Digestive enzyme and alkaline phosphatase activities during the early stages of Silurus soldatovi development
LIU Wei, ZHANG Xiu-Mei, WANG Li-Bo
(1. College of Fisheries, Ocean University of China, Qingdao 266003, China;2. Heilongjiang River Fishery Research Institute, Chinese Academy of Fishery Sciences, Harbin 150070, China;3. The College of Animal Science and Technology, Northeast Agriculture University, Harbin 150030, China)
Digestive enzyme and alkaline phosphatase activities during the early stages ofSilurus soldatovidevelopment
LIU Wei1,2, ZHANG Xiu-Mei1,*, WANG Li-Bo3
(1.College of Fisheries,Ocean University of China,Qingdao266003,China;
2.Heilongjiang River Fishery Research Institute,Chinese Academy of Fishery Sciences,Harbin150070,China;
3.The College of Animal Science and Technology,Northeast Agriculture University,Harbin150030,China)
To provide a theoretical basis to improve the survival and growth rate and optimize diet of sheatfish(Silurus soldatovi), the activities of certain digestive enzymes and alkaline phosphatases were investigated during larval development of one − ten day old individuals. Results indicated that sheatfish larva (~ three days after hatching) had high levels of alkaline protease activity, which peaked at five days old and dipped by eight days old, although the trend was generally upward. Acid protease activity at one − eight days old was low, after which it increased rapidly. Amylase activity reached the highest value at five days old, after which it began to decline. Lipase activity fluctuated markedly and showed two peaks at three − four days old and six − eight days old. Larval digestive enzyme activity and alkaline phosphatase activity were higher when fed live food than when fed an artificial diet. Throughout the early development process, alkaline protease activity was higher than acid protease, alkaline protease and amylase specific activity decreased significantly for eight-day-old transition larvae, while acid protease activity increased rapidly. These results indicate that the changes in digestive enzyme activity were relevant to digestive function conversion during fish larvae development.Alkaline phosphatase activity showed an upward trend over the first ten days of life, which indicated that the gastrointestinal function of sheatfish improved gradually.
Silurus soldatovi; Larva; Juvenile; Digestive enzymes; Alkaline phosphatase
The activity of digestive enzymes is an important indicator of digestive physiology in fish species. The level of digestive enzyme activity in fish determines the capacity of digestion and absorption of nutrients, which influences the speed of growth and development in fish.In recent years, digestive research on fish has focused on the occurrence and activity changes of digestive enzymes during larvae and juvenile stages (Chen et al, 2004).Studies on sea breamPagrosomus major(Chen et al,1998) and Amur sturgeonAcipenseride schrencki(Su &Zhao, 2005) found that types, time of occurrence, and activity levels of digestive enzymes in larvae and juveniles differ significantly. Additionally, the occurrence of digestive organs in fish is not synchronous in early development and gradually improves during later development (Pan et al, 2009). There are also differences and similarities in food composition for different fish species during individual development (Specker, 1988).Nutrient strategies and survival rates can be improved if the physiological mechanism of digestive enzymes is acquired during the fish larvae stage. Alkaline phosphatase is an important regulative enzyme in bio-metabolic processes (Ram & Satyyanesan, 1985),and plays a vital role in digestion, absorption, and transition of nutrients (Swarup et al, 1981). As the stability of enzyme activity can influence the body’s biological metabolism and adaptive capacity (Wang et al,2001), it is essential to quantify its activity during the early developmental stages of fish.
Sheatfish (Silurus soldatovi), which belong to the Siluridae family andSilurusgenus, are large-scale northern freshwater commercial fish, characterized as a fast-growing predator with rapid early development and difficult larval rearing (Pan et al, 2000). Previous research on this species has reported on the relationship between the digestive tract, post-embryonic development of the liver and pancreas, and changes in food ingestion(Guan et al, 2006; Wang et al, 2007). However, early developmental stages of digestion and adaptation evolution and many other issues still remain unclear, and studies on their larval and juvenile period of transformation and nutritional digestive physiology have not been reported yet. To a certain extent, this affects the nutrient supply of fry breeding and the stability of seedling quality. To understand the digestive mechanism and development characteristics of digestive function,and to resolve physiological nutrition problems between the mouth-opening and the nutritional transition period during early development of sheatfish, we investigated activity changes in digestive enzymes and alkaline phosphatase and their roles in live and artificial diet digestion during the larval period. Results from this study provide a theoretical basis for artificial diet preparation and seedling breeding of sheatfish.
1 Materials and Methods
1.1 Larvae rearing
Sheatfish larvae were reproduced artificially in Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences, in temperature controlled glass tanks (80 cm × 60 cm × 50 cm). Experimental groups were fed either live or artificial food in three tanks, which each contained ~900 larvae. Groundwater was used after full aeration and filtration, one third of the water was changed every three days, and the rearing temperature was controlled at 20 − 22°C. Feeding started at two days old. The live-food group was fed with small live daphnia (two − five days old) and water silk earthworms (six − ten days old) four times a day. The artificial group was fed with mouth-opening commercial Sturgeon feed containing 50% crude protein and 8%crude fat at a frequency of 8 − 10 times daily during the mouth-opening period, followed by 3 − 4 times daily after more than 76% of larvae began to feed. Samples of 1 − 10 day-old larva were collected and stored in a 1.5 mL centrifuge tube in a −80°C refrigerator. Three parallel samples were set in each group.
1.2 Enzyme analysis
1.2.1 Preparation of crude enzyme solution
Whole larva were added to 10 times body weight pre-cooling double-distilled water, were homogenized using a FSH-II high-speed tissue homogenizer, and then centrifuged at 3,500 r/min for 15 min. The supernatant was then obtained and stored in 4°C.
1.2.2 Measurement of enzyme activity
Acid protease:A total of 3.5 mL of reaction liquid,including 2 mL of 0.5% casein solution, 0.1 mL of 0.04 mol/L EDTA-Na2, 0.4 mL of Na2HPO4-citric acid buffer solution (pH2.6), 0.4 mL of enzyme solution, and 0.6 mL of redistilled water was placed into a test tube and incubated in a 37°C water bath for 15 min before the addition of 1 mL 30% trichloroacetic acid to terminate the reaction. The solution was then centrifuged at 4,000 r/min for 15 − 20 min, and approximately 1 mL of supernatant in 5 mL 0.55 mol/L sodium carbonate solution and 1 mL forint reagent was added before incubation in a 37°C water bath for 15 min. Enzyme activity was measured at 680 nm wavelength using 751 spectrophotometer, before termination by adding trichloroacetic acid.
Alkaline protease:The method used was the same as for acid protease, except for the usage of reaction buffer pH10.3 of borax-NaOH buffer solution according to Pan et al (1997).
Total protein determination:Enzyme protein content was determined by biuret assay and bovine serum albumin (Wang et al, 2007).
Amylase:Soluble starch as substrate 3,5-dinitrosalicylic acid color method was used. One unit of enzyme activity was defined as the amount of enzyme able to catalyze starch generating to 1 μg one-water-maltose per min at 25°C.
Lipase:Measurement was conducted with a kit supplied by Nanjing Jiancheng Bio-engineering Institute.One unit of enzyme activity was defined as the amount of enzyme able to generate 1 µg of molecular fatty acids per minute at 37°C.
Alkaline phosphatase:Measurement was conducted with a kit supplied by Nanjing Jiancheng Bio-engineering Institute. One unit of enzyme activity was defined as the amount of enzyme able to catalyze one gram protein generating 1 mg phenol in 15 min at 37°C.
1.3 Data analysis
Analysis of variance (ANOVA) was used to test significant differences of various sample groups (P=0.05).
2 Results
The weight of larvae fed with different food showed a significant difference (P<0.05) (Tab. 1). The weight of larvae fed a live diet showed an increase three times that of artificial food. Moreover, the survival rate for the live diet group was over 85%, while for the artificial diet group was about 62%.
After feeding, acid protease activity decreased to the lowest level at four days old, increased at five days old, then decreased gradually and increased rapidly at eight days old (Fig.1). The activity of alkaline protease was higher than that of acid protease throughout the early development stage. Activity increased to (24.80 ± 4.9)U·(mg protein)−1at five days old, then declined to (11.82± 0.43) U·(mg protein)−1at eight days old, followed by another upward trend. The activity of alkaline protease in the live diet group was higher than the artificial dietgroup (Fig.1).
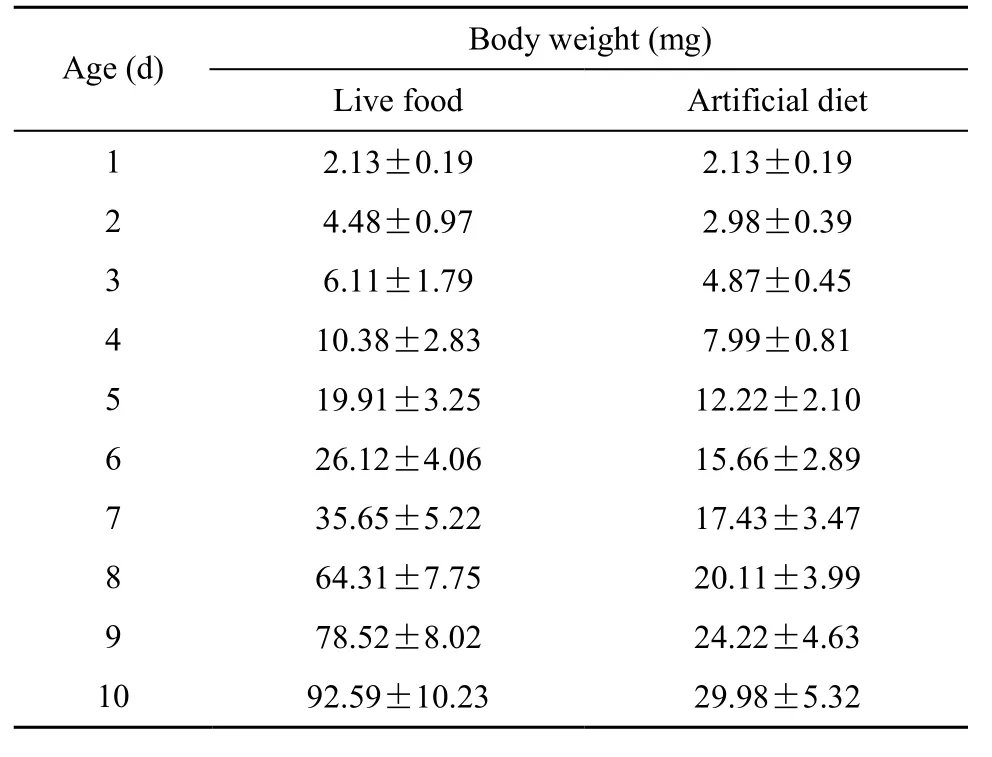
Tab. 1 Average live weights of fish larvae from different sampling points
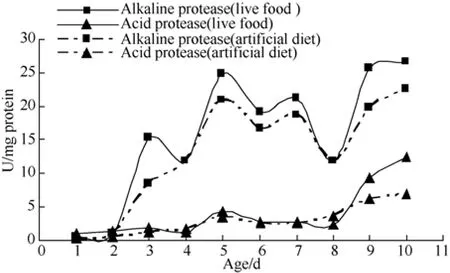
Fig. 1 Protease activities during larval development of sheatfish
Amylase activity was relatively high during the opening mouth stage. Activity reached the highest level(40.76 ± 3.43) U· (mg protein)−1at five days old, then declined to the lowest level (5.51 ± 1.51) U·(mg protein)−1by eight days old. Amylase activity showed a generally downward trend at the beginning and end stages with three upward trends in the middle. There was no obvious difference in amylase activity between the live-food group and the artificial diet group (Fig.2).

Fig. 2 Amylase activity during larval development of sheatfish
Lipase activity was bimodal during the early development of sheatfish. Lipase activity of the live-food group changed stably. The first activity peak appeared around four days old (58.57 ± 6.22) U·(g protein)−1,while the second peak appeared at around six days old(52.39 ± 2.18) U·(g protein)−1. Lipase activity of the artificial diet group fluctuated remarkably, however, with activity lower at one to two days old and four to eight days old (Fig.3).
Alkaline phosphatase activity initially increased slowly, with the highest value at eight days old (3.91 ±0.93) U·(g protein)−1before it began to decline at nine days old. Alkaline protease activity in the live diet group was significantly higher than in artificial diet group(Fig.4).

Fig. 3 Lipase activity during larval development of sheatfish

Fig. 4 Alkaline phosphatase activity during larval development of sheatfish
3 Discussion
In natural waters, the time and activity levels of digestive enzymes can be regarded as physiological indicators of individual growth and development during fish larvae-juvenile period. The type and activity of digestive enzymes also reflects the physiological function of the optimum diet selected during early larvae development. A mutual adaptation relationship also exists between the production and activity of the enzyme changes and diet selection (Wguolev & Kuzmina, 1993).All fish species live on zooplankton during the mouth opening stage of life (Oozeki & Bailey, 1995; Ribeiro &Sarasquete, 1999), a phenomenon that could help confirm the molecular biological mechanisms of digestive enzyme reproduction in fish species(Cuvier-Peres & Kestemont, 2002) and increase understanding of digestive enzyme activity mechanisms.We found that sheatfish larvae had a variety of digestive enzymes and a high level of activity before ingestion.This indicated that larvae opened mouth at around three days old and had the ability to digest alien food when it shifted from endogenous to exogenous nutrients(Munilla-Moran & Barbour, 1990). There are two main reasons why digestive enzyme activity changed in sheatfish larvae. Firstly, digestive enzyme activity was affected by the digestive organs at different developmental stages and functions. Secondly, digestive enzyme activity depended on the early nutrients or bait species. A crucial period for quantitative changes in digestive enzyme activity occurred at the nutrition transition from inside to outside, as well as the larvae to juvenile transformation period. In this study, acid protease activity appeared low for a few days after mouth opening, which may be related to the nutrition metabolic mechanism after yolk sac absorption, and to the developmental process and function of the stomach(Guan et al, 2006). Alkaline protease activity declined during the nutrition transition period, but its level was higher than that of acid protease during the whole larval period. The occurrence and activity of digestive enzyme production was related to body structure, function, and functional transition. In this study, endogenous nutrients of larvae, as well as acid and alkaline protease activity,were at their lowest level at four days old, which may be one of the reasons of larvae death. A low value appeared again at eight days old, which may indicate the digestive system of larvae was not fully developed and was in a nutrition transformation period from mixed to exogenous based on the histology and growth performance of larvae.Digestive enzyme activity declines significantly in a nutrition transition period in many fish species (Bong,2001), which indicates that activity changes of digestive enzymes are closely related to organ development and function.
While newly hatched larvae have higher alkaline protease activity irrespective of diet, the live-food group had higher activity, suggesting that digestive enzyme activity may be related to food type. Studies have found that the presence of live bait can increase trypsin and alkaline phosphatase activity inDicentrarchus labraxjuveniles (Cahu & Zambonino-Infante, 1998). Some research has indicated that 40% − 80% of enzyme activity comes from live bait (Kolkovski, 2001), while other research has suggested that larvae have sufficient digestive enzymesin vivoto digest exogenous nutrients(Zambonino-Infante and Cahu, 2001). According to these two points of view, the activity value of digestive enzymes from the live food group was related to endogenous activation of fish and exogenous diet such as water flea and limnodrilus. It is possible, therefore, that both these sources of protease existed in this study.
Our results showed that amylase activity remained high in sheatfish larvae, with a peak at five days old.Previous research has indicated that ingestion of flea-borne nutrients stimulates the digestive tract and leads to higher activity, while changing feeding patterns can lead to significantly lower levels (Cousin &Gabaudan, 1987). A significant reduction in amylase activity appeared at eight days old, however, which may be related to the carnivorous feeding habits of sheatfish.Many carnivorous fish larvae demonstrate the same changing pattern of amylase (Ribeiro & Sarasquete 1999;Cahu & Zambonino Infante, 1994), showing that high amylase activity during larvae developmental stages appears universal.
In this study, lipase activity of larvae reached the highest value at four days old, which may be related to the absorption of the yolk sac (Guan et al, 2006; Wu et al,2007). The increase in lipase activity at eight days old may be related to fat content of feed intake. Studies onTheragra chalcogrammashow similar changes in lipase activity to our study. Oozeki & Bailey (1995) considered that the two observed peaks were probably caused by two kinds of enzymes.
Alkaline phosphatase distributed in the shallow and striated border in fish intestinal epithelial cells, is a kind of metal enzyme that has a positive correlation with lipid,glucose, calcium, and inorganic phosphorus absorption(Tengjaroenkul & Smith, 2000; Feng et al, 2003).Sheatfish larvae showed alkaline phosphatase activity in an upward trend, indicating that mature intestinal cells and intestinal digestive functions improved in sheatfish larvae.
Understanding the activity of various digestive enzymes and alkaline phosphatase during the developmental stage of sheatfish larvae can help clarify the biological basis of natural bait in natural waters and improve diet preparation during artificial reproduction and breeding. This can, in turn, enhance digestive enzymes activity, digestion, and nutrient utilization,thereby contributing to fish larvae growth and the survival rate of seedlings. According to our data, the enzyme activity changes in the live-feed group showed a gradual upward trend, while the activity of the artificial diet group fluctuated greatly. Additionally, numerous enzyme activities were lower in the artificial group than the live diet group. Our results suggest that live bait better promotes the growth and development of sheatfish larvae than artificial food.
Bong GK, Divakaran S, Brown CK, Os-Trowski AC, Divakaran S.2001.Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin,Polydactylus sexfilisand bluefin trevally,Caranx melampygus[J].Fish Physiol Bioch, 24:225-241.
Cahu CL, Zambonino Infante JL. 1994. Early weaning of sea bassDicentrarchus labraxlarvae with a compound diet: Effect on digestive enzymes [J].Comp Bioch Physiol, 109A: 213-222.
Cahu CL, Zambonino Infante JL. 1998. Algal addition in sea bass(Dicentrarchus labrax) larvae rearing: Effect on digestive enzymes [J].Aquaculture, 161(14): 479-489.
Chen MY, Zhang XM. 2004. Recent advances in digestive physiology of marine fish larvae-juvenile [J].Mar Fish Res,25(3): 81-88. (in Chinese)
Chen PJ, Wang ZG, Huang CN, Gu Y, Lu H. 1997. Variation of digestive enzyme activity in different development periods ofPagrosomus major[J].J Ocean Taiwan Strait,16(3): 245-248.
Cousin JCB, Baudin-Laurencin F, Gabaudan J. 1987. Ontogeny of enzymatic activities in fed and fasting turbotScophthalmus maximusL [J].Fish Biol,30: 15-33.
Cuvier-Peres, Kestemont. 2002. Development of some digestive enzymes in Eurasian perch larvaePerca fluviatilis[J].Fish Physiol Bioch, 24: 279-285.
Feng XY, Zheng JS, Wang ML. 2003. A study of the histochemistry on the digestive tract of theSebastes schlegeli[J].J Ocean Univ Qingdao, 33(3): 399-404. (in Chinese)
Guan HH, Pan WZ, Chen J, Zhao CG, Liu W. 2006. Post-embryonic development of liver and pancreas and absorption of yolk inSilurus soldatovi,Silurus asotusand F1 [J]J Fish Sci Chn, 13(3):460-464. (in Chinese)
Kolkovski S. 2001. Digestive enzymes in fish larvae and juvenile-implications and applications to formulated diets [J].Aquaculture, 200: 181-201
Munilla-Moran R, Stark JR, Barbour A. 1990. The role of exogenous enzymes in digestion incultured turbotScophthalmus maximuslarvae [J].Aquaculture, 88: 337-350.
Oozeki Y, Bailey KM. 1995. Ontogenetic development of digestive enzyme activities in larval walleye pollockTheragra chalcogramma[J].Mar Biol, 122:177-186.
Pan LQ, Wang KX. 1997. The experimental studies on activities of digestive enzyme in the larvaePenaeus chinensis[J].J Fish Chn,21(1): 26-31. (in Chinese)
Pan WZ,Yin HB, Liu W, Lu TY, Ren B, Zhao CG. 2000. Northern sheatfish × catfish distant cross-breeding technology research [J].Chn J Fish,13(2): 74-79. ( in Chinese)
Pan XF, Yang JX, Li ZY, Chen XY. 2009. Feeding changes and growth performance ofSinocyclocheilus grahami(Pisces,Barbinae) larvae and juveniles in farm environment [J].Zool Res,30(4): 433-437. (in Chinese)
Ram RN, Satyyanesan AG. 1985. Mercurie chloride, cythion and ammonium sulfate induced changes in the brain, liver, and ovarian alkaline phosphatase content in the fish [J].Channo Puntactus Emir Ecol,(3): 263-268.
Ribeiro L, Sarasquete C, Dinis MT. 1999. Histological and histochemical development of the digestive system ofSolea senegalensislarvae [J].Aquaculture, 191: 293-308.
Specker JL. 1988. Preadaptive role of thyrold hormones in larval and juvenile salmon: Growth the gut and evolutionary considerations[J].Am Zool,28(2): 337-349.
Su SP, Zhao XW. 2005. The influence of different diets on the digestive enzyme activities of amur sturgeonAcipenseride schrenckiBrandt larvae [J].J Biol, 22(1): 27-29. (in Chinese)
Swarup G. 1981. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatasess [J].Biol Chem, 256:8197-8201.
Tengjaroenkul B, Smith BJ, Caceci T, Smith SA. 2000. Distribution of intestinal enzyme activities along the intetinal tract of cultured Nile tilapia,Oreochromis niloticusL[J].Aquaculture, 182:317-327.
Wang HT, Xu YL, Zhang PJ. 2001. Specific activities of alkaline phosphatase in digestive organ of pseudomale flounder(Paralichthys olivaceusT.ETS) [J].Bull Mar Sci, 43(6): 157-160.(in Chinese)
Wang LB, Liu W, Chen J, Zhi BJ. 2007. Changes in digestive enzyme activities of northern sheatfish after feeding [J].Chn J Fish, 20(1):36-41. (in Chinese)
Wu XF, Zhan JB, Qing YZ, Wu C. 2007. Histological study of the digestive system organogenesis for the mandarin fish,Siniperca chuatsi[J].Zool Res, 28(5): 511-518. (in Chinese)
Zambonino-Infante JL, Cahu CL. 2001.Ontogeny of the gastrointestinal tract of marinefish larvae [J].Comp Bioch Physiol Part C, 130:477-487.
怀头鲇早期发育阶段消化酶及碱性磷酸酶活性变化
刘 伟1,2, 张秀梅1,*, 王立波3
(1. 中国海洋大学 水产学院, 山东 青岛 266003; 2. 中国水产科学研究院黑龙江水产研究所, 黑龙江 哈尔滨 150070;3. 东北农业大学 动物科学技术学院水产养殖系, 黑龙江 哈尔滨 150030)
采用动物性饵料和人工饲料培育1~10日龄怀头鲇(Silurus soldatovi)仔稚鱼,分析测定了全鱼酸性、碱性蛋白酶、淀粉酶、脂肪酶以及碱性磷酸酶的活性。结果表明:孵化后3天开口期仔鱼已具有较高的碱性蛋白酶活性, 5日龄时碱性蛋白酶比活力达到较高值, 8日龄时出现低值, 总体变化呈波动上升趋势; 酸性蛋白酶活性在1~8日龄处于较低水平, 8日龄后开始迅速升高; 淀粉酶活性在5日龄左右达到最高值, 随后酶活性开始下降至较低水平; 脂肪酶活性变化波动较大, 表现为双峰型, 两个峰值分别出现在3~4日龄和6~8日龄。摄食动物性饵料仔稚鱼消化酶活性和碱性磷酸酶活性均高于摄食人工饲料。在整个早期发育过程中, 碱性蛋白酶比酸性蛋白酶活性高, 碱性蛋白酶、淀粉酶比活力在约8日龄仔稚鱼转变期明显下降, 而酸性蛋白酶活性开始迅速升高, 这说明消化酶活性的变化与仔稚鱼发育过程中消化机能转换具有相关性。怀头鲇在 10日龄内碱性磷酸酶活性呈上升趋势,表明怀头鲇胃肠道功能的逐步发育完善。
怀头鲇; 仔稚鱼; 消化酶; 碱性磷酸酶
Q175; Q133
A
0254-5853(2010)06-0627-06
2010-02-24;接受日期:2010-10-08
刘 伟, 女, 从事渔业资源与鱼类生态生理研究。E-mail:liuwei_1020@yahoo.com.cn
date: 2010-02-24;Accepted date: 2010-10-08
Agricultural Science and Technology Fund of Department of Science and Technology (02EFN216900727)*
(通讯作者), E-mail: gaozhang@ouc.edu.cn
The authors wish to thank Prof.WAN Zhen-zhen of the College of Fisheries, Ocean University of China, and CHEN Jun from the research group, for their assistance during the experiments.
- Zoological Research的其它文章
- Shape change in viable eggs of the collembolan Folsomia candida provides insight into the role of Wolbachia endosymbionts
- Isolation and characterization of Hainantoxin-II, a new neurotoxic peptide from the Chinese bird spider (Haplopelma hainanum)
- Morphological changes of silver and bighead carp in the Yangtze River over the past 50 years
- Positive influence of traditional culture and socioeconomic activity on conservation: A case study from the black-and-white snub-nosed monkey (Rhinopithecus bieti) in Tibet
- Bats and marsupials as indicators of endemism in the Yungas forest of Argentina
- A new record of Dasyatid fish in China: Dasyatis laosensis

