金属酞菁仿生催化儿茶酚胺氧化性能研究及其用于肾上腺素浓度的光学检测
李明田 黄 俊 杨瑞嵩 喻兰英 周 璇
(1材料腐蚀与防护四川省重点实验室,自贡 643000)(2四川理工学院材料与化学工程学院,自贡 643000)(3武汉理工大学光纤传感技术与信息处理教育部重点实验室,武汉 430070)
金属酞菁仿生催化儿茶酚胺氧化性能研究及其用于肾上腺素浓度的光学检测
李明田*,1,2黄 俊3杨瑞嵩1,2喻兰英1,2周 璇3
(1材料腐蚀与防护四川省重点实验室,自贡 643000)
(2四川理工学院材料与化学工程学院,自贡 643000)
(3武汉理工大学光纤传感技术与信息处理教育部重点实验室,武汉 430070)
采用电子吸收光谱法研究了5种金属酞菁MPcs(M=Mn(Ⅱ),Fe(Ⅱ),Ni(Ⅱ),Cu(Ⅱ))仿生催化肾上腺素(Adrenaline,AD)和去甲肾上腺素(Noradrenaline,NA)2种儿茶酚胺的氧化性质,相应的氧化产物分别为三羟基-N-甲基-吲哚和三羟基-吲哚。用氧化产物的特征吸收峰强度评价金属酞菁的催化能力,实验表明,在最佳催化条件下,金属酞菁催化效率有以下顺序ηMnPc>ηFePc>ηNiPc>ηCuPc>ηCoPc。以酞菁锰仿生酶为催化剂,采用锁相放大技术构建了一种新型光纤生物传感器实现对肾上腺素浓度的测定,系统地研究了光纤肾上腺素传感器的性质:在2.0×10-6~9.0×10-5mol·L-1范围,滞后相移φ与肾上腺素的浓度有较好的线性关系,检测下限为4.0×10-7mol·L-1,响应时间为10 min,该传感器有良好地重复性和稳定性。
金属酞菁;仿生酶;儿茶酚胺;催化氧化;光纤生物传感器
Catecholamines (CAs),such asepinephrine(adrenaline,AD),noradrenaline (NA)and dopamine,play a key role in central and sympathetic nervous systems.Some catecholamine drugs have been used to treat hypertension,bronchial asthma,organic heart disease,and used in cardiac surgery and myocardial infarction[1-3].CAs monitoring has become of increasing interest in medical diagnosis.Many methods are applied to determine the CAs concentration,such as flow injection analysis[4],spectrophotometry[5],flowinjection chemiluminescence[6-7],high performance liquid chromatography (HPLC)[8-9],and electrochemical detection[10].However,these methods are timeconsuming,unsatisfactory for on-line and real-time monitoring.
The fiber optic biosensors are composed of a biologicalrecognition element (biocomponent),a transducer converting the biocomponent response to an optical signal and associated electronics.The ones have many advantages such as high sensitivity,low cost,immunity from electrical and magnetic disturbances,small size,lightweight,and can be used to monitor many parameters online and real-time.In our previous investigation,the fiber optic biosensor based on laccase catalysis was applied into the detection of AD[11].However,the application is seriously restricted due to the very limited enzyme source and poor stability.Contrastively,metallophthalocyanines (MPcs)with a perfectly symmetrical 18-electron aromatic macrocycle are easily accessible,very stable to degradation and cost effective[12],and have been extensively used to mimicenzymesto catalyzea varietyof organic reactions[13-20].
As a part of the investigation of phthalocyanine and the derivatives,MPcs[M=Mn(Ⅱ) Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ)]were mimickedenzymetocatalyzethe oxidation of ABTS[2,2′-azino-bis-(3-ethylthiazoline-6-sulphonate)][21],and the nano-composites formed from Fe3O4and metaltetraaminophthalocyanines were used as carriers of immobilized laccase to catalyze AD oxidation[11,22].As potential effective alternatives to laccase,five MPcs[M=Mn(Ⅱ),Fe(Ⅱ),Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ)]were tested to catalyze the oxidation of CAs(AD and NA)by dioxygen.Based on the fluorescence quenching and MnPc catalysis,a novel fiber optic biosensor was fabricated and studied for AD concentration detection.The experimental results indicated that MPcs were good catalyst for the CAs oxidation and could be used effe ctively in the fiber optic AD biosensor.
1 Experimental
1.1 Materials and instruments
Adrenaline hydrochloride injection(1.0 mg·mL-1)and noradrenaline bitartrate injection (2.0 mg·mL-1)were purchased from Wuhan Pharmaceutical Co.Ltd.[Ru(bipy)3]Cl2·6H2O and cellulose aceate were obtained from Sigma-Aldrich.All other reagents were analytical grade.Simulated body fluid (SBF)was prepared by deionized water.Dual-distilled water was used throughout the experiment.The UV/Vis spectra were measured on a Shimadzu UV-2450 spectrophotometer(Shimadzu,Japan)with 10 mm quartz cell.Lock-in amplifier(SR830,Standford Research Systems,U.S.A)was used to measure the phase delay of the sensor.MPcs were synthesized and purified according to the literatures[23-25],and a schematic structure of the complex was presented in Fig.1.

1.2 Procedure for estimation of catalytic activity
A certain amount of MPc was dispersed into the buffer solution of disodium hydrogen phosphate-citric acid(PBS)(3.0 mL,0.1 mol·L-1).After incubated about 10 min,the solution of AD or NA was added into the buffer containing MPc,then the mixture was incubated for a period of time.MPc was filtered off and UV/Vis spectrum of the filtrate was recorded on spectrophotometer.The procedure of the oxidation reaction can be estimated by the characteristic peak of the oxidation product,and the rates of catalytic oxidation were evaluated by η=It/Ic,where Itand Icwere the absorbances of the mixture solution at 298 nm after t minutes reaction and after complete oxidation of AD or NA,respectively.
1.3 Principle of biosensors
CAs are o-diphenol and can be oxidized easily by O2with catalysis of enzyme[26],the structures of AD and NA were shown in Fig.2.If the sensor head with an oxygen-sensitive membrane was put into the AD or NA solution containing catalyst,an oxygen gradient would be created on the membrane due to the consumption of the dissolved oxygen caused by the oxidation of AD or NA,which would lead to a fluorescence change of the oxygen-sensitive membrane since O2serves as a dynamicquencheroffluorescence.Thiscan be mathematically described by using Stern-Volmer equation:

Where I0and I, τ0and τ are the fluorescence intensities and lifetimes ofthe oxygen-sensitive membranein theabsence and presence ofthe quencher,respectively,and Ksvis the Stern-Volmer constant.cQis oxygen concentration in the solution.
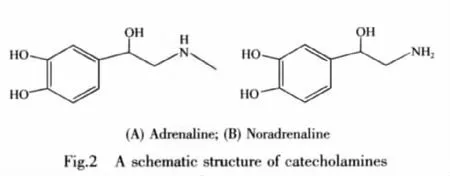
Since a novel lock-in amplifier is used,light signal from LED is supplied as sine modulated signal,and the fluorescence signal thus also appears a sine signal but a phase delay.The change of light signal containing the fluorescence lifetime can be converted into phase change in the lock-in amplifier according to the relationship between τ and φ as shown in Eq.(2):

When φ is very small,tanφ can be substituted by φ.By collecting the data of phase delay φ,the quantification of AD or NA is achieved.
1.4 Preparation of biosensor
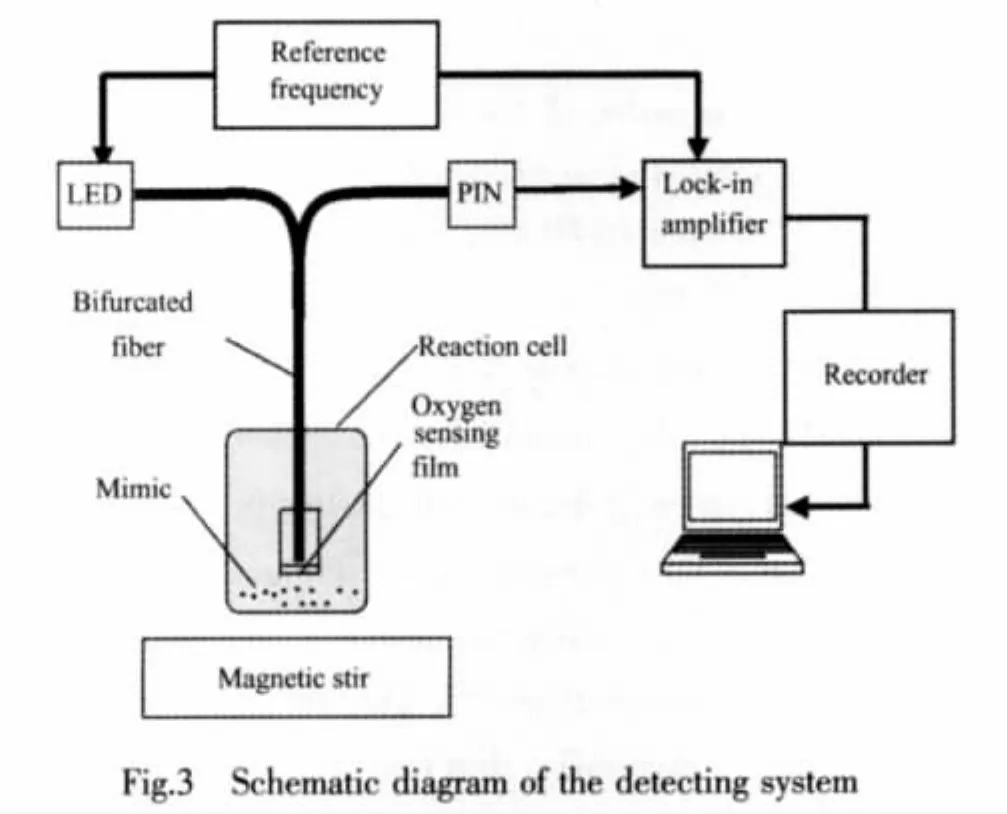
The oxygen-sensitive membrane was prepared by using [Ru(bipy)3]2+as the fluorescence indicator and cellulose acetate as the matrix[27].The detecting system consists of a lock-in amplifier,a LED with the excitation wavelength of 416 nm as the light source,a sensor head with a sensing membrane and a computer for data processing(Fig.3)[11].The modulation frequency was set for 40.0 kHz.
2 Results and discussion
2.1 Absorption spectra
In 0.1 mol·L-1PBS at pH 8.0,the two compounds of CAs showed two bands in the UV range at 217 and 279 nm for AD and NA shown in Fig.4.Barreto assigned the transitions at 220 and 280 nm in CAs to a π-π*and an La-Lbcoincident transition,respectively[28].

Fig.4A(b~g)showed the spectra obtained from the oxidation of AD catalyzed by MnPc.It can be seen that MnPc can catalyze the oxidation of AD effectively.The same spectrum patterns were observed for AD and NA during the oxidation processes catalyzed by the other MPcs.During the oxidation,two bands appeared progressively at ca.298 and 266 nm(for NA at 298 and 267 nm)until they completely overlapped the band at 280 nm,which were assigned to a1Lb←1A1and a1La←1A1transition,respectively[28].However,the spectral patterns were obviously different from those of the reported literatures(new peaks at ca.300 and 475 nm in the visible region of their UV/Vis spectra)[28-32],and the new peaks of 298 and 266 nm were considered as the characteristic absorption of trihydroxyl-N-methylindole (THMI) and trihydroxyl-indole (THI),respectively[33].
The experimental results also showed that the suitable concentration was 5.0×104mol·L-1for AD and NA.When the concentration was lower,the change of the UV/Vis spectra of CAs could not be observed evidently.Due to the similar properties of oxidation reactions with AD and NA as the substrates,AD was only discussed in the followings.
2.2 Effects of incubation time
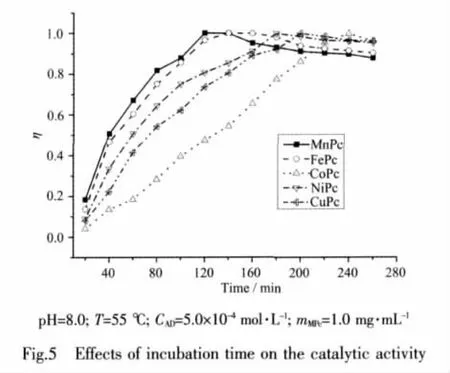
The results of evaluation on incubation time were shown in Fig.5.At pH 8.0 and T=55℃,five MPcs catalyzed the oxidation of AD effectively.η first increased remarkably for all MPcs,then increased slowly.However,η decreased after about 120 min for MnPc,and 140,180,200,240 min for FePc,NiPc,CuPc and CoPc,respectively.The decline might be that THMI proceeded with the structural transformation.The results also indicated that the catalytic abilities of MPcs to AD oxidation were different and they had the order of ηMnPc>ηFePc>ηCuPc>ηNiPc>ηCoPc.This difference might be probably due to the different d-electron configurations of metal ions in MPcs[32,34-36].
2.3 Effects of pH
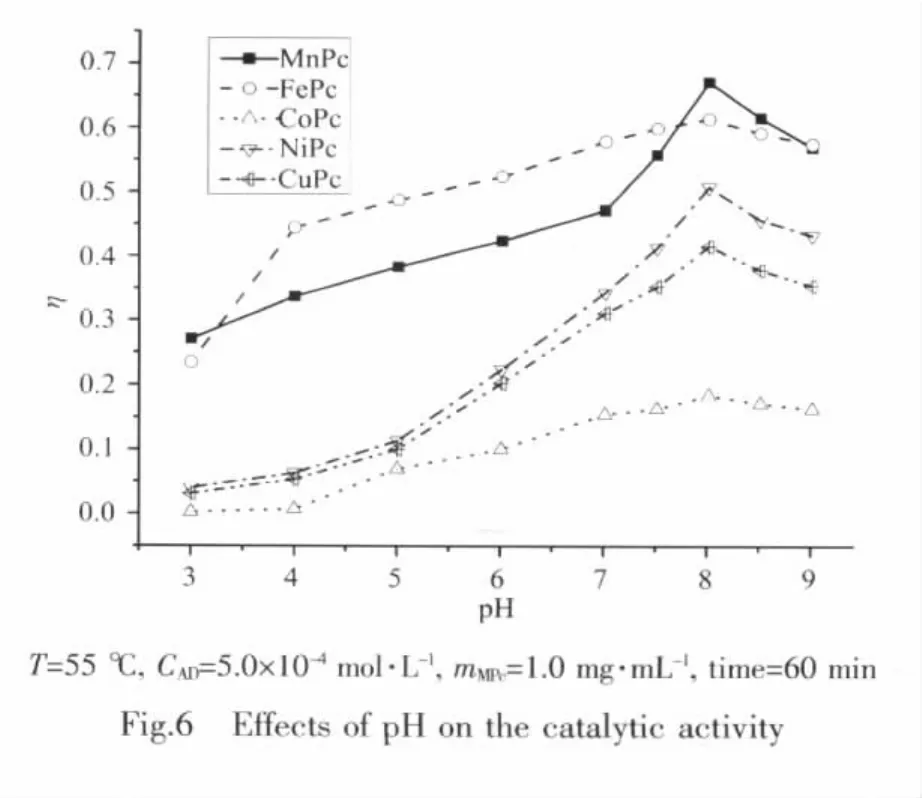
Fig.6 presented the effects of pH values on the catalytic activities of MPcs for the oxidation of AD.η increased gradually in the range from pH 3.0 to 8.0,and then declined when pH was over 8.0 for all five MPcs.Thus,the optimal pH values for the catalytic reaction of all MPcs were the same at 8.0.Although it had the best catalytic activity under the optimal conditions,MnPc showed worse activity in low pH environment than FePc,and the other three MPcs(M=Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ))shared the similar catalytic tendency.FePc had better catalytic activity in the range from pH 4.0 to 9.0,indicating that FePc can be used in larger pH range.The reason for the poor catalyzing ability of MPc in low pH environment is probably that the acid environment is not advantageous to the coordination of AD and O2to metal center and the formation of AD2+[34-35].
2.4 Effects of temperature
The temperature dependence of MPcs catalytic activities to the oxidation of AD was shown in Fig.7.As temperature increased,η increased rapidly in the temperature range from 40 to 55℃,then decreased from 55to 65 ℃ for four MPcs(M=Mn(Ⅱ),Fe(Ⅱ),Ni(Ⅱ),Cu(Ⅱ)).These were different from the catalytic activity of CoPc which increased gradually in the temperature range from 40 to 65℃.Therefore,the optimal temperatures for the catalytic reaction of the four MPcs were the same at 55℃.It might be explained in the following way:The rate of reaction would increase with the increase of temperature.In addition,AD and O2should coordinate to M(Ⅱ)in MPc along the vertical axis direction before the oxidation occurred at higher temperature,AD and O2would dissociated themselves from M(Ⅱ)in MPc before the accomplishment of electronic transfer.These two factors caused the reaction rate to reach a maximum at about 55℃.[32,34,36].

As for CoPc,the electron transfer rate between O2and the substrate was quickened with temperature increased,which accelerated the speed of the catalytic reaction,however,the change of temperature less influences the stability of transitional products than the electron transfer rate,so the catalytic activity increased gradually ranging from 40℃to 65℃.
2.5 Effects of MPc amount
The effects of MPcs amount on the catalytic activities were shown in Fig.8.It indicated that η increased with the addition of MPcs.The catalytic activities for MnPc and FePc showed some degree above 50%at 1.0 mg·mL-1and the increasing magnitude was not notable once the quantity exceeded 1.0 mg·mL-1.The similar results were occurred to the others.Hence,1.0 mg·mL of MPc was used throughout the other parts of our experiments[37-38].

2.6 Mechanism of catalytic oxidation
The oxidative pathways of CAs have been well studied in order to investigate the catalytic mechanism[28-32].As for MPc acting as the catalyst to the oxidation of AD or NA,the following steps were proposed taking MnPc catalyzing AD as the example and were shown in Fig.9:(1)AD and O2coordinated to Mn(Ⅱ)in MnPc along the vertical axis direction[29];(2)one electron transferred from Mn(Ⅱ) to O2,Mn(Ⅱ) was oxidized to Mn(Ⅱ) and O2was reduced to O2-;(3)Mn(Ⅱ)obtained one electron from AD,Mn(Ⅱ)was reduced to Mn(Ⅱ)and ADwas formed;(4)another one electron of AD transferred to O2-via Mn(Ⅱ),thus AD2+and O22-were formed,and then dissociated from Mn(Ⅱ)in MnPc;(5)AD2+transferred continuedly to THMI.

Under the optimal conditions of pH 8.0,T=55℃,mMPc=1.0 mg·mL-1,MPcs have the catalytic activity order of ηMnPc>ηFePc>ηNiPc>ηCuPc>ηCoPc,which is followed with the changed rule of d electron number of central metal cations except CoPc,and is accorded with the order ofthe oxidation ofpinane to 2-pinane hydroperoxide by using MPc as catalyst[17].Obviously,the electron populations of M(Ⅱ)is vital to the catalytic rate.Because the peaks of o-quinone framework were not found during the oxidation,the rate-determining step of the catalytic reaction might lie in before the formation of AD,hence the oxidization and reduction of M(Ⅱ)might be the determinate factor of the reaction rate.
2.7 Properties of biosensor
According to our studies,the oxygen-sensitive membrane could only be used below 40℃[11].The biosensor could effectively work at pH 7.0 and T=30℃using MnPc as the catalyst,because the activity is about 35%at 30℃and pH 7.0 obtained from the discussed above.
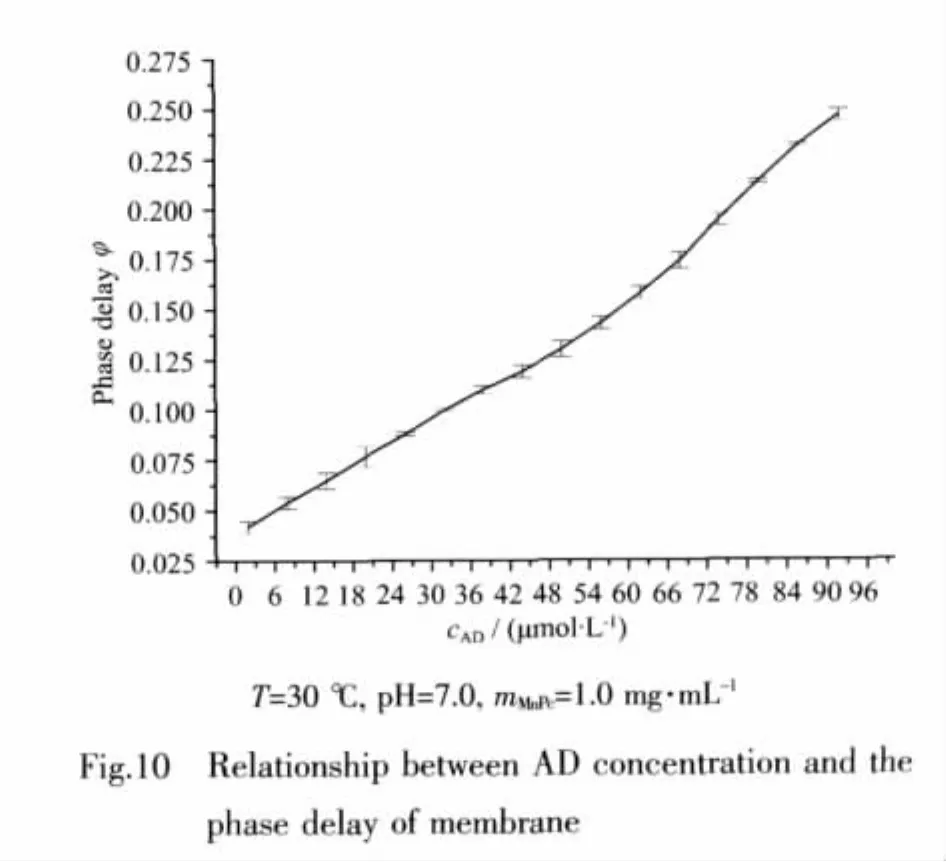
The concentrations of dissolved oxygen and AD were evaluated by the phase delay φ.A linear relationship between φ and AD was observed in the concentration range from 2.0×10-6to 9.0×10-5mol·L-1,as shown in Fig.10,and the linear graph was defined by the equation of y=0.002 2x+0.029 8(R2=0.993),while the detection limit was 4.0×10-7mol·L-1.It would be possible to perform the determination of lower AD concentration if the catalyzing characteristics of mimic enzyme were improved.
Repeatability of the sensor was tested,and the standard deviation(SD)value was 5.6×10-7(n=5)mol·L-1for the concentration range from 2.0×10-6to 9.0×10-5mol·L-1.While the response time of the sensor was 10 min.It would be possible to reduce the response time if suitable mediator was used.
The reusability and stability of the fiber optic AD biosensor were investigated,and the results were shown in Fig.11 and 12.The stability of the biosensor depended on the stabilities of the oxygen-sensitive membrane and MnPc.The oxygen-sensitive membrane was immersed in the reaction cell containing working buffer solution for 72 h on optimized working conditions and its oxygen sensitivity had no detectable change.Due to the immunity from strong acid,strong base and high temperature,MnPc has good stabilities.Hence,the biosensor has good reusability and stability.


To test performance of the fiber optic biosensor,the SBF and rabbit serum were used as the test medium,and the results were listed in Tables 1 and 2.Viewed from Table 1,the test results were consistent with the actual values,indicating the surroundings of SBF had little influence on the biosensor.However,the deviations were large in the rabbit serum listed in Table 2.The reasonable cause are as follows:first,there are no interactions between the anions or cations within SBF and MnPc or the sensitive membrane;second,the rabbit serum contains various inorganic ions and organic complexes,which is more complicated than SBF and the solvent for the standard curves,and the sensitive membrane is influenced to some degree by organic complexes.Thus,the performance can be optimized by improving the functions of MPc and the sensitive membrane,and the deviation can be reduced greatly through simulating more close to the real serum conditions.

Table 1 Results of AD in SBF for the fiber optic biosensor1

Table 2 Results of AD in the rabbit serum1
3 Conclusions
MPcs[M=Mn(Ⅱ),Fe(Ⅱ),Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ)]were used as catalyst for the oxidation of adrenaline(AD)and noradrenaline(NA)by dioxygen.The optimal pH values were the same at 8.0 for all five MPcs.The best suitable temperature were 55 ℃ for four MPcs[M=Mn(Ⅱ),Fe(Ⅱ),Ni(Ⅱ) ,Cu(Ⅱ) ],while the catalytic activity of CoPc increased gradually from 40℃to 65℃.MPcs had the catalytic activity order of ηMnPc>ηFePc>ηNiPc>ηCuPc>ηCoPcunder the optimal catalytic conditions with pH 8.0,T=55 ℃,mMPc=1.0 mg·mL-1.The conceivable mechanism was proposed.Based on the fluorescence quenching,the fiber optic sensor by using MnPc as catalyst to the oxidation of AD by dioxygen was constructed and studied.It had the detecting range from 2.0×10-6to 9.0×10-5mol·L-1with the detection limit of 4.0 ×10-7mol·L-1.The sensor also has good reproducibility and stability,showing great prospect of its application.
[1]Sorou raddin M H,Manzoori J L,Kargarzadeh E,et al.J.Pharm.Biomed.Anal.,1998,18:877-881
[2]Cohn J N,Levine T B,Olivari M T,et al.N.Engl.J.Med.,1984,311(13):819-823
[3]Fernstrom J D,Fernstrom M H.J.Nutr.,2007,137:1539S-1547S
[4]Palop S G,Romero A M,Calatayud J M.J.Pharm.Biomed.Anal.,2002,27:1017-1025
[5]Nagaraja P,Vasantha R A,Sunitha K R.J.Pharm.Biomed.Anal.,2001,25:417-424
[6]Zhang C X,Huang J C,Zhang Z J,et al.Anal.Chim.Acta,1998,374:105-110
[7]Loay K A,Anas M A,Mayada H A.Anal.Chim.Acta,2005,538(1/2):331-335
[8]Kartsova L A,Sidorova A A,Kazakov V A,et al.J.Anal.Chem.,2004,59:737-741
[9]Fotopoulou M A,Ioannou P C.Anal.Chim.Acta,2002,462:179-185
[10]Chernyshov D V,Shvedene N V,Antipova E R,et al.Anal.Chim.Acta,2008,621:178-184
[11]Huang J,Fang H,Liu C,et al.Anal.Lett.,2008,41:1430-1442
[12]Obirai J,Nyokong T.Electrochim.Acta,2005,50:3296-3304
[13]Meunier B,Sorokin A.Acc.Chem.Res.,1997,30:470-476
[14]Prasada R T,Murali D G Ed.Recent Advances in Basic and Applied Aspects of Industrial Catalysis,Amsterdam:Elsevier,1998,113:921-926
[15]Valente A A,Vital J.J.Mol.Catal.A Chem.,2000,156:163-172
[16]NiñoME,GiraldoSA,Páez-MozoEA.J.Mol.Catal.A Chem.,2001,175:139-151
[17]Nyokong T.Coord.Chem.Rev.,2007,251:1707-1722
[18]Sulman E M,Romanovskii B V.Russ.Chem.Rev.,1996,67:609-616
[19]QIANG Min-Jie(钱敏杰),JIANG Xiang-Fen(蒋相芬),WANG Xi-Tong(王喜童),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(9):1278-1283
[20]KONG De-Jing(孔德静),SHEN Shui-Fa(沈水发),YU Hai-Yang(于海洋),et al.Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(5):817-821
[21]TANG Yan(汤 雁),HUANG Jun(黄 俊),FANG Hua(方 华),et al.J.Wuhan Univ.Tech.(Wuhan Ligong Daxue Xuebao),2008,9:46-48
[22]Huang J,Xiao H Y,Li B,et al.Biotechnol.Appl.Biochem.,2006,44:93-100
[23]Kimer J F,Dow W,Scheidt W R.Inorg.Chem.,1976,15:1685-1690
[24]Linstead R P,Robertson J M.J.Chem.Soc.,1936,1736-1738[25]Robertson J M.J.Chem.Soc.,1935,615-621
[26]Palop S G,Romero A M,Calatayud J M.J.Pharm.Biomed.Anal.,2002,27:1017-1025
[27]ZHANG Jian-Biao(张建标),CHEN Xing(陈 兴),HUANG Jun(黄 俊),et al.J.Transducer.Tech.(Chuanganqi Jishu),2002,21(10):4-7
[28]Barreto W J,Ponzoni S,Sassi P.Spectrochim.Acta Part A,1999,55:65-72
[29]CHEN Bin(陈 彬),DU Xi-Guang(杜锡光),YANG Shu-Qing(杨树卿),et al.Chin.J.Mol.Sci.(Fenzi Kexue Xuebao),1996,3(9):211-217
[30]Graham D G.Mol.Pharmacol.,1978,14:633-643
[31]Heacock R A.Adv.Heterocycl.Chem.,1965,5:205-290
[32]Kitamura Y,Mifune M,Takatsuki T,et al.Catal.Commun.,2008,9:224-228
[33]TrautnerEM,BradleyTR.Austral.J.Sci.,1951,1-4:303-306
[34]CHEN Bin(陈 彬),SHAO Yun(邵 允),LI Lian-Zhong(李连忠),et al.Chin.J.Mol.Sci.(Fenzi Kexue Xuebao),1996,3(9):218-223
[35]SONG Xu-Feng(宋旭峰),JI Hong-Bing(纪红兵),ZHOU Xian-Tai(周显太),et al.Fine Chem.(Jingxi Huagong),2004,12(6):474-476
[36]Sorkin A,Fraisse L,Rabion A,et al.J.Mol.Catal.A Chem.,1997,117:103-114
[37]Jiang Q,Hu H Y,Guo C C,et al.J.Porphyrins Phthalocyanines,2007,11(07):524-530
[38]XIANG Yu-Zhi(项玉芝),SI Xi-Qiang(司西强),ZHANG Yan-Sheng(张衍胜),et al.Fine Chem.(Jingxi Huagong),2008,25(12):1240-1244
Oxidation of Catecholamines Catalyzed by Metallophthalocyanines and Application to the Fiber Optic Biosensor for Adrenaline Concentration Detection
LI Ming-Tian*,1,2HUANG Jun3YANG Rui-Song1,2YU Lan-Ying1,2ZHOU Xuan3
(1Key Laboratory of Material Corrosion and Protection of Sichuan Province,Zigong,Sichuan 643000)
(2Institute of Materials Science and Chemistry Engineering,Sichuan University of Science and Engineering,Zigong,Sichuan 643000)
(3Key Laboratory of Fiber Optic Sensing Technology and Information Processing(Ministry of Education),Wuhan University of Technology,Wuhan 430070)
The oxidation ofcatecholamines (adrenaline,AD;noradrenaline,NA)by oxygen using metallophthalocyanines[MPcs,M=Mn(Ⅱ),Fe(Ⅱ),Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ)]as the catalyst were studied by electronic absorption spectra,and the consequent products were trihydroxyl-N-methyl-indole and trihydroxyl-indole,respectively.The catalytic activities of the MPcs were evaluated by the absorbance ratios at the characteristic peak of the oxidation products.The results showed that MnPc had the best catalytic activity under the optimal conditions.The fiber optic AD biosensor based on MnPc catalysis and fluorescence quenching was fabricated and studied.The dissolved oxygen and AD content were evaluated by the phase delay φ.A linear relationship between φ and AD concentration was observed in the range from 2.0×10-6to 9.0×10-5mol·L-1,and the detection limit was 4.0×10-7mol·L-1.The results indicated that the fiber optic biosensor exhibited good reproducibility and stability.
metallophthalocyanines;mimic enzyme;catecholamines;catalytic oxidation;fiber optic biosensor
O625.8;O643.36+1
A
1001-4861(2010)11-2069-08
2010-04-26。收修改稿日期:2010-06-06。
国家自然科学基金(No.60877048)和四川理工学院科技项目(2009xjkRL007)资助。
*通讯联系人。 E-mail:limt63646616@yahoo.com.cn
李明田,男,31岁,讲师;研究方向:仿生功能材料。

