具有kgd拓扑结构的二维铅配位聚合物的合成、结构及性质研究
刘 萍 曹超初 梁燕萍 吴振森*,
(1西安电子科技大学理学院,西安 710071)(2西北大学化学与材料科学学院,合成与天然功能分子化学教育部重点实验室,陕西省物理无机重点实验室,西安 710069)
具有kgd拓扑结构的二维铅配位聚合物的合成、结构及性质研究
刘 萍1,2曹超初2梁燕萍1吴振森*,1
(1西安电子科技大学理学院,西安 710071)
(2西北大学化学与材料科学学院,合成与天然功能分子化学教育部重点实验室,陕西省物理无机重点实验室,西安 710069)
醋酸铅(Pb(Ac)2·3H2O)和咪唑乙酸(Hima)通过溶胶凝胶扩散合成二维配位聚合物[Pb(ima)2]n(1)的单晶。X-射线单晶衍射分析表明,晶体属于单斜晶系,P21/n 空间群,晶胞参数为:a=0.875 0(2)nm,b=5.122 6(12)nm,c=13.29 2(3)nm,β=93.570(4)°,V=594.6(2)nm3,Z=2。配合物[Pb(ima)2]n是一个具有二维kgd拓扑结构的配位聚合物,二维层间又通过C-H…O氢键连接形成三维超分子结构。对配合物的红外、元素、热重及荧光等性质进行了研究。
铅配合物;柔性配体;晶体结构;荧光性质
0 Introduction
During the last two decades,the field of metalorganic coordination polymershasaroused great interest for chemists not only because of their intriguing structural topologies but also due to their potential applications in gas adsorption,separation,ion exchange,heterogeneous catalysis,and so on[1-6].The related work in this field has mostly focused on the assembly of d-block metal-organic open frameworks so far[5-9],however,the analogous chemistry of the main group element remains less developed.In contrast to the transition metals,lead also has a tendency to form metal-organic complexes and can provides a stable framework structure.As an intermediate acid in Pearson′s HSAB(hard and soft acids and bases)classification,Pb(Ⅱ)forms very stable complexes with both hard ligands as oxygen donors and soft ligands like sulfur donors[10-13],and the Pb(Ⅱ)have large ion radius,variable coordination numbers from 2 to 10,and diverse coordination geometries for Pb(Ⅱ)ions,which indicate great potential in the construction of new polymers and functional materials[14-18].In particular,the intrinsic features of Pb(Ⅱ)ions,the presence of a 6s2outer electron configuration,make Pb(Ⅱ)frameworks unique application in coordination chemistry,photophysics and photochemistry[19-22].Furthermore,Pb(Ⅱ) cations are the most commonly encountered toxic metal pollutant in the environment,and exploiting the unique coordination properties of Pb(Ⅱ)provides opportunities for the development of practical ligands as extractants or leadpoisoning treatment agents and understanding the mechanisms of its toxic actions.
The functionalized imidazolium-containing polycarboxylate,containing azole heterocycle and carboxylate groups,may be efficient coordinative ligand and lead-poisoning treatment candidates to deposit or extract the weight metal Pb(Ⅱ)ion from environmental solutions.On the other hand,the 2-(1H-imidazole-1-yl)acetate (Hima)ligand possesses a longer bridging spacer and richer coordination modes to form a fascinating structure.Thus,this type of ligand is expected to more likely form novel hybrid materials with porous network architectures by the self-assembly with lead ion.Inspired by the aforementioned considerations,we have attempted to study this flexible functionalized ligand as bridging ligands in our work.Herein,we describe the syntheses and characterization of a novel porous coordination polymers[Pb(ima)2]n(1).Complex 1 represents a(3,6)-connected 2D layer with kgd topology generated from a unsymmetrical flexible ligand ima,which further stack into 3D supramolecular networks through C-H…O weak interactions.
1 Experimental
1.1 Materials and physical measurements
All reagents were of commercial grade and were used as received.IR spectra were measured with KBr pellets on a Nicolet 170SX FTIR spectrometer(4 000~400 cm-1).Elemental analyses were determined with a Perkin-Elmer model 240C automatic analytical instrument.Fluorescence experiments used a HITACHI F-4500 Fluorescence Spectrophotometer.Thermal analysis was performed on a NETZSCH STA 449C microanalyzer in a nitrogen atmosphere at a heating rate of 10℃·min-1.
1.2 Synthesis of complex[Pb(ima)2]n(1)
A methanol solution(10 mL)of Hima(0.254 g,2 mmol)was added to a solution of Pb(Ac)2·3H2O(0.379 g,1 mmol)in MeOH (30 mL).The mixture was stirred at room temperature.A white precipitate appeared immediately,and the resulting solution was stirred continuously for 2 h.Then the precipitate was filtered off,washed with methanol and diethyl ether,and dried in air(yield:0.402 g,88%).Anal.Calcd.for C10H10Pb N4O4(%):C 26.26,H 2.20,N 12.25;found(%):C 26.32,H 2.09,N 12.02.The single crystals were obtained by diffusing methanol solution of Pb(Ac)2·3H2O and Hima in 1∶1 ratio through agar gel aqueous solution for 4 weeks in U-shaped tube.The elemental analysis of the precipitate is consistent with the crystal structures of single crystals.
1.3 Crystal structure determination
X-ray diffractions were collected with graphite monochromated Mo Kα radiation(λ=0.071073 nm)on BRUKER SMART 1000 CCD diffratometer for the complex at 298(2)K.The structures were solved by the direct methods and refined by full-matrix least-squares methods on F2with SHELXTL-97 program[23].All hydrogen atoms were added according to theoretical modes.Crystal data and structure refinement details are summarized in Table 1.Selected bond lengths and bond angles,and hydogen-bonded parameters are listed in Table 2.
CCDC:773324.
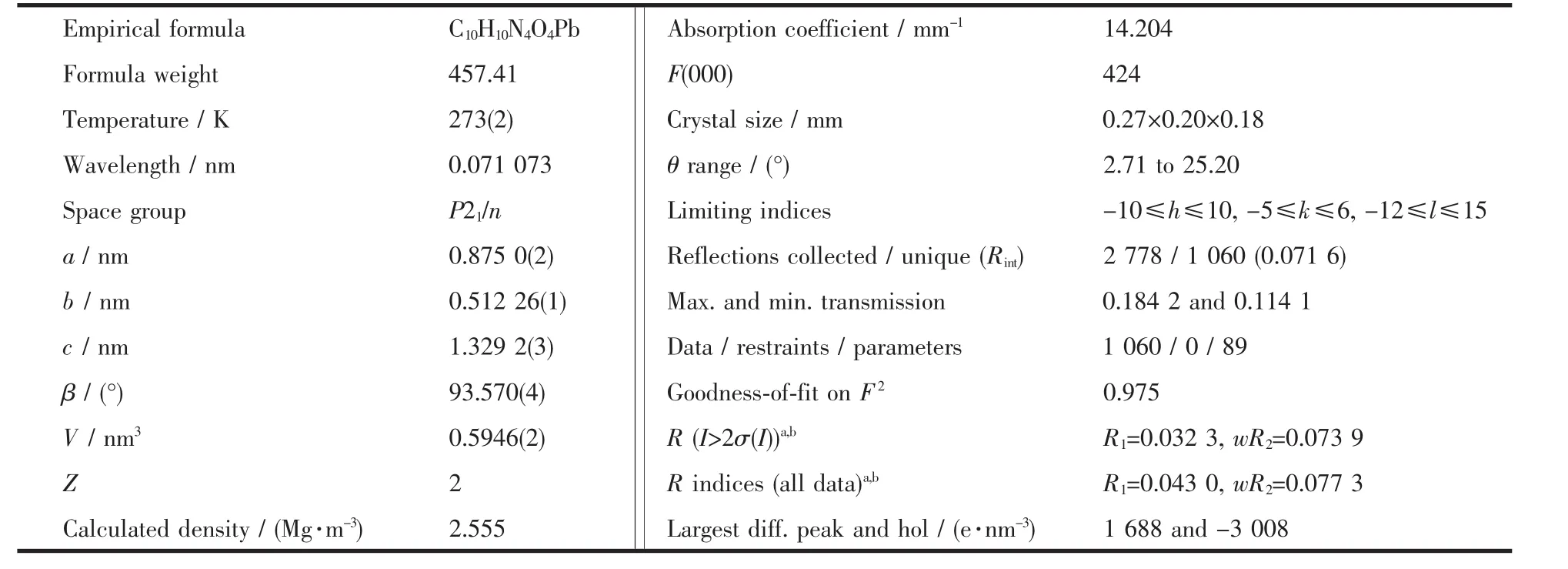
Table 1 Crystallographic data of complex 1


Table 2 Selected bond distances(nm)and bond angles(°)for complex 1
2 Results and discussion
2.1 Crystal structure of[Pb(ima)2]n(1)
A single-crystal X-ray diffraction study revealed that 1 is a 2D polymeric structure crystallizing in the monoclinic space group P21/n.Each Pb(Ⅱ)atom is sixcoordinated to four carboxylate oxygen atoms and two imidazole nitrogen atoms that come from six different ima ligands (Fig.1).The local coordination geometry around the Pb center can be described as a slightly distorted octahedral geometry with the Pb-N distance of 0.2623(7)nm and the Pb-O bond distances ranging from 0.254 0(5)to 0.257 0(6)nm.The bond angles around the neighboring atoms range from 77.18°to 102.82°.For the ima carboxylic groups,the two coordinated C-O bond distances of the carboxylic group are almost equivalent,0.123 9(7)nm for C1-O1 and 0.1252(1)nm for C1-O2,which is consistent with its structural feature(syn-anti coordination).

The carboxylate group bridges two metal centers so that each ima ligand adopts an tridentate bridging fashion to link three Pb(Ⅱ)metal centers giving a 2D MOFs,as shown in Fig.2a.Firstly,neighboring Pb(Ⅱ)centers are linked to each other by the carboxylate groups to form a one-dimensional(1D)chain that runs along the b axis with the Pb…Pb distance of 0.512 3 nm.The neighboring 1D chains are interconnected by the ima ligands via coordination with the imidazole nitrogen atoms to result in a 2D lamellar framework layer.From the topologicalview,an interesting structural feature in 1 is that the 2D layer can be rationalized as a noninterpenetrating(3,6)-connected net with kgd topology (Fig.2d)by simplifying the ima ligand as a 3-connecting node (vertex symbol 43)(Fig.2b)and each Pb atom as a 6-connecting node(vertex symbol 466683)(Fig.2c).

It is interesting to note that two ima ligands link two Pb atoms to form a 14-membered macrocycle ring with an opposite Pb…Pb with the nearest distance of 0.857 3 nm,when viewed down the b axis(Fig.3).But there is no included solvent molecule in 1,which was confirmed by TGA.These 2D layers are stacked with ABAB sequence(Fig.3).The supramolecular framework of 1 is held together by C-H…O hydrogen bond interactions of the carboxyl O atom and the imidazole C atom(the C4…O2 distance of 0.336 7 nm)between two nearly parallel neighboring layers.

2.2 IR spectra of complex 1
In IR spectrum,the polymer shows the strong bands corresponding to ν(COO)asand ν(COO)sat 1 558 and 1 386 cm-1,which is the characteristic ν(COO)asand ν(COO)sstretching vibrations of the carboxylate groups.In comparison to the characteristic stretching vibration bands of carboxylate groups of free ligand molecules,the significant blue-shift of both the ν(COO)asand ν(COO)sstretching vibration peaks may be attribu-ted to the coordination interactions.The Δν value is 172 cm-1,which indicates that the bridging modes of the carboxylate groups of ima is present,being consistent with the crystal structures of 1[24].
2.3 Thermal analyses
High thermal stability is an important precondition in the conversion of coordination frameworks from laboratory curiosities to practical materials.Thus,thermogravimetric analyses (TGA)were performed to determine the thermal stability of the precipitate.The TGA results of the compound show that no obvious decomposition was observed until about 240℃and proves no solvent molecules in polymers.Then the TGA shows MOFs remained intact until two consecutive weight losses occur in the range of 250~600 ℃.The final product is Pb2O3.The experimental residual percentage weight(51.07%)at the end of the decomposition of the polymer is consistent with the calculated values of 49.10%.
2.4 Luminescent properties
The fluorescent properties of the pure ligand and the complex 1 were investigated in the solid state at room temperature (Fig.4).The pure ligand shows an intense emission band at 350 nm (λex=280 nm).The emission of pure ligand may be attributed to the π→π*transition.There are quite strong emissions at 356 nm for complex 1,which are red-shifted about 6 nm compared with that of free ima ligand.We assume that the emissions may arise from the Pb2+lone pair to ligand charge transfer[25-26].The study of crystal structures in 1 gives evidence for the stereochemically active 6s2lone pair on the lead(Ⅱ) ion.

In this article,we have utilized the unsymmetrical flexible functionalized bridging ligand Hima for the synthesis of lead coordination networks.The porous coordination polymer[Pb(ima)2]nadopts a(3,6)-connected 2D net with kgd topology,in which the carboxylate groups bridge the adjacent Pb centers.The 2D layer further stack into 3D supramolecular networks through C-H…O weak interactions.The polymer is also characterized by IR, elemental analysis, fluorescent properties and thermogravimetric analysis.
[1]Kitagawa S,Kitaura R,Noro S I.Angew.Chem.,Int.Ed.,2004,43:2334-2375
[2]Deng H,Doonan C J,Furukawa H,et al.Science,2010,327:846-850
[3]Uemura T,Yanaia N,Kitagawa S.Chem.Soc.Rev.,2009,38:1228-1236
[4]Ohara K,Kawano M,Inokuma Y,et al.J.Am.Chem.Soc.,2010,132:30-31
[5]Férey G,Serre C.Chem.Soc.Rev.,2009,38:1380-1399
[6]Xie Z G,Ma L Q,Kathryn E,et al.J.Am.Chem.Soc.,2010,132:922-923
[7]Min K S,Suh M P.Chem.Eur.J.,2001,7:303-313
[8]LIU Yu-Juan(刘玉娟),ZUO Jing-Lin(左景林),CHE Chi-Ming(支 志 明 ),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(4):610-614
[9]Bradshaw D,Prior T J,Cussen E J,et al.J.Am.Chem.Soc.,2004,126:6106-6114
[10]Gasque L,Verhoeven M A,Bernès S,et al.Eur.J.Inorg.Chem.,2008:4395-4403
[11]Zhao Y H,Xu H B,Shao K Z,et al.Cryst.Growth Des.,2007,7(3):513-520
[12]Martell A E,Smith R M.Critical Stability Constants:Vol.3.New York:Plenum Press,1977.
[13]Trindade T,Brien P,Zhang X M,et al.J.Mater.Chem.,1997,7(6):1011-1016
[14]Yang J,Ma J F,Liu Y Y,et al.Inorg.Chem.,2007,46:6542-6555
[15]Fan S R,Zhu L G.Inorg.Chem.,2007,46:6785-6793
[16]Zhao Y H,Xu H B,Fu Y M,et al.Cryst.Growth Des.,2008,8(10):3566-3576
[17]Yang J,Ma J F,Liu Y Y,et al.Cryst.Growth Des.,2009,9(4):1894-1911
[18]Yang J,Li G D,Cao J J,et al.Chem.Eur.J.,2007,13:3248-3261
[19]Jack M H,Saeed M,Ali A S.Inorg.Chem.,2004,43:1810-1812
[20]Shi Y J,Li L H,Li Y Z,et al.Polyhedron,2003,22:917-923
[21]Duan H Y,Ai X P,He Z K.Spectrochim.Acta A,2004,60:1447-1451
[22]Li L K,Song Y L,Hou H W,et al.Eur.J.Inorg.Chem.,2005:3238-3249
[23]Sheldrick G M.SHELXS97,Program for Crystal Structure Solution,University of Göttingen,Germany,1997.
[24]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.New York:Wiley,1978.
[25]Zhang K L,Liang W,Chang Y,et al.Polyhedron,2009,28:647-652
[26]Li S H,Gao S K,Liu S X,et al.Cryst.Growth Des.,2010,10(2):495-503
Syntheses,Structure and Properties of 2D Pb(Ⅱ)Complex with kgd Topology
LIU Ping1,2CAO Chao-Chu2LIANG Yan-Ping1WU Zhen-Sen*,1
(1School of Science,Xidian University,Xi′an 710071)
(2Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education,Shaanxi Key Laboratory of Physico-Inorganic Chemistry,College of Chemistry&Materials Science,Northwest University,Xi′an 710069)
The reaction between Pb(Ac)2·3H2O and 2-(1H-imidazole-1-yl)acetate(Hima)produced a neutral 2D coordination polymer[Pb(ima)2]n(1).The single crystals were obtained by diffusing through agar gel aqueous solution and structurally characterized by single-crystal X-ray diffraction analysis.The polymer crystallized in the monoclinic space group P21/n with the cell parameters:a=0.8750(2)nm,b=5.1226(12)nm,c=13.292(3)nm,β=93.570(4)°,V=594.6(2)nm3,Z=2.The[Pb(ima)2]nrepresents a(3,6)-connected 2D layer with kgd topology,which further stacks into 3D supramolecular networks through C-H…O weak interactions.The polymer is also characterized by IR,elemental analysis,fluorescent properties and thermogravimetric analysis.CCDC:773324.
lead complex;flexible ligand;crystal structure;luminescent properties
O614.43+3
A
1001-4861(2010)11-2057-06
2010-05-04。收修改稿日期:2010-06-25。
国家自然科学基金(No.20771090、20931005),陕西省自然科学基金(No.2006B07)和陕西省教育厅专项基金(No.07JK390)资助。
*通讯联系人。 E-mail:liuping@nwu.edu.cn,Tel:+86-29-88302604
刘 萍,女,36岁,副教授,会员登记号:S06N7345M1006;研究方向:配位化学。

