metabolism has been reported to be closely associated with the development of hypertension and its complications[39].Compared with the SHR-Untreated group,malonic acid,barbituric acid,deoxycytidine,and thymine,which were involved in pyrimidine metabolism,were increased in the SHR-NCW group by 2.349-fold,2.162-fold,1.645-fold,and 2.203-fold,respectively.And the level of UMP was decreased by 0.558-fold.Malonic acid can reduce the production of mitochondrial ROS in Ang II-induced hypertension,which contributes to improving oxidative stress and endothelial dysfunction[36].However,the anti-hypertensive mechanism of barbituric acid (a condensation metabolite of malonic acid and urea)is still not clear.Deoxycytidine is the main nucleoside of DNA,thymine is the pyrimidine base,and UMP is the uracil nucleotide,which participate in almost all biochemical reactions[40].This suggested that the anti-hypertensive mechanism of peptide NCW might be related to the regulation of deoxycytidine,thymine,and UMP levels in pyrimidine metabolism.Importantly,pyrimidine metabolism andβ-alanine metabolism were well connected by malonic acid.Inβ-alanine metabolism,the levels ofL-aspartate and spermidine were decreased by 0.642-fold and 0.423-fold in the kidney of SHRs after intervention with peptide NCW.Spermidine was converted to 4-aceylaminobutyric acid by complex multi-step reaction.4-Aceylaminobutyric acid as the precursor of 4-aminobutyric acid (GABA) was increased by 1.909-fold in the SHR-NCW group.Reportedly,GABA inhibits the release of norepinephrine from sympathetic nerve fibers by acting on presynaptic GABABreceptors,thereby suppressing the increase of blood pressure in SHRs[41].In short,pyrimidine metabolism andβ-alanine metabolism have complex multifaceted effect in hypertension,the anti-hypertensive effect of peptide NCW in this study might be the result of comprehensive action of many active substances in pyrimidine metabolism andβ-alanine metabolism.
Retinol (vitamin A) is a vital regulator of cell proliferation,differentiation,apoptosis,immune function,and visual system.The kidney is a target organ for retinol action[42].11-cis-retinol was involved in retinol metabolism,which was increased by 1.645-fold after intervention with peptide NCW.Emerging findings indicated that retinol seemed to have a vital inhibitory effect on inflammation and might improve endothelial function by regulating NO related pathway[43].This suggested that retinol might play an important role in lowering blood pressure,and peptide NCW intervention increased the expression level of 11-cis-retinol,which contributes to inhibiting inflammation and regulating NO production in the kidney of SHRs.
4. Conclusion
In summary,the widely targeted kidney metabolomics analysis indicated kidney metabolites disorder caused by hypertension was partially alleviated by peptide NCW intervention.Peptide NCW might exert anti-hypertensive effect by suppressing inflammation and improving NO production in the kidney of SHRs under the regulation of linoleic acid metabolism,folate related pathways,synthesis and degradation of ketone bodies,pyrimidine metabolism,β-alanine metabolism,and retinal metabolism.This study will provide an important clue and insight for the anti-hypertensive mechanism of peptide NCW,and provide a theoretical basis for the development and application of ACE inhibitory peptides in the prevention and improvement of hypertension.In future studies,the relationship between the changes in kidney metabolites and the underlying anti-hypertensive mechanism will be further validated by target metabolomic studies,cell experiments,and other biological technologies.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (No.31901635).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250041.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

