A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
Qioyun Li,Zumn Dou,b,Qingi Dun,Chun Chn,c,d,*,Ruihi Liu,Yuming Jing,Bo Yng,Xiong Fu,b,d,*
a SCUT-Zhuhai Institute of Modern Industrial Innovation,School of Food Science and Engineering,South China University of Technology,381 Wushan Road,Guangzhou 510640,China
b Guangzhou Institute of Modern Industrial Technology,Nansha 511458,China
c Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety,Guangzhou 510640,China
d Overseas Expertise Introduction Center for Discipline Innovation of Food Nutrition and Human Health (111 Center),Guangzhou 510640,China
e Department of Food Science,Stocking Hall,Cornell University,Ithaca 14853,USA
f South China Botanical Garden,Chinese Academy of Sciences,Guangzhou 510650,China
Keywords:Sea buckthorn Extraction method Structure Rheological properties Antioxidant activity Bile acid binding capacity
ABSTRACT In this study,the structural characters,antioxidant activities and bile acid-binding ability of sea buckthorn polysaccharides (HRPs) obtained by the commonly used hot water (HRP-W),pressurized hot water (HRP-H),ultrasonic (HRP-U),acid (HRP-C) and alkali (HRP-A) assisted extraction methods were investigated.The results demonstrated that extraction methods had significant effects on extraction yield,monosaccharide composition,molecular weight,particle size,triple-helical structure,and surface morphology of HRPs except for the major linkage bands.Thermogravimetric analysis showed that HRP-U with filamentous reticular microstructure exhibited better thermal stability.The HRP-A with the lowest molecular weight and highest arabinose content possessed the best antioxidant activities.Moreover,the rheological analysis indicated that HRPs with higher galacturonic acid content and molecular weight showed higher viscosity and stronger crosslinking network (HRP-C,HRP-W and HRP-U),which exhibited stronger bile acid binding capacity.The present findings provide scientific evidence in the preparation technology of sea buckthorn polysaccharides with good antioxidant and bile acid binding capacity which are related to the structure affected by the extraction methods.
1. Introduction
The abnormal increase in blood lipids such as total cholesterol(TC),triglycerides (TG) and low-density lipoprotein cholesterol(LDL-C) were the major factors in the occurrence of hypertension,obesity,diabetes and other chronic diseases,which have become one of the main public health problems all over the world[1].Current therapeutic treatments for these chronic diseases usually have adverse side effects or high rates of secondary failure[2,3].Therefore,developing potential natural products to prevent these chronic diseases has attracted increasing attention from the scientific community[4].In recent years,accumulating evidences indicated that the natural polysaccharides could effectively bind to bile acids to promote the excretion of bile acids in the intestine and transformation of cholesterol into bile acids in the liver,leading to the decrease of blood lipids which is good for the cardiovascular diseases[5,6].
Sea buckthorn (Hippophae rhamnoidesL.),as a member of Elaeagnaceae family,is widely planted in different part of Asia and Europe[7].Sea buckthorn can survive in saline-alkali soils due to its ability to resist drought,wind and sand stress,so it is widely used in soil and water conservation and possessed high economic and environmental value.Besides,sea buckthorn,particularly berries,possess good antioxidant,anti-inflammatory and intestinal protection activity for rich in phenolic compounds,polysaccharides,vitamins,unsaturated fatty acids and carotenoids[8,9].Currently,it has been widely used in Chinese,Mongolian and Tibetan traditional medicines for cardiovascular disease[10],and sea buckthorn polysaccharide played an important role.Nevertheless,to our best knowledge,there is limited information on the bile acid binding capacity of sea buckthorn polysaccharides.In addition,previous studies indicated that the binding ability of polysaccharides to bile acids was closely related to the rheological properties of polysaccharides[6,11],which are greatly affected by the extraction method[12],and various extraction solvents were applied to isolate polysaccharide,such as hot buffer (HBSS),chelating agent (CHSS),dilute alkaline (DASS) and concentrated alkaline (CASS),etc.[13-15].For example,polysaccharide extracted from blackberry with higher viscosity exhibited stronger binding ability to bile acids[16],which was consistent with the result reported by Chen et al[17].Chen et al[12]reported that polysaccharides from mulberryfruits obtained by hot water extraction (MFPh) had the highest viscosity,while that extracted with ultrasonic-assisted extraction (MFPu)exhibited relatively low viscosity.Thus,the selection of extraction method is important for the bioactive polysaccharides.Furthermore,the activity of enzyme was easily affected by pH,temperature and storage time ect.,the reproducibility of enzymatic-assisted method was poor.Compared with enzymatic-assisted method,physical and chemical extraction methods exhibited better reproducibility and were more helpful in controlling the molecular weight and yield of polysaccharides[18,19].However,the information on comprehensive evaluation of the relationship between rheological properties and bile acid binding capacity of sea buckthorn polysaccharide extracted with different methods is still limited.
Therefore,the present study aims to clarify the effects of five commonly used physical and chemical methods on the physicochemical properties,structure,rheological properties,antioxidant and bile acid-binding capacity of sea buckthorn polysaccharide.The findings provide information on the relationship among structure,property and bioactivities of sea buckthorn polysaccharide extracted with different methods.
2. Materials and methods
2.1 Materials and chemicals
Sea buckthorn berries were collected from a local fruit market in horticultural farm in Lvliang (Lvliang,Shanxi province,China).2,2’-Azobis [2-methylpropionamidine] dihydrochloride(AAPH),1-phenyl-3-methyl-5-pyrazolone (PMP),1,1-diphenyl-2-picrylhydrazyl (DPPH),trifluoroacetic acid (TFA) were obtained from Aladdin Biochemical Technology Co.,Ltd (Shanghai,China).Monosaccharide standard and Dextrans with different molecular weights were purchased from Sigma-Aldrich Chemical Co.(St.Louis,MO,USA).All other regents and chemicals used in the present study were analytical or chromatographic grade.
2.2 Extraction of sea buckthorn polysaccharides (HRPs)
The sea buckthorn berries were washed,dried and ground into powder.The oil in the powder (500 g) was removed by petroleum mether,and the ethanol-soluble impurities were removed by 95% (V/V)ethanol.The pretreated sea buckthorn powder was used for further extraction according to previous studies with slight modifications[11,20].The detailed extraction and purification procedures of sea buckthorn polysaccharides are shown in Fig.1.Pressurized hot water extraction was conducted in a high pressure steam sterilizer (YXQ-LS-50A,Shanghai Boxun Industrial Co.,Ltd.Medical Equipment Factory,Shanghai,China),and ultrasonic-assisted enzymatic extraction was performed by an ultrasonic cleaner (KQ-800KDE,Kunshan Ultrasonic Instrument Co.,Ltd,Kunshan,China).The crude sea buckthorn polysaccharides were deproteinization with Sevag reagent(chloroform:n-butylalcohol=4:1) and decolored using macroporous resin AB-8.After dialysis (molecular cut-off 3 500 Da) for 48 h,the solution was freeze-dried and the samples were named HRP-W,HRP-H,HRP-U,HRP-C and HRP-A,respectively.

Fig.1 The process of polysaccharide isolated from sea buckthorn with different extraction methods.
2.3 Chemical analysis of HRPs
The neutral sugar content in the HRPs was measured by the phenol-sulfuric acid method withD-glucose as a standard[21-23].Contents of uronic acid were quantized by the meta-hydroxydiphenyl method[24,25],and the galacturonic acid (GalA) was used to make a standard curve at a wavelength of 525 nm.The total protein content was analyzed by the method of Coomassie brilliant blue with bovine serum albumin (BSA) as a standard[26].
2.4 Homogeneity and molecular weight determination
High performance gel permeation chromatography (Agilent 1260,Agilent Co.,USA) coupled with a refractive index detector (RID,Agilent) was used to analyze the homogeneity and molecular weight of the HRPs.Dextrans with different molecular weights were used to establish molecular weight calibration curve,and the detailed analysis conditions were referred to reported method[27].
2.5 HPLC analysis of monosaccharide composition
The monosaccharide composition of HRPs were analyzed by HPLC method after hydrolysis with TFA and pre-column derivatization with PMP[24,28].The chromatographic analysis was performed with an Agilent ZORBAX Eclipse XDB-C18column(5 μm,4.6 mm × 250 mm) and the data was collected at 245 nm.The quantitative analysis of monosaccharides was performed by comparing the corresponding calibration curves established by monosaccharide standards.
2.6 Structural characterization
2.6.1 Particle size determination
The particle size distributions of HRPs were determined by a Micron Laser Particle Sizer Mastersizer 3000[29,30].The measurements were conducted at 25 °C with three replicates.The refractive indexes of the continuous phase and dispersed phase were set as 1.330 and 1.414,respectively.
2.6.2 FT-IR spectroscopy
The FT-IR spectra of HRPs was analyzed on a Bruker VERTEX 33 spectrometer (Bruker Corporation,Germany),and the spectra were recorded in the region of 4 000-600 cm-1.
2.6.3 Triple-helix conformation analysis
The triple-helix conformation of HRPs was analyzed with Congo red method according to the previous report with minor modifications[31].
2.6.4 XRD (X-ray diffraction) analysis
The amorphous or crystalline structures of HRPs were determined with an X-ray diffractometer (D8 Advance,Bruker,Germany) and the spectra were recorded with a scan range from 5° to 70° with a step of 0.01° intervals.
2.7 Scanning electron microscopy (SEM)
The surface micro-structures of the HRPs were observed on a JSM-7500F scanning electron microscopy (JEOL,Japan) at an accelerating voltage of 5.00 kV with magnification at 500× or 1 000×[32,33].
2.8 Thermal analysis
The thermal properties of the HRPs were investigated by thermogravimetry/differential thermogravimetry on a simultaneous thermal analyzer (Netzach,STA 449 F3 Jupiter,Netzach,Germany).The HRPs samples (3-5 g) were heated from 40 °C to 700 °C at a rate of 20 °C/min with nitrogen as the carrier gas.
2.9 Rheological characterization
2.9.1 Apparent viscosity analysis
A Discovery Hybrid Rheometer-2 (DHR-2) (TA instruments,New Castle DE,USA) equipped with a parallel steel plate (40 mm diameter,1.0 mm gap) was used to investigate the rheological properties of HRPs according to previously reported procedures[34,35].The HRPs samples were dissolved in deionized water and prepared with 50 mg/mL polysaccharide solution.The apparent viscosity was detected at a shear rate range of 1 to 100 s-1at 25 °C.The Power-Law model was applied to fit the flow curves[16,36]:
Power-Law model equation:η=k(γ)n
wherenis the flow behavior index;ηis the apparent viscosity;γis the shear rate (s-1);kis the viscosity coefficient (mPa·sn).
2.9.2 Oscillation frequency sweep analysis
The dynamic rheological properties of HRPs were determined by oscillation frequency sweep from 0.1 to 100 rad/s at 25 °C with strain of 2%,the oscillation frequency was set as 2 Hz.For comprehensive study on the rheological properties of complex viscosity (η*),elastic(storage) modulus (G’),loss angle tanδ(tanδ=G”/G’),viscous(loss) modulus (G”) and the complex modulus (G*) were measured.The Power-Law model was used to fit the obtained data (G’,G” andG* vsω):
whereωis the angular frequency;n’ (n”) was frequency exponent;k’ (k”) was intercepts exponent;Aαis the strength of the polysaccharide network in simple shear (Pa·sa) andαis order of relaxion function.
2.9.3 Temperature sweep analysis
The gelling properties of the HRPs samples were determined by temperature ramp test.The heating (cooling) temperature range was 20–80 °C (80–20 °C) with a heating (cooling) rate of 10 °C/min.
2.10 In vitro antioxidant activity assay
The antioxidant activities of HRPs samples were comprehensively evaluated using DPPH radical,ABTS radical cation scavenging assays and oxygen radical absorption capacity (ORAC) as described previously with slight modifications[33].
2.11 Bile acid-binding capacity assay
The hypolipidemic activity of HRPs was determined using bile acid binding assay.The bile acid binding ability of HRPs was calculated according to a standard curve generated from a sodium taurocholate solution at an absorbance of 387 nm.The detailed experiment procedures as described in published literature[16,37].
2.12 Statistical analysis
All experiments were replicated at least three times and the data were expressed as means ± standard deviations (SD).Analysis of variance (ANOVA) was conducted using the Duncan and LSD test in SPSS 22 software (SPSS Inc.,Chicago,USA).A difference withP<0.05 was considered as statistically significant.
3. Results and discussion
3.1 Extraction yields and physicochemical properties of HRPs
In this study,the extraction yields of HRPs extracted by five different methods were displayed in Table 1.The yields of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were 4.74%,4.54%,6.05%,3.75% and 6.59%,respectively.Obviously,the extraction yields of HRPs were greatly affected by extraction methods.The HRP-A had the highest yield,followed by HRP-U,HRP-W,HRP-H and HRP-C.Evidence has shown that insoluble polysaccharide can be effectively released from the cell wall and converted into soluble polysaccharide by breaking hydrogen bonds between cellulose and hemicellulose during the alkali extraction,thereby increasing the final recovery rate[38],which could explain the highest extraction yield of HRP-A.Ultrasonic wave migration could generate cavitation,resulting in the destruction of cell wall to improve the yield of polysaccharide[12].In addition,the yield of HRP-H was lower than HRP-W,which was contrary to the findings of Gao et al[39]that the extraction yield of polysaccharide fromL.japonicaby pressurized hot water extraction (LP-PH)was higher than that of hot water (LP-H),suggesting extraction methods had different effects on different plant materials,and the high temperature might cause degradation of HRP-H,leading to a lower extraction yield[11].Furthermore,the lowest extraction yield of HRP-C may be due to the fact that the acid solution could degrade polysaccharide into free sugars[40].
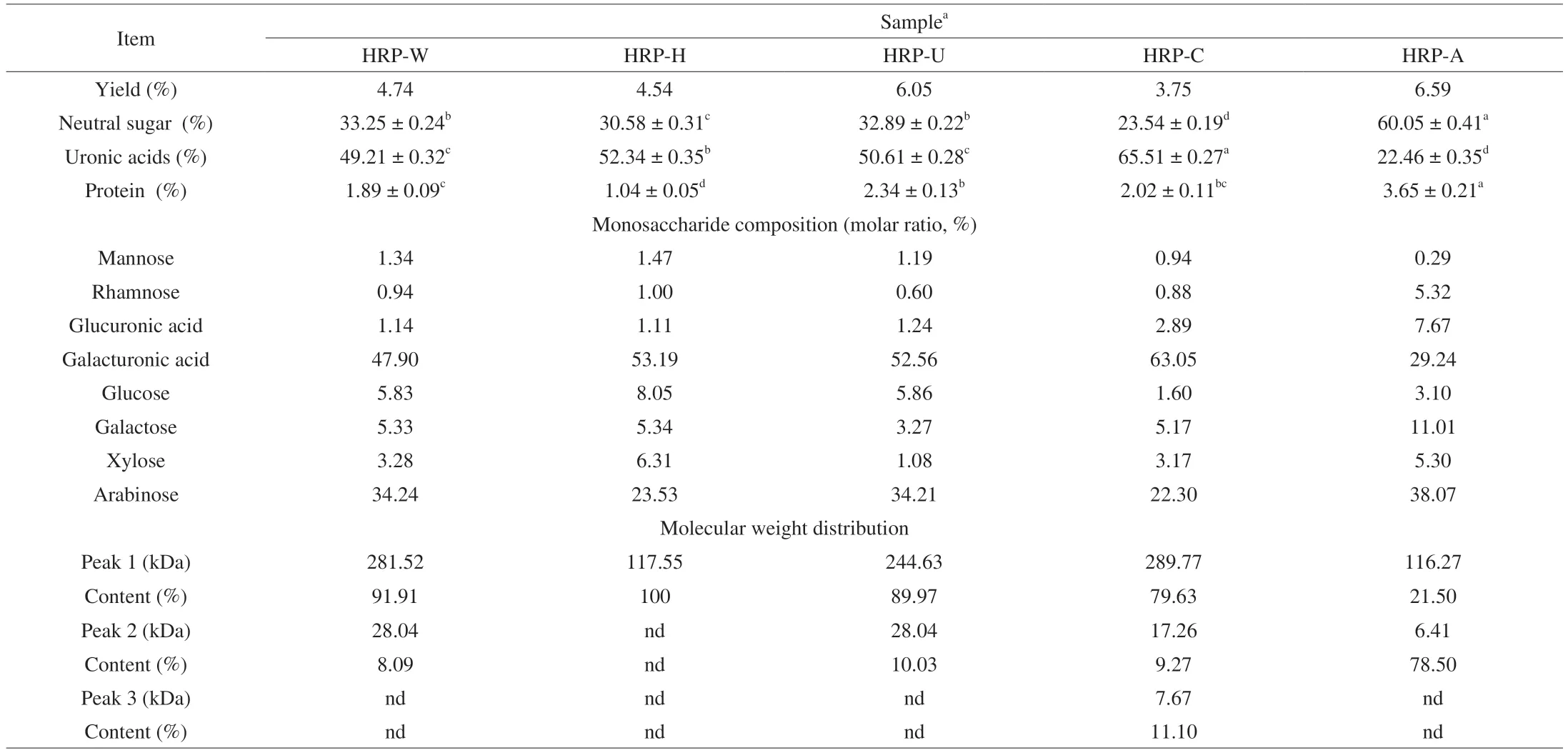
Table 1 Chemical properties and compositions of HRPs extracted from sea buckthorn using different extraction methods.
As shown in Table 1,the neutral sugar and uronic acid content of different HRPs varied significantly (P<0.05).The neutral sugar content in HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were(33.25 ± 0.24)%,(30.58 ± 0.31)%,(32.89 ± 0.22)%,(23.54 ± 0.19)%and (60.05 ± 0.41)%,respectively,indicating the HRP-A contained highest neutral sugars while the HRP-C had the lowest.The uronic acid content of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were 49.21 ± 0.32,52.34 ± 0.35,50.61 ± 0.28,65.51 ± 0.27 and 22.46 ± 0.35,respectively.Clearly,the lowest uronic acid content was observer in HRP-A,which might be due to the destruction of carboxyl groups in uronic acid under alkali conditions[41].The highest protein content was observed in HRP-A,which may be related to the hydrolysis of amide bonds of protein by alkaline media[12],which was consistent with the report by Yan et al[40].Furthermore,the lowest protein content in HRP-H could be explained as theo-glycosidic linkage between the protein and polysaccharide was destroyed by high temperature[42].
3.2 Monosaccharide composition
Chromatograms of monosaccharide composition of HRPs were depicted in Table 1.According to the monosaccharide standard,all five HRPs were typical acidic heteropolysaccharide and composed of mannose,rhamnose,glucuronic acid,galacturonic acid,glucose,galactose,xylose and arabinose with different molar ratios.Galacturonic acid,glucose,galactose and arabinose were predominant components in each polysaccharide (Table 1).The HRP-C contained the highest galacturonic acid content (63.05%),while HRP-A possessed highest arabinose content (38.07%),and no obvious difference was observed in monosaccharide composition between HRP-W and HRP-U.The results suggested that extraction methods had significantly impacts on the molar ratios of monosaccharides inHRPs,and similar results were observed in extraction of mulberry fruit and okra polysaccharide by different methods[12,43].The differences in monosaccharide composition suggested differences in structure which may affect the biological activities of HRPs[44].
3.3 Molecular weight analysis
The molecular weight of polysaccharide is closely related to its bioactivities that large molecular weight polysaccharide hardly enters into cells while low hardly form advance structures[44].In the present study,the molecular weight distributions of HRPs were determined using HPGPC method,and the results were shown in Table 1.Noteworthily,all five HRPs exhibited a wide signal peak,indicating that the molecular weights of the various polysaccharide were heterogeneous.According to the standard curve of dextrans,the molecular weight distribution of HRP-W,HRP-U,HRP-C and HRP-A ranged from 28.04 to 281.52 kDa,28.04 to 244.63 kDa,7.67 to 289.77 kDa and 6.41 to 116.27 kDa,respectively.In contrast,HRP-H had a narrower molecular weight distribution,showing only a major peak with a molecular weight of 117.55 kDa.In addition,HRP-H and HRP-A showed lower molecular weight,as presented in Table 1.This might be due to the degradation of polysaccharide caused by alkali solution and high temperature during the extraction process[12,24].The results were consistent with previous studies that alkali media and high temperature could effectively decrease glycosidic linkages of polysaccharide[11,24].
3.4 Particle size determination
The particle size represents the aggregation degree of polysaccharide molecules in solution,which could indirectly reflect the molecular weight of polysaccharide to a certain extent[24].In general,the high molecular weight polysaccharide tends to have a larger particle size[21].As shown in Fig.2A,all five HRPs polysaccharide solution exhibited a wide particle size distribution,which were consistent with the results of molecular weight determinations.TheZ-average particle size of five HRPs were illustrated in Fig.2B.Noticeably,HRP-C had the largestZ-average particle size of 854.6 nm.Evidence has shown that the crosslinking between galacturonic acids may lead to the aggregation of polysaccharide molecules,resulting in an increase in particle size[24].TheZ-average particle size of HRP-W and HRP-U showed no significant difference,while the HRP-H and HRP-A had a relatively low particle size with 350.3 nm and 188.4 nm,which may be related to the lower molecular weight[21].

Fig.2 Particle size distributions (A) and Z-average particle size (B) of HRPs;FT-IR spectra of HRPs (C,D).The highlighted part of Fig.2C is enlarged in Fig.2D.
3.5 FT-IR spectra
The functional groups of HRPs were identified by FT-IR spectra.As shown in Figs.2C-D,no obvious difference was observed among the characteristic functional groups of HRPs obtained from different extraction methods.A strong and wide absorption peak at 3 353 cm-1was assigned to the O-H stretching vibrations.The absorption peak at 2 917 and 1 441 cm-1was related to the stretching vibrations of C-H[11].The peak at around 1 744 cm-1was contributed to the symmetric stretching vibration of C=O in acetyl groups.The absorption band at approximately 1 640 cm-1was related to the COO-deprotonated carboxylic acid,indicating the presence of uronic acid[45].The strong extensive absorption peaks at 1 227,1 100 and 1 015 cm-1were attributed to asymmetric vibrations (C-O-C,C-O-H and C-C)and ring vibrations[40].Furthermore,the peak at 970 cm-1indicated the presence ofβ-glycosidic linkages[44].The FT-IR spectra revealed that the major linkage bands and functional groups of HRPs were uninfluenced by extraction methods,although the monosaccharide composition was significantly different.
3.6 Triple-helix conformation analysis
The maximum absorption wavelength (λmax) of Congo red-polysaccharide complex will produce red-shift when the polysaccharide had a triple-helix structure.In the present study,the curdlan with typical triple-helix conformation was selected as positive control (Fig.3A).With the increase of the alkaline concentration from 0 to 0.5 mol/L,λmaxof HRP-H,HRP-U,HRP-C and HRP-A all decreased and the same trend was observed in blank control,suggesting HRP-H,HRP-U,HRP-C and HRP-A had no triple-helix structure.However,λmaxof HRP-W increased first and then decreased with the alkaline concentration increase,indicating that HRP-W possessed a weak triple-helix structure.
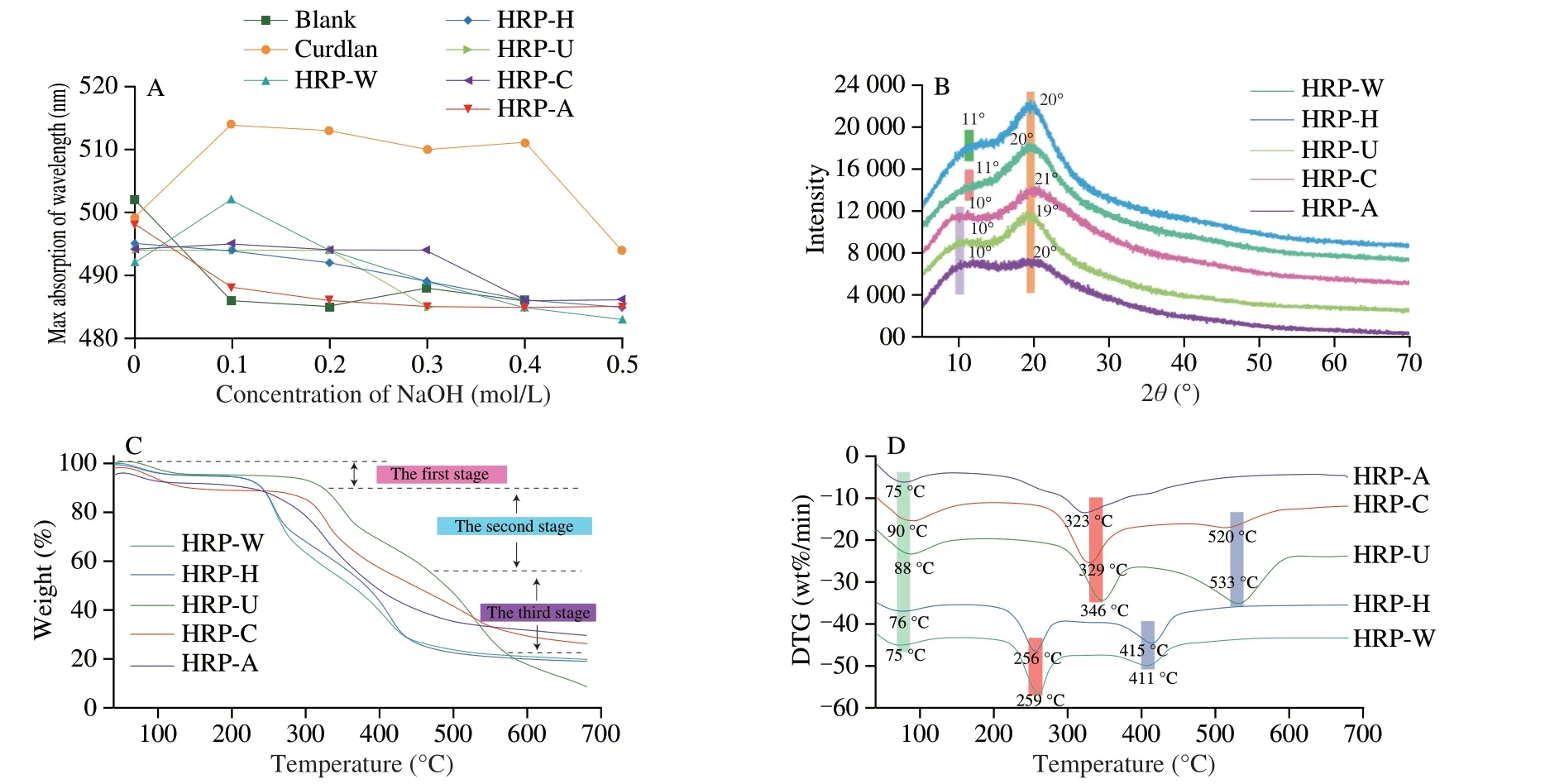
Fig.3 Triple-helix conformation analysis (A),XRD spectra (B),thermogravimetry (C) and differential thermogravimetry (D) curves of HRPs.
3.7 Crystal structure analysis
The amorphous or crystalline structure of HRPs was further characterized by X-ray diffraction (XRD) analysis.As shown in Fig.3B,all HRPs presented two passivated single bread-like peaks,manifesting that all HRPs were semi-crystalline substances.However,the exact position and intensity of the diffraction peak of HRPs was different,especially HRP-A,suggesting the detailed microcrystalline structure of HRPs was different.Combined with the results of Congo test,the advance structure of HRPs was significantly affected by extraction methods.
3.8 Morphological characterizations
Scanning electron microscopy (SEM) is an effective tool to investigate the morphologies of polysaccharides.The surface images of five HRPs observed by SEM at magnifications of 500× and 1 000×were shown in Fig.4.The morphologies of HRP-W and HRP-H exhibited a mixture of rough fragments and irregular filaments with some small pores.However,the size of fragments and length of filaments of HRP-H were smaller than that of HRP-W,indicating that the polysaccharide has been degraded during the pressurized assisted extraction,which was consistent with the results of particle size distributions and molecular weight determinations.HRP-U showed a long filamentous network with loose structure.The morphologies of HRP-C exhibited a dense,rough and irregular porous surface with a large sheet-like structure,which could be attributed to the crosslink aggregation of galacturonic acids.Furthermore,a relatively small,smooth and uniform fragment observed in HRP-A might be due to the higher content of neutral sugars.Our findings confirmed that the morphologies and microstructures of polysaccharide could be affected by extraction methods,which might be closely correlated with their physicochemical properties and bioactivities[44].
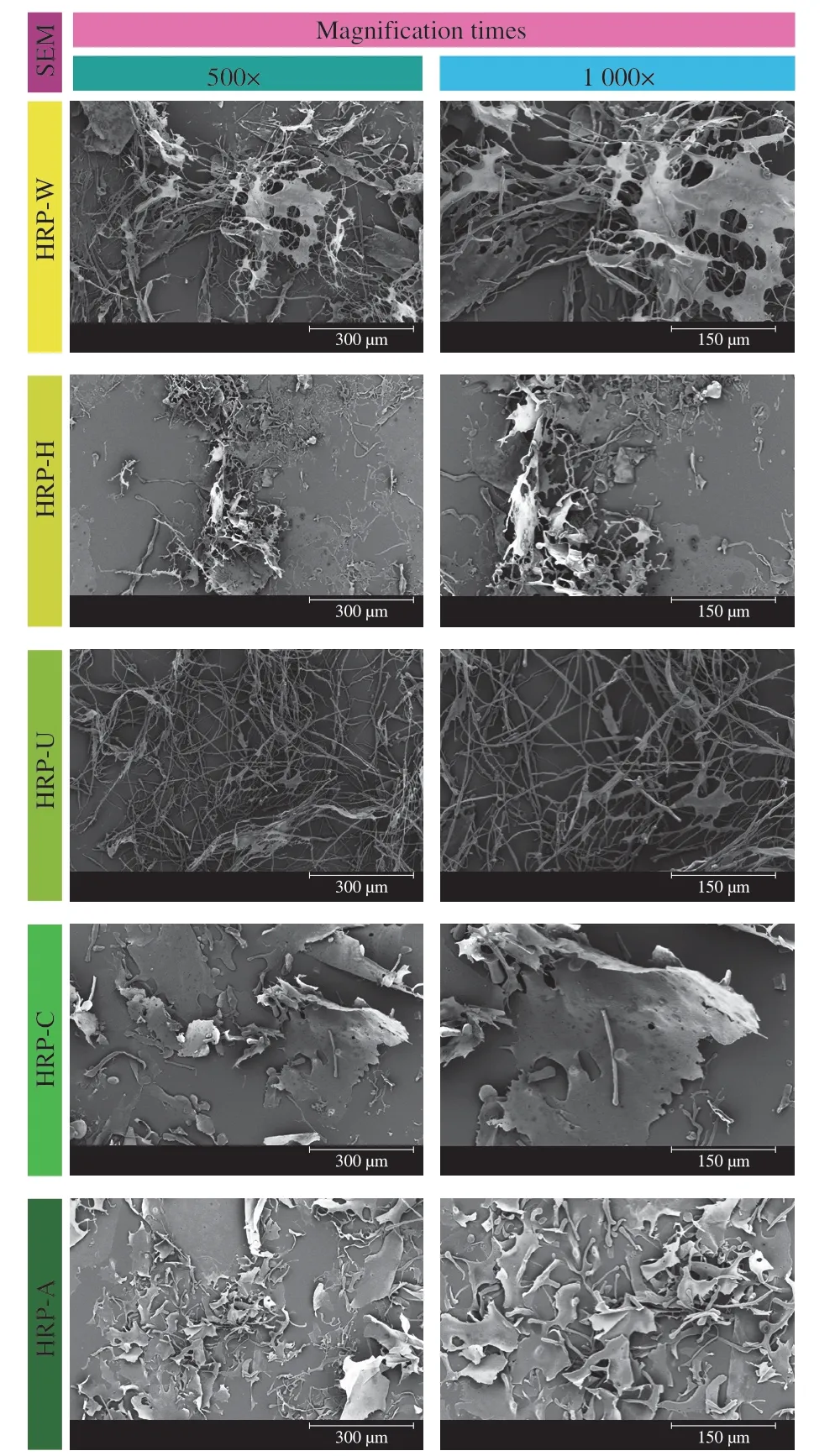
Fig.4 SEM images of HRPs from different extraction methods at magnifications of 500× and 1 000×.
3.9 Thermodynamic properties
Thermal stability is a vital physicochemical property of polysaccharides for industrial and commercial applications.As shown in Fig.3C,the thermogravimetry curves (TG) indicated that all HRPs had three major weight loss stages.The first stage in TG curves showed a slight decrease in weight,which was due to the evaporation of free and bound water present in the polysaccharide[46].The maximum rate of weight loss was observed in the second stage,which was attributed to the scission of the glycosidic bond together with degradation of side-chain residues of polysaccharides[47].The weight loss of the third stage was related to the further cleavage of the depolymerized polysaccharide fragments[48].The differential thermogravimetry (DTG,Fig.3D) curves showed that all HRPs had three endothermic peaks,except for HRP-A.The first endothermic peak of HRP-C (90 °C) and HRP-U (88 °C) was close,and higher than that of HRP-W (75 °C),HRP-H (76 °C) and HRP-A (75 °C),indicating that HRP-C and HRP-U had stronger free water binding ability,which might be related to higher galacturonic acid contents and filamentous reticular microstructure.Compared with other HRPs,the second and third endothermic peak of HRP-U had higher temperatures of 346 and 533 °C,respectively.This might be related to filamentous reticular microstructure of HRP-U,which might be resistant to the thermal degradation[49].Overall,the results revealed that HRP-U had the better thermal stability.
3.10 Rheological properties
3.10.1 Apparent viscosity
As displayed in Fig.5A,with the increase of shear rate,the apparent viscosity of HRPs declined rapidly,suggesting that all HRPs were pseudoplastic fluids and exhibited shear-thinning behavior[24,50].The parameters of steady flow curves were further calculated by Power-Law model,and theR2values of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were calculated to be 0.840 8,0.879 9,0.852 4,0.883 5 and 0.991 2,respectively.Clearly,theR2values of all HRPs were near to 0.9,indicating that Power-Law model could be used to explain the rheological behavior of HRPs well.In general,the higher thekvalue,the greater the viscosity.In the present study,thekvalue of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were 0.094 5,0.070 3,0.094 3,0.178 7 and 0.062 2,respectively.The HRP-C exhibited the greatest viscosity,possibly due to the higher content of galacturonic acid[24,39].Previous study demonstrated that the viscosity of polysaccharide was closely related to its molecular weight,which may explain the lowest viscosity of HRP-A[21].In addition,the higher the degree of pseudoplasticity of the fluid,the lower thenvalue.The n value of all HRPs were far below 1,showing obvious pseudoplasticity,especially those with low relative molecular weight,such as HRP-H (-0.553 8) and HRP-A (-0.589 4).
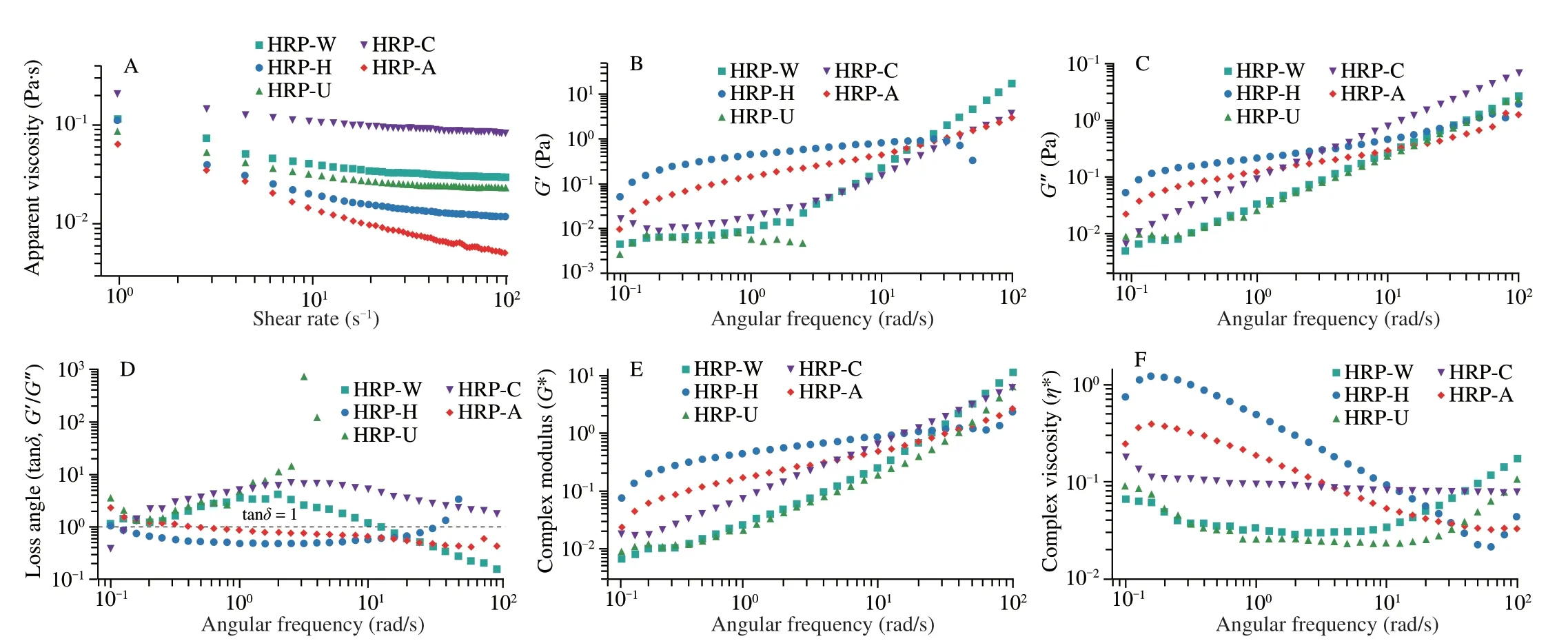
Fig.5 (A) The apparent viscosity of HRPs;(B) storage modulus (G’),(C) loss modulus (G”),(D) loss angle,(E) complex modulus (G*) and (F) complex viscosity (η*) of HRPs.
3.10.2 Frequency sweep
The dynamic rheological properties of HRPs were determined by oscillatory measurements and the results were shown in Figs.5B-F.The modulusG’ andG” values of all HRPs increased with increasing frequency,except for the HRP-U with lowerG’,which could not be detected (Figs.5B-C).The tanδvalue (loss angle) of all HRPs were between 0.1 to 100,including tanδ=1 (G’=G”),indicating all HRPs samples exhibited both liquid viscous and solid elastic properties within the frequency region (0.1-100 rad/s)[51].Complex viscosity (η*)is always used to describe the total resistance to flow of a material considered to be a viscous liquid.As shown in Fig.5F,the complex viscosity of all HRPs declined with the increase in angular frequency,especially lower molecular weight polysaccharide (HRP-H,HRP-A).The behavior was similar to other polysaccharide,which suggested that all HRPs had non-Newtonian shear-thinning behavior[35].
The intercepts (k’,k”) and frequency (n’,n”) exponents are always used to describe viscoelastic characteristics of food materials[52].Thek’ value of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were 0.005 05,0.001 35,0.003 85,0.007 83 and 0.001 17,respectively.Obviously,thek’ value of HRP-C was highest,andk” exhibited the similar trend,manifesting that HRP-C solution formed the stronger crosslinking network.It has been reported that the stronger the polysaccharide crosslinking network,the stronger the bile acid binding ability[24].Hence,the HRP-C could be used to lower the cholesterol by binding to bile acid.In addition,then’ value of all HRPs were greater than 0,demonstrating that all HRPs solutions could be identified as a viscous solution[52].
The relationship between complex modulus (G*) and frequency could be used to evaluate the stability of three-dimensional network structures[53],and the results were shown in Fig.5E.The strength of the polysaccharide network of HRPs solutions could be described in terms of theAα.TheAαvalues of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were calculated to be 0.072 97,0.026 44,0.040 88,0.090 39 and 0.008 84,respectively.Compared with other HRPs,the Aα value of HRP-C was highest,manifesting that HRP-C solution formed the strongest crosslinking network,which was consistent with the results ofkvalue analysis.Theαvalue is usually used to evaluate the three-dimensional structure[54].In this study,theαvalue of all HRPs was much higher than 0.1,which indicated that HRPs solutions exhibited pronounced viscous characters[54].
3.10.3 Temperature sweep
The thermal stability of rheological properties of HRPs was measured during heating and cooling process.As shown in Fig.6,theG’ andG” of all HRPs samples declined sharply with the temperature increased,andG” of HRP-W,HRP-U and HRP-C was higher thanG’ from 20 to 80 °C,suggesting liquid-like behavior.However,a cross point (G’=G”) was detected at around 65 °C for heating curve of HRP-H and HRP-A.When beyond this temperature,a typical gel-like system would be formed.During the cooling process,theG’ andG” of all HRPs increased and cross point appeared,implying a transition tendency for solid-like behavior.Furthermore,the tanδvalues of HRPs almost all decreased with the increase of temperature,except for HRP-C and HRP-W,while tanδvalues of HRPs remained basically unchanged during the cooling process(Fig.6F).Combined with the results of steady rheological and frequency sweep experiment,the results implied that HRPs could be used as a thickening agent in functional food industry,especially HRP-C with high galacturonic acid content.Besides,the rheological properties of HRPs were greatly affected by extraction methods.

Fig.6 Variation in storage modulus (G’) and loss modulus (G”) of HRPs during the heating and cooling process (A-E),loss angle (tanδ,G”/G’) of temperature dependence for HRPs (F).A-E represent HRP-W,HRP-H,HRP-U,HRP-C and HRP-A,respectively.
3.11 Antioxidant activities of HRPs
Oxidative stress has been proved to be closely related to cardiovascular disease,neurodegeneration,cancer,diabetes mellitus,aging and other chronic diseases[11,45].It has been reported that plant polysaccharides could effectively remove oxidative stress[31].Hence,in the present study,the antioxidant activity of HRPs was comprehensively investigated by three different methods,including ABTS radical cation,DPPH radical scavenging activity and oxygen radical absorption capacity (ORAC).
ABTS radical cation was scavenged by antioxidants through donation of hydrogen to form a stable ABTS radical cation molecule.As shown in Fig.7A,all HRPs demonstrated scavenging ability in a concentrationdependent manner within the tested concentration range(0.062 5-2 mg/mL).However,the scavenging capacity of each sample was different.The ABTS radical cation scavenging ability of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were 31.0%,35.9%,23.0%,29.4% and 88.6%,respectively,at a concentration of 0.5 mg/mL.It is noteworthy that the HRP-H and HRP-A with lower molecular weight exhibited higher ABTS radical cation scavenging activity,which were consistent with the previous study that the antioxidant activity of oka polysaccharide declined as the decrease of molecular weight[43].In addition,Yi et al[42]reported the polysaccharide extracted fromAchyranthis bidentataeradix (ABPS) with higher arabinose content exhibited higher antioxidant activity,which could explain the highest ABTS radical cation scavenging activity of HRP-A.DPPH radical is a stable free radical that has been widely used to evaluate the antioxidant capacity of bioactive compounds[11].As shown in Fig.7B,all HRPs showed significant scavenging ability on DPPH radical in a concentration dependent manner over the concentration range of 0.062 5 to 2 mg/mL.Obviously,the HRP-A and HRP-H exhibited higher DPPH radical scavenging activity compared with other HRPs,which was consistent with the result of ABTS radical cation scavenging assay.
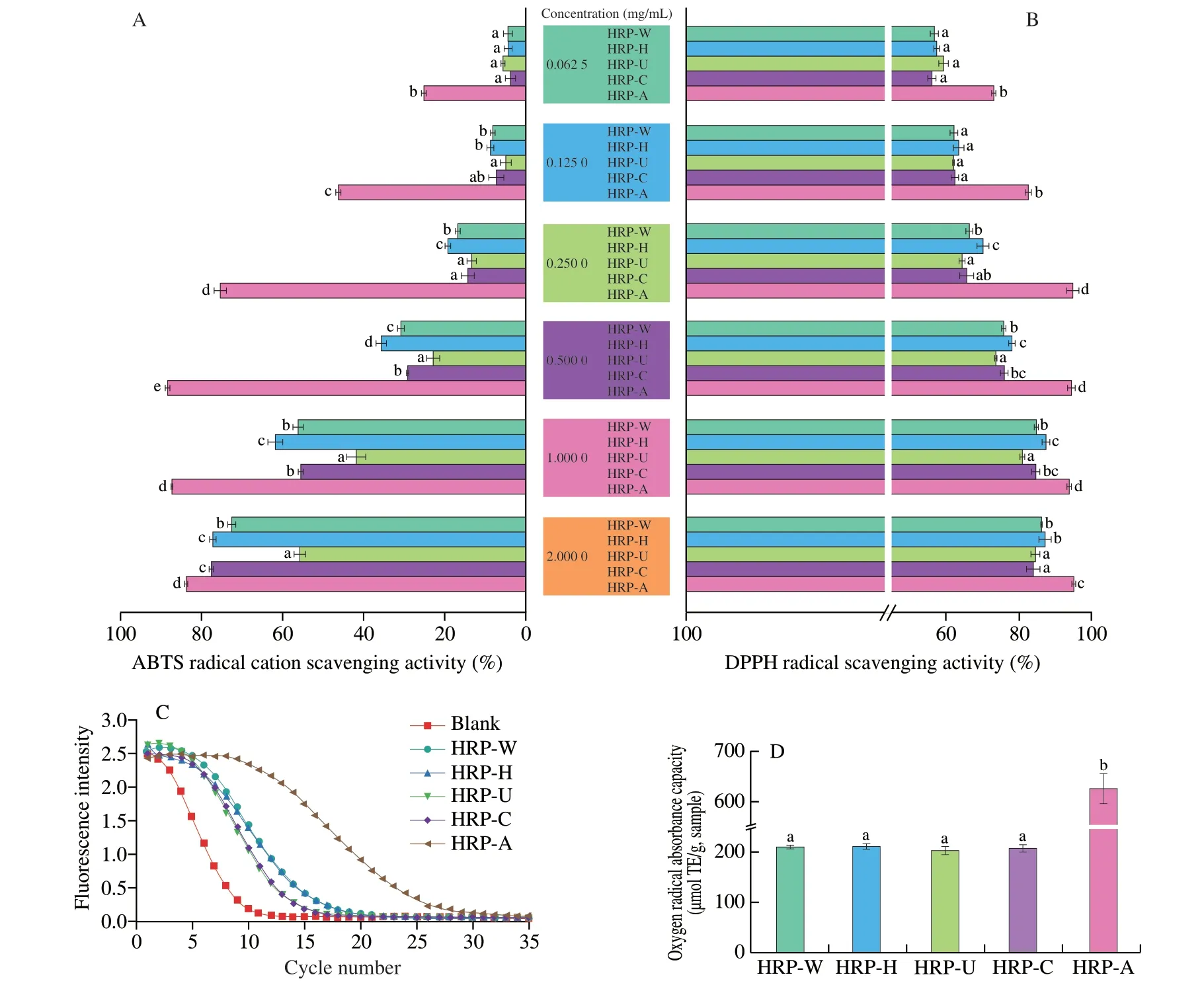
Fig.7 ABTS radical cation scavenging activity (A),DPPH radical scavenging activity (B) and oxygen radical absorption capacity (C) of HRPs.Different letters are used in the same indicator to show statistical significance (P <0.05).
ORAC method determines the hydrogen supplying capacity and free radical chain breaking capacity of antioxidants by monitoring their inhibition of peroxy-free radical induced oxidation.As demonstrated in Fig.7C and Table 2,the ORAC value of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were 218.21,221.29,205.48,213.00 and 627.49 μmol TE/g,respectively.The ORAC value of HRP-A was almost 3-fold higher than that of other HRPs,suggesting HRP-A with lowest molecular weight and highest arabinose content possessed strongest oxygen radical absorption capacity.The result was consistent with DPPH radical and ABTS radical cation scavenging ability.
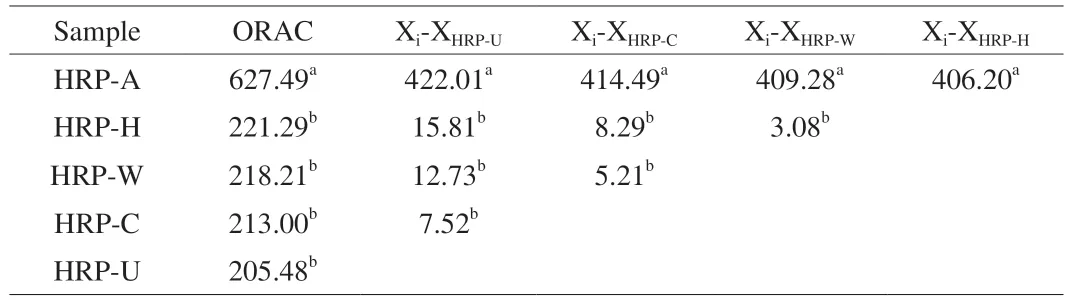
Table 2 Comparative analysis of different extraction method on ORAC by LSD
3.12 Bile acid-binding capacity
In the present study,the bile acid-binding abilities of HRP-W,HRP-H,HRP-U,HRP-C and HRP-A were (0.72 ± 0.02),(0.56 ±0.03),(0.64 ± 0.04),(0.95 ± 0.03) and (0.39 ± 0.08) mmol,respectively.Obviously,HRP-C had the strongest bile acid-binding capacity,followed by HRP-W and HRP-U.In comparison with other HRPs,HRP-H and HRP-A showed the weakest bile acid-binding ability (Fig.8).

Fig.8 Binding ability of HRPs to bile acids.Different letters are used in the same indicator to show statistical significance (P <0.05).
The difference between polysaccharides in their ability to bind bile acids may be due to their different structure,such as molecular weight,viscosity and monosaccharide composition[11].Correlations between molecular weight,monosaccharide composition,rheological properties and bile acid binding ability of HRPs were analyzed using Pearson’s correlation coefficient,and the results were normalized by column,as shown in Fig.9.Noteworthily,the bile acid binding ability of HRPs was strongly correlated with molecular weight(0.888*),apparent viscosity (0.948*),k(0.962**) andAα(0.965**)values.In addition,a strong correlation was also observed between the molecular weight and the apparent viscosity (0.718),k(0.868)andAα(0.907*) values,which was in agreement with previous reports that the molecular weight of polysaccharide was correlated with its viscosity[16,17].Hu et al.[6]reported that the high molecular weight polysaccharides have hydrodynamic restrictions on bile acid-binding due to their high viscosity.Combined with Pearson correlation analysis results,we deduced the binding mechanism of HRPs to bile acids,as shown in Fig.10.HRPs could effectively bind to bile acids to improve the excretion of bile acids in the intestine and promote the transformation of cholesterol into bile acids in the liver,thus significantly reducing the level of cholesterol,especially,HRP-C with highest molecular weight and galacturonic acid content possessed the highest viscosity and strongest crosslinking network,which showed strongest hydrodynamic restrictions on bile acids,resulting in the strongest binding ability to bile acid.

Fig.9 Pearson correlation analysis of molecular weight,monosaccharide composition and rheological properties of HRPs with bile acid binding ability.
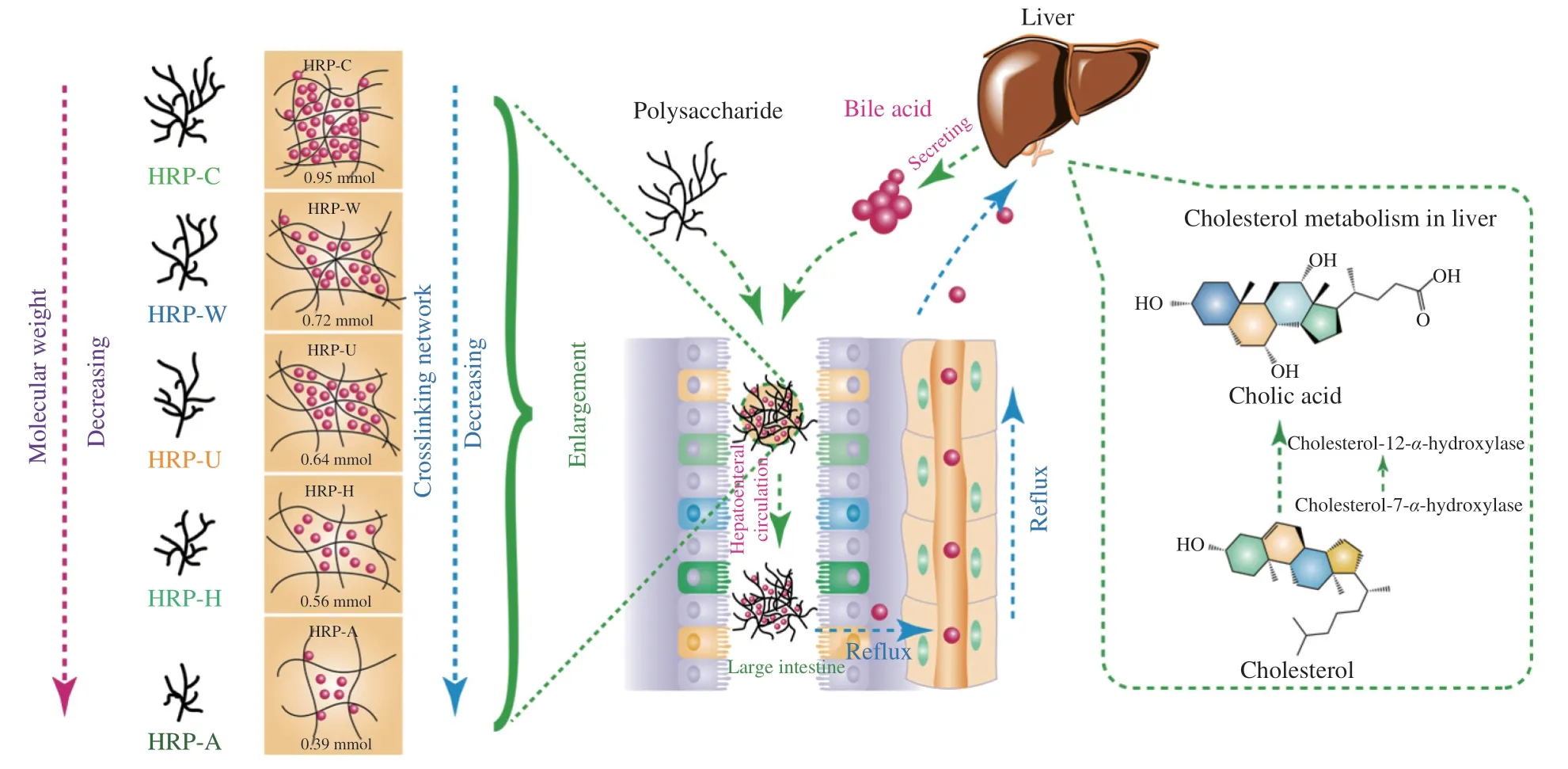
Fig.10 The binding mechanism of HRPs to bile acids.
4. Conclusion
In the present study,the structural characterizations,antioxidant activities and bile acid-binding abilities of HRPs prepared by five different extraction methods were investigated.The results indicated that the physicochemical properties,structures,rheological properties and bioactivities of HRPs were greatly affected by extraction methods.Exactly,HRP-A had the highest yield,arabinose and protein content,while HRP-C had the highest galacturonic acid content.The molecular weight determinations and particle size distributions both indicated that the polysaccharide was degraded by alkali solution(HRP-A) and high temperature (HRP-H) during the extraction process.The HRP-U with filamentous reticular microstructure had better thermal stability,and HRP-A with the lowest molecular weight and highest arabinose content exhibited the highest antioxidant activities.Furthermore,HRP-C had the highest binding ability to bile acids due to its highest viscosity and strongest crosslinking network,followed by HRP-W and HRP-U.The results demonstrated that high viscosity and molecular weight had good effects on the bile acid binding ability of sea buckthorn polysaccharides.Generally,the acid extraction was more beneficial to obtain polysaccharides from sea buckthorn with good bile acid binding capacity.
Conflicts and interest
Chun Chen is an editorial board member and Ruihai Liu is the founding editor-in-chief forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare no conflict of interest.
Acknowledgments
The Guangdong Basic and Applied Basic Research Foundation(2022A1515010730),National Natural Science Foundation of China (32001647),National Natural Science Foundation of China (31972022),Financial and moral assistance supported by the Guangdong Basic and Applied Basic Research Foundation(2019A1515011996),and 111 Project (B17018) to conduct the project are gratefully acknowledged.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

