介孔碳/聚苯胺修饰电极的制备及其对Cu2+的响应研究
郭 卓 郭 彤 赵常礼 高云鹏 李 莎
(沈阳化工大学材料学院,辽宁省教育厅聚合物材料应用技术重点实验室,沈阳 110142)
介孔碳/聚苯胺修饰电极的制备及其对Cu2+的响应研究
郭 卓*郭 彤 赵常礼 高云鹏 李 莎
(沈阳化工大学材料学院,辽宁省教育厅聚合物材料应用技术重点实验室,沈阳 110142)
制备了一个新的电极-聚苯胺掺杂介孔碳修饰电极(PANI-MC),并且研究了电极的电化学性质。在介孔分子筛SBA-15的孔道中沉积蔗糖,然后在氮气的保护下,1200℃热裂解,生成孔道规则排列的介孔碳(MC);XRD、N2吸附-脱附、TEM等方法表征了介孔碳的结构,用SEM表征了PANI-MC修饰电极的形貌。结果表明:复合电极膜与修饰前的聚苯胺膜形貌不同,与介孔碳形貌相似,介孔碳纳米微粒的大小清洗可辨,长度大约为20~40 μm。复合电极循环伏安结果显示:峰电位向负电位方向移动,这可能是因为介孔碳的孔道结构阻碍了离子的转移。同时,还研究了复合电极对Cu2+的相应,表明:电极对低浓度的Cu2+有很好的线性相应,可以作为Cu2+的感应器。
介孔碳;Cu2+分析;电极
0 Introduction
Recently,the preparation of a new series of ordered mesoporous carbon materials has been reported via the nanocasting technique using mesoporous silicas as the templates[1-5].Insertion of carbon precursors within the ordered mesoporous of the silica materials,carbonization at high temperature and subsequent removal of the silica template result in the ordered mesoporouscarbon materials.Compared with the typical porous carbon materials such as activated carbon,this new type of mesoporous carbons possess a well-ordered pore structure,high specific surface area,large pore volume,electrical conductivity which can be used in many fields.Moreover,the pore structure of ordered mesoporous carbon materials can be controlled by the carbon framework source,silica template and carbonization temperature.
Considerable interest has been devoted to the electrochemical study of conducting polymers,such as polyaniline(PANI),polypyrrole and polyacetylene.Of thesePANIhasbeen the subjectofnumerous investigations.PANI is unique among conducting polymers,primarily due to its high chemical durability and reversible controlofconductivity both by protonation and bycharge-transferdoping.These features make it a fascinating subject for scientific study and possible applications,such as electrode materials[6-11].
Chemically modified electrodes (CMEs)have attracted considerable attention for their application in analytical chemistry in recent years.The use of CMEs coupled with Anodic Stripping Voltammetry(ASV)is an expanding field of investigation.There is a great variety of CMEs which has been used in the heavy metal determination[12-19].As an example,MWNT/PANI electrode was described for the simultaneous determination of trace heavy metal ions by ASV[20].Among the existing solid electrodes,for many electrochemical applications carbon has been the electrode material of choice.A variety of new carbon-based materials have been developed in recent years and these electrodes have been employed successfully in fuel cells[21-22],as analytical sensors[23-24],for energy storage[25-27],etc.For instance,many attempts have been made to synthesize CNT/PANI and MWNTS/PANI composites for electronic applications[28-31].Based upon that,the aim of this work was to investigate the possibility of use of the ordered mesoporous carbon in electrodes with polyaniline in the determination of Cu2+.This sensing and determining system have the following advantages:free of mercury,low detection limit,fast response and good reproducibility and stability.
1 Experimental
1.1 Synthesis of mesoporous carbon(MC)
Mesoporous carbon was prepared using silica SBA-15 as template.The synthesis of mesoporous silica template SBA-15,was performed following procedures described elsewhere[32].Triblock polymer Pluromic P123(EO20-PO70-EO20,Mav=5 800,BASF)was adapted as the structure-directing agentand tetraethylorthosilicate(TEOS,Aldrich)was used as the silica source.The procedure for the synthesis of mesoporous carbon materials is very similar to that of CMK-3 materials[1].The SBA-15 was filled with an aqueous solution containing sucrose and sulfuric acid by the impregnation method.The mixtures were dried at 100℃and subsequently at 160℃for 6 h each.The carbonization was carried out by heating at 1 200℃under nitrogen flow,and finally,silica template was dissolved at room temperature in ethanol solution of HF and isolated by suction filtration.After the extraction procedure,the silica-free mesoporous materials obtained were dispersed in deionized water while stirring,and filtered more than 3 times to completely remove HF.
1.2 Characterization of mesoporous carbons
X-ray powder diffraction patterns were obtained on a Siemens D5005 X-ray powder diffraction apparatus using Cu Kα radiation(λ=0.15418 nm).
Nitrogen adsorption/desorption isotherms were performed at 77 K with a Micromeritics ASAP 2010 analyzer.Thesurfaceareawascalculated using Brunauer-Emmett-Teller (BET)model.The pore volume,pore size distributions and the average pore diameter were obtained by Barrett-Joyner-Halenda(BJH)calculations.Before the nitrogen adsorption/desorption measurements,the mesoporous carbon was outgassed at 150℃ for 15 h.
Transmission electron micrographs were obtained on a JEM-3010 using a copper grid type sample holder.
1.3 Working electrode preparation
The surface of the glassy carbon electrode was cleaned by scrubbing on an alumina polishing pad,followed by immersing in HCl and rinsing with the deionised water.
Polyaniline was synthesize by oxidative polymerization of aniline using ammonium persulfate as oxidizing agent in a 1.0 mol·dm-3HCl solution.Deprotonated polyaniline was dissolved in 1-methyl-2-pyrrolidone(NMP)to yield a 5wt%solution.
1.0 mg of the mesoporous carbon was added in 5 mL of 5wt%polyaniline solution.This suspension was sonicate for 5 min to achieve a homogeneous dispersion of the mesoporous carbon particles.Working electrode was fabricated by coating 500 μL of the slurry on glassy carbon electrode (GC),followed by evaporating the solvent,NMP,with a blow dryer.This form of working electrodeisdenotedasPANI-MC electrode.By contrast,the PANI film coated GC electrode was prepared by the same procedure just explained but without mesoporous carbon.This electrode is denoted as PANI electrode.
1.4 Reagents
Stock solution of 1×10-2mol·L-1Cu2+was prepared by dissolving Cu(NO3)2(Shanghai Reagent Corporation,China)into redistilled water,and then diluted to various concentration working solution.NMP was purchased from Fluka Chemical Reagent Corporation.Other chemicals used were of analytical reagents.
1.5 Apparatus
The electrochemical measurements were performed with a conventional three-electrode glass cell.The auxiliary electrode was a platinum foil and the reference was a saturated calomel electrode(SCE).The working electrodes were mesoporous carbon modified glassy carbon(GC)working electrode.
All the voltammetric determinations were carried out with CHI 660A Electrochemical workstation(CH Instruments,USA).
The morphology of the PANI and PANI/mesoporous carbon were characterized using Scanning electron microscopy(SEM).SEM images were obtained on JSM-6700 field emission instrument.Transmission electron micrographs(TEM)were obtained on a JEM-3010 using a copper grid type sample holder.
2 Result and discussion
2.1XRD of MC
Fig.1 shows the powder X-ray diffraction patterns of MC.MC possesses a hexagonally ordered mesostructure as evident from the presence of at least three XRD peaks that can be indexed to (100),(110)and (200)reflections of two-dimensional hexagonal space group P6mm.The XRD pattern of MC indicates that the sample has highly ordered uniform mesopores.

2.2 Nitrogen adsorption/desorption
Fig.2 shows the adsorption/desorption isotherms of nitrogen at 77 K on MC.The isotherm exhibits a typeⅣprofile according to the BET classification.The isotherm shows a distinct hysteresis loop,which is characteristic of mesoporous adsorbents.This phenomenon is associated with capillary condensation in mesopores.Poreswithin porousmaterialsare classified as micropores(<2 nm),mesopores(2~50 nm),and macropores (>50 nm),in accordance with the classification adopted by the IUPAC.The pore size distribution curves suggest that mesopores are predominant,owing to the presence in the pore size distribution curves of large peaks at pore diameters greater than 2 nm.The mesopore of the MC is concentrated in a relatively small size range.

2.3 TEM images of MC
The transmission electron microscopy image of the MC is shown in Fig.3.The TEM shows the regular hexagonal array of uniform channels for MC with pore size 3.7 nm agreed with nitrogen adsorption results.

2.4 Surface morphology
Fig.4 shows typical SEM images for the PANI(a)and PANI-MC(b)films.It is possible to observe that the morphology of the film is drastically modified in the presence of the mesoporous carbon.PANI films are smooth, and PANI-MC films possess rod-like morphology with length from 20 μm to 40 μm.For PANI-MC films,it morphological structure is very similar to that observed for pure MC.
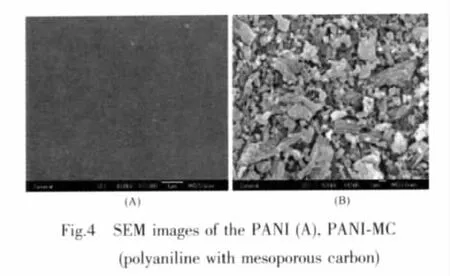
2.5 Electrochemical behavior of PANI-MC electrode
Fig.5 shows the cyclic voltammograms(CV)of the pure PANI(a),PANI-MC composite(b),and pure MC(c)electrode between-0.20 and 1.0 V versus SCE taken at a sweep rate of 50 mV·s-1,which are performed in 0.5 mol·L-1H2SO4solution.
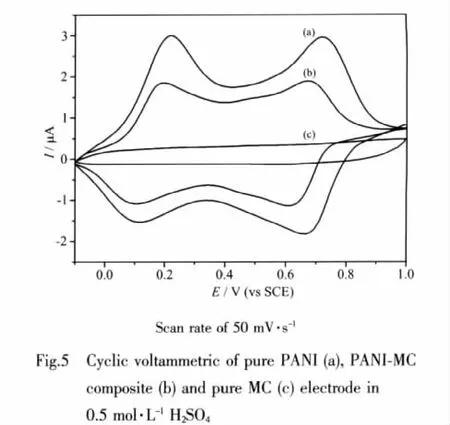
The voltammograms of the PANI-MC and pure PANI electrodes show two main redox processes,which are related to the interconversion reaction of PANI upon varying the potential (leucoemaldine/emeraldine and emeraldine/pernigraniline)[33].
There are two redox pairs in the voltammograms with the anodic peaks.The first peak corresponds to the removal of electrons from the amine units,accompanied by HSanion binding.The amine units convert to semiquinone radical cation in this oxidation peak.The second represents the oxidation of semiquinone radical cation to quinine didimine with release of a proton.The voltammogramsreflectthatthe potentialwindow employed in the measurements involves transfer of two electrons per repeating unit of PANI[34].
The potential shift toward negative direction for the anodic peaks of the PAIN-MC electrode,and vice versa for the cathodic peaks,can be attributed to the porous feature of the mesoporous carbons,which imposes resistance for ion migration in mesoporous.Similar behavior was observed in other paper[34].
The CVs of MC show almost rectangular-like response to the zero-current line and a rapid current response on voltage reversal at each end potential.
2.6 Effect of accumulation potential and time
The dependence of the pulse anodic stripping voltammetric peak currenton the accumulation potential and time for Cu2+concentrations on the PAINMC is shown in Fig.6a.

The peak currentincreaseswith increasing accumulation time,indicating an enhancement of Cu2+ion at the surface of the electrode.At the lower concentration (curve□),the stripping peak current increases at the first 150 s,and then remains constant.For the higher concentration (curve(Ⅱ)),the peak current increases rapidly at the first 150 s,and levels off.The curve indicates that a limiting value of the amount of reduced Cu2+has been achieved on the PANI-MC electrode.Hence for all subsequent measurements preconcentration time of 150 s was employed.
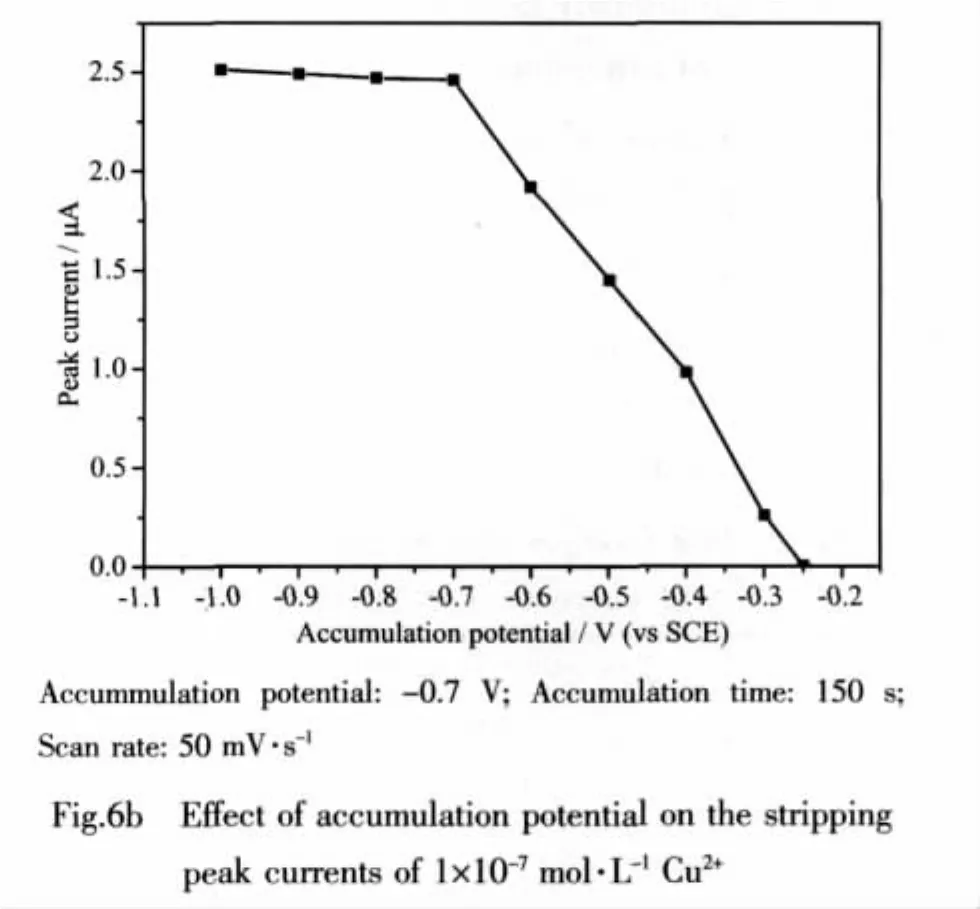
Fig.6b depicts the effect of the accumulation potential on the stripping peak currents after 150 s of accumulation.When the accumulation potential shifts from-0.3 to-0.7 V,the stripping peak currents increase greatly.As the accumulation potential becoming more negative,Cu2+is reduced more completely and consequently the stripping peak currents increases.When the accumulation potential is negative than-0.7 V,the stripping peak currents change very slightly.
2.7 Cu2+analysis
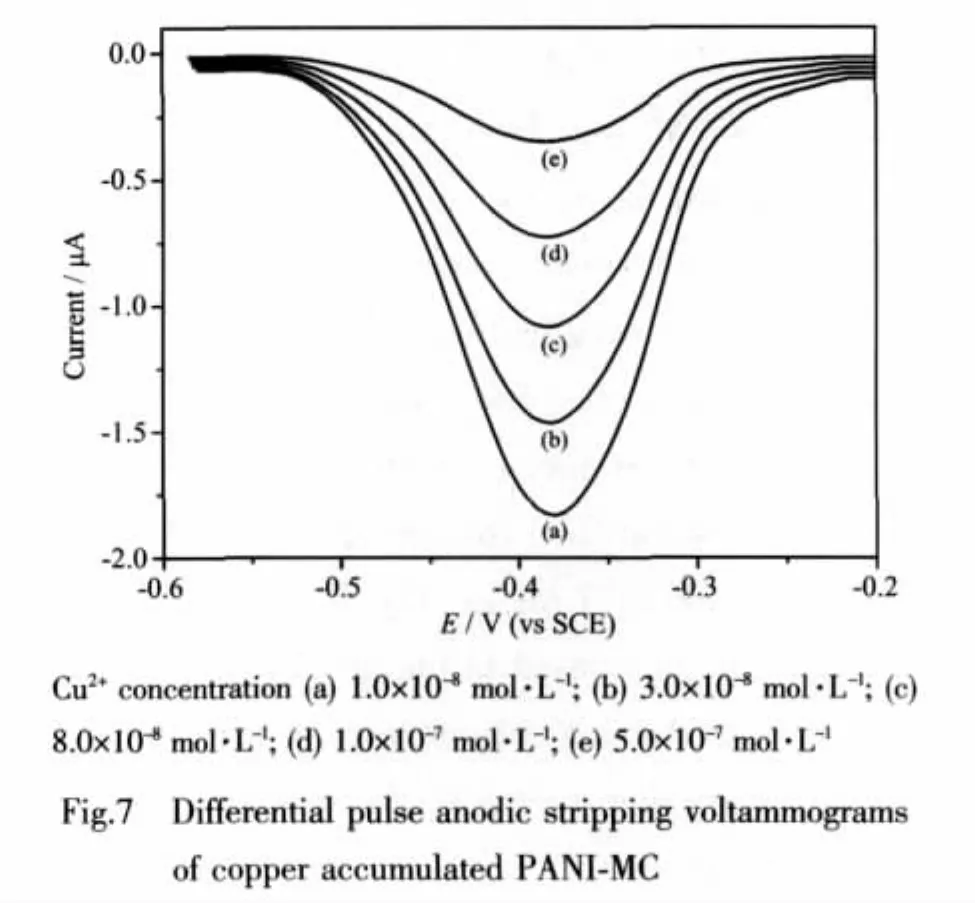
The electrochemical response obtained for solutions containing Cu2+is shown in Fig.7.The peak potential was found to shift towards more positive potential with increasing concentration of Cu2+.According to the figure,there is one peak located near-0.38 V,corresponding to Cu dissolution.The peak current increased with increasing concentration of Cu2+.The response was linear in the concentration range 1.00×10-8~1 ×10-6mol·L-1.The plot leveled off at higher concentration due to saturation of the electrode surface.The response was well reproducible.The regression equation wasip=179+200C,r=0.996,ipin nA,Cin μmol·L-1.The lowest detectable concentration of Cu2+at 150 s accumulation are estimated to be 6×10-9mol·L-1.
2.8 Effects of other ions
The influence of other metal ions on the current response of Cu2+is shown in Table 1.Table 1 suggests that Ca2+,Zn2+,Mn2+,Co2+,Fe2+,Al3+,Hg2+have no influences on the signals of 5×10-7mol·L-1Cu2+,even up to a 100-fold molar excess with respect to Cu2+,with deviation below 4%.
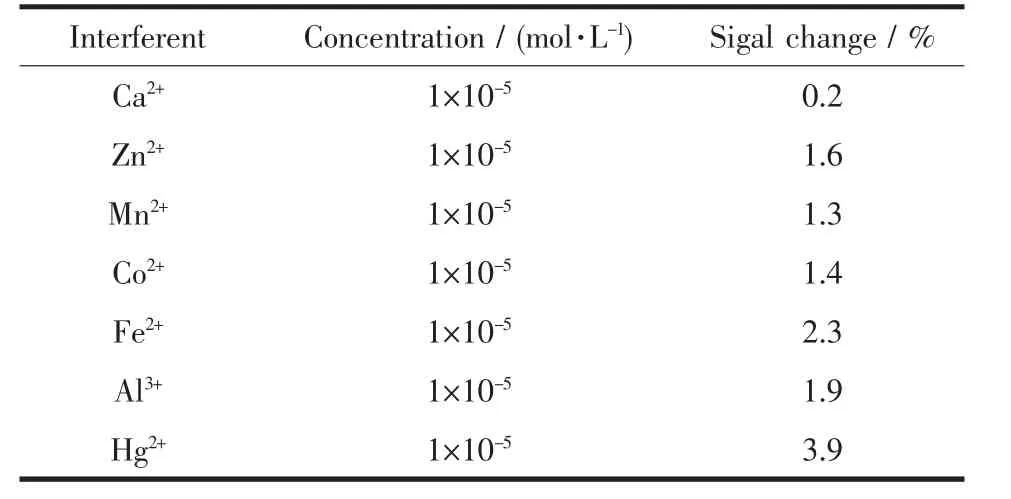
Table 1 Interferences of some ions on the stripping peak currents of 5×10-7mol·L-1Cu2+
2.9 Precision
The relative standard deviations of 10 measurements of 5×10-7mol·L-1Cu2+,which determined at one single modified electrode,is 4.7%.After every measurement,the PANI-MC electrodes are remade,and the relative standard deviations of 5×10-7mol·L-1Cu2+is 5.2%(n=9).This result suggests that the PANI-MC electrode exhibits excellent reproducibility towards the determination of Cu2+.The long-term stability of the PANI-MC electrode was evaluated by measuring the current responses at Cu2+concentration of 5×10-7mol·L-1over a period of 2 weeks.The PANI-MC electrode was used daily and stored in the air.The experimental results indicate that the current responses deviated only 5.7%,revealing that the PANI-MC electrode possesses long-term stability.
3 Conclusion
ASV analysis utilizing the PANI-MC electrode for the determination of Cu2+dissolved in aqueous solutions has been demonstrated.The deposition potential and deposition time have been optimized for determination of Cu2+in aqueous solution.In addition,combining the subtle properties ofordered mesoporous carbon(ordered structure,high surface area,large pore volume)with the high electronic conductivity of PANI,a sensitive electrochemical technique for determination of Cu2+has been illustrated.
Acknowledgements:The authors are grateful to the financial support from the National Science Foundation of China(No.20771049).
[1]Joo S H,Jun S,Ryoo R,et al.Mesopor.Mater.,2001,44-45(1):153-158
[2]Ryoo R,Joo S H,Kruk M,et al.Adv.Mater.,2001,13(9):677-681
[3]Jun S,Joo S H,Ryoo R,et al.J.Am.Chem.Soc.,2000,122(43):10712-10713
[4]Shin H J,Ryoo R,Kruk M,et al.Chem.Commun.,2001,4:349-350
[5]Ryoo R,Joo S H,Jun S,et al.Stud.Surf.Sci.Catal.,2001,135:150-157
[6]Yahya A I,Su R S,Kwang M S,et al.Sens.Actuators B,2008,129(2):834-840
[7]Rasa P,Christopher M A B,Andrew P,et al.Electrochimica Acta,2004,50(1):159-167
[8]Zoran M,Marijana K R,Tomislav P,et al.Electrochim.Acta,2009,54(10):2941-2950
[9]Xu G C,Wang W,Qu X F,et al.Eur.Polym.J.,2009,45(9):2701-2707
[10]Li X X.Electrochim.Acta,2009,54(24):5634-5639
[11]Wang F,Wang W B,Liu B H,et al.Talanta,2009,79(2):376-382
[12]Zeng X D,Liu X Y,Kong B,et al.Sens.Actuators B,2008,133(2):381-386
[13]Tan J,Yan X P.Talanta,,76(1):9-14
[14]Lok P S,Jitendra M B.Talanta,2004,64(2):13-319
[15]Senthilkumar S,Saraswathi R.Sens.Actuators B,2009,141(1):65-75
[16]Rihab N,Didier F,Florence G.Electrochem.Commun.,2010,12,(1):98-100
[17]Pandey P C.Sens.Actuators B,1999,54(3):210-214
[18]Xin Y L,Gao Y,Guo J,et al.Biosens.Bioelectron.,2008,24(3):369-375
[19]FAN Li-Fang(范丽芳),FAN Yue-Qin(樊月琴),MENG Shuang-Ming(孟双明),et al.J.Instrumental Anal.(Fenxi Ceshi Xuebao),2010,2:180-184
[20]Zen J M,Annamalai S K,Tsai D M,et al.Electroanalysis,2003,15:1073-1087
[21]Takashi K,Hokuto O,Mizuki K,et al.Bioelectrochemistry,2008,74(1):66-72
[22]Sanjeev M.R,Xing Y.J.Power Sources,2008,185(2):1094-1100
[23]Marcos F S,Teixeira E R D,Éder T G.C,et al.Sens.Actuators B,2005,106(2):619-625
[24]Behzad R,Zohreh S M.Sens.Actuators B,2008,134(1):292-299
[25]Elzbieta F,Franois B.Carbon,2001,39(6):937-950
[26]Elzbieta F,Franois B.Carbon,2002,40(10):1775-1787
[27]Jan M S,Jan U N.Energy Convers.Manage.,2008,49(9):2455-2460
[28]Elena N,Jaroslav S,Miroslava T,et al.Polymer,2006,47(16):5715-5723
[29]Qiao Y,Li C M,Bao S J,et al.J.Power Sources,2007,170(1):79-84
[30]Chetna D,Sunil K A,Monika D,et al.Anal.Biochem.,2008,383(2):194-199
[31]Wang C Y,Mottaghitalab V,Too C O,et al.J.Power Sources,2007,163(2):1105-1109
[32]Zhao D Y,Feng J L,Huo Q S,et al.Science,1998,279(5350):548-552
[33]MacDiarmid A G,Yang L S,Huang W S,et al.Synth.Metals,1987,18(1/2/3):393-398
[34]Alexsandro M Z,Roberto B,Marco T,et al.Electrochem.Commun.,2003(5):983-988
Preparation of Ordered Mesoporous Carbon/Polyaniline Electrodes and as Electrochemical Sensors for Cu2+
GUO Zhuo*GUO Tong ZHAO Chang-LiGAO Yun-Peng LI Sha
(Education Department of Liaoning Province,Key Laboratory of Applied Technology of Polymer Materials,Shenyang University of Chemical Technology,Shenyang 110142)
A new electrode,obtained by the dispersion of ordered mesoporous carbon (MC)nanoparticles onto polyaniline(PANI)was presented in this paper.Ordered mesoporous carbon was prepared by pyrolysis of sucrose filled in the mesoporous channels of SBA-15 at 1 200℃,and followed by dissolution of the silica matrix in hydrofluoric acid.The pore structure of the MC material was evaluated using XRD,nitrogen adsorption,TEM.SEM showed that PANI-MC films presented a wholy modified morphology when compared to PANI film.Electrochemical characterization indicated that the potential shift toward negative direction.This can be attributed to the porous feature of MC,which imposes resistance for ion migration in mesoporous.The deposition potential and time had been optimized for determination of Cu2+in aqueous solution.ASV analysis revealed that the new electrode presented a linear response to low concentrations of Cu2+.Under optimized conditions,the current showed a linear dependence with concentration in the range 1.00 ×10-8~1×10-6mol·L-1.The detection limit was 6×10-9mol·L-1.The results showed good reproducibility and stability.
mesoporous carbon;copper determination;electrodes
O657.14
A
1001-4861(2010)11-1927-07
2010-05-13。收修改稿日期:2010-06-26。
国家自然科学基金(No.20771049)资助项目。
*通讯联系人。 E-mail:guozhuochina@sina.com.cn
郭 卓,女,35岁,副教授;研究方向:多孔复合材料。

