Simultaneous Removal of Thiophene and Dibenzothiophene by Immobilized Pseudomonas delafieldii R-8 cells*
TANG Huang (唐煌), LI Qiang (李强), WANG Zelong (王泽龙), YAN Daojiang (闫道江)and XING Jianmin (邢建民),**
1National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
2Graduate University of Chinese Academy of Sciences, Beijing 100049, China
Simultaneous Removal of Thiophene and Dibenzothiophene by Immobilized Pseudomonas delafieldii R-8 cells*
TANG Huang (唐煌)1,2, LI Qiang (李强)1, WANG Zelong (王泽龙)1,2, YAN Daojiang (闫道江)1,2and XING Jianmin (邢建民)1,**
1National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
2Graduate University of Chinese Academy of Sciences, Beijing 100049, China
Biodesulfurization (BDS) is a promising technology for deep desulfurization. In this work, Pseudomonas delafieldii R-8 cells are immobilized in calcium alginate beads and used for BDS of transportation fuels. It is found that thiophene and dibenzothiophene (DBT) can be simultaneously metabolized by immobilized R-8 cells. The initial sulfur content in the model oil is 300 mg·kg−1(thiophene∶DBT=1∶1). After 10 h of treatment, the thiophene concentration is reduced by 40%, while DBT is reduced by 25%. The utilization rate of thiophene is faster than that of DBT. Moreover, the oil/water ratio of alginate immobilized cells is studied to reduce the water volume in desulfurization systems. Long-term recycling of BDS by alginate immobilized cells is carried out with oil/water ratio at 5∶1. The immobilized cells are successfully reused over 15 batch cycles. In the last batch, the desulfurization activity remains at least 75% of the first batch.
biodesulfurization, simultaneous removal, alginate immobilization, long-term recycling
Chinese Journal of Chemical Engineering,20(1) 47—51 (2012)
1 INTRODUCTION
Ultra-deep desulfurization of transportation fuels has become very important in petroleum refinery industry, due to the stricter environment regulations and steady increases in the average sulfur content of crude oil [1]. Presently, biodesulfurization (BDS) has attracted intensive interest due to its benefits of cost-effectiveness, mild reaction conditions, high selectivity, and small impact on the environment [2-5].
Both thiophene and dibenzothiophene (DBT) are present in most of oil products and usually considered as the model compound in research for desulfurization of fuels. There are numerous reports on the treatment of transportation fuels by using suspensions of growing or resting cells [6-9]. The treatment of oils using free cells has encountered some limitations such as high cost of the biocatalyst and low volumetric ratio between the organic phase and the aqueous one. Also, separation of oil product from oil-water-biocatalyst emulsion is troublesome and the free cells are difficult for recycling [10, 11]. In view of industrial application of BDS, cell immobilization is considered to be one of the most promising approaches [12, 13].
So far, few papers are available on BDS by immobilized cells and the available recycling times are less than 10 times [14, 15]. Calcium alginate is currently one of the most widely used entrapment carriers for immobilization of enzymes and entire cells for its advantages of biocompatibility, cheapness and simplicity [16, 17]. Our group has done some research on immobilized biocatalysts to minimize the diffusion limitations and promote the kinetics of biodesulfurization by alginate immobilized cells, which was conducted by entrapment in calcium alginate [14, 18-20]. In the present study, thiophene and DBT are simultaneously removed by immobilized R-8 cells. A simple calcium alginate cell-immobilization technology is developed, aiming to increase the production rate of immobilized biocatalysts. A triporate injector is designed and produced for cells immobilization. The oil/water ratio is optimized. Moreover, desulfurization of high sulfur concentration oils and long-term recycling of immobilized cells are also studied.
2 EXPERIMENTAL
2.1 Chemical materials
DBT (98%) was purchased from Alfa Aesar Company (Tianjin, China). 2-Hydroxybiphenyl (2-HBP) was purchased from Shanghai Tixiai Huawei Co. Thiophene was purchased from Beijing Xingjin Chemical Plant. Sodium alginate was purchased from Shantou (Guangdong) Xilong Chemical Plant. Methanol and n-octane of chromatography grade were bought from Tianjin Siyou Reagent Company. Other materials were analytical grade and available commercially.
2.2 Bacterial strain and cultivation
P. delafieldii R-8 (CGMCC No. 0570) was isolated from the sewage pool of Shengli Oil Field of China, and was capable of desulfurizing DBT to 2-HBP [14]. The cells were incubated in 500 ml flasks containing 120 ml basal salt medium (BSM) supplemented with 0.2 mmol·L−1DBT as the source of sulfur.Cell cultivation was carried out in a rotary shaker at 30 °C and 180 r·min−1. The inoculum concentration was 2% (by volume). The BSM was composed of KH2PO42.44 g, Na2HPO4·12H2O 12.03 g, NH4Cl 2.0 g, MgCl2·6H2O 0.4 g, CaCl20.75 mg, FeCl3·6H2O 1 mg, MnCl2·4H2O 4 mg and glycerol 10 g in one liter of deionized water.
2.3 Cell immobilization in calcium alginate beads
Cells were harvested in the late exponential phase by centrifugation for 5 min. The harvested cell pellets were washed twice with deionized water and re-suspended in it. The concentration of cells solution is about 20 g dry cells mass per unit volume (DCW·L−1). Sodium alginate was dissolved in deionized water (4%, by mass) and mixed well with equal volume of cell suspension.
Resting cells were immobilized with conventional methods by extruding the alginate as drops into a calcium salt solution for gelation. The resultant slurry was extruded into a stirred 0.1 mol·L−1CaCl2gelling solution by a self-made triporate injector. The slurry was intruded as discrete droplets so as to form calcium-alginate beads with standard size (4 mm in diameter). The beads were left for aging in calcium chloride solution about 2 h. Then, the beads were washed with saline [8% (by mass) NaCl] to remove the residual calcium ions and then kept in saline at about 4 °C overnight. Activation was carried out at 30 °C on a 180 r·min−1rotary shaker. To avoid the dissolution of alginate immobilized cells in the BSM, MBSM was used as the aqueous phase for the activation of the immobilized cells. The phosphate components of MBSM were changed to KH2PO40.24 g and Na2HPO4·12H2O 1.20 g. Other components were the same as those in BSM.
One batch of cells was divided into two same parts. The volume of as-produced free resting cells in saline solution was 100 ml, and the immobilized beads were about 38 g. Consider that a small amount of cells was lost during the immobilization process, it is estimated that the cell numbers are equal either in 20 ml saline containing resting cells or 8 g alginate immobilized cells.
2.4 Biodesulfurization in model oil by free resting R-8 cells
The model oil was n-octane containing 10 mmol·L−1sulfur compounds. The reaction mixture contained 10 ml model oil and 20 ml saline containing resting cells at the concentration is 20 g DCW·L−1. Biodesulfurization was carried out in 100 ml flasks at 30 °C and 180 r·min−1in a rotary shaker. The time course of sulfur compounds utilization was obtained by analyzing sulfur concentrate with HPLC at set time intervals. These experiments were performed at least three times and representative data are presented.
2.5 Biodesulfurization in model oil by immobilized R-8 cells
The model oil consisted of 3 mmol·L−1sulfur compounds (thiophene∶DBT=1∶1) in n-octane. The reaction mixture contained 8 g alginate immobilized cells, 10 ml model oil and 2 ml saline [8% (by mass) NaCl]. Biodesulfurization was carried out in 100 ml flasks at 30 °C and 180 r·min−1in a rotary shaker. The time course of sulfur compounds utilization was obtained by analyzing sulfur concentrate with HPLC sampling at set time intervals. These experiments were performed at least three times and representative data are presented.
2.6 Recycling BDS by alginate immobilized cells
Alginate immobilized cells were reused for sulfur removal in the biphasic systems. After a run of DBS of several days, the activation of immobilized cells was carried out in BSM aqueous solution for 2 h to refresh the cells. The model oil is n-octcane with 2 mmol·L−1sulfur compounds (thiophene and DBT). Each cycle of desulfurization lasted for 24 h. By comparison, the same amount of resting cells could only be used once, and the recycled ones retained only 50% density and 15% activity.
2.7 Analytical methods
Cell density was calculated from the optical density at 600 nm, referenced to a calibration curve constructed with scalar dilutions of a cell suspension of known density. To determine the average size of the alginate beads, 10 individual wet beads were measured with vernier calipers.
High-performance liquid chromatography (HPLC) was used for the quantitative assay of DBT and thiophene in the n-octane phase. HPLC was performed on an Agilent 1100 (HP1100, Agilent, USA) liquid chromatography equipped with a diode array detector. The mobile phase was composed of methanol-water (volume ratio of 90∶10) with a flow rate of 1.0 ml·min−1. For the quantification of thiophene and DBT, the external standard method was used at 235 nm.
3 RESULTS AND DISCUSSION
3.1 Biodesulfurization in model oil by free resting cells
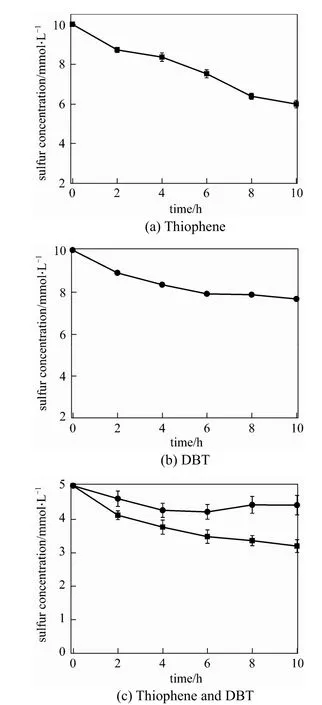
Figure 1 Biodesulfurization of 10 mmol·L−1sulfur compounds by resting R-8 cells■ thiophene; ● DBT
Three model oils with the same total 10 mmol·L−1sulfur compounds in n-octane are prepared. As shown in Fig. 1, the concentrations of thiophene and DBT decrease respectively to 6.0 mmol·L−1and 7.6 mmol·L−1after 10 h of BDS. R-8 cells are more efficient in removing thiophene from model oil than for removal of DBT. R-8 cells can simultaneously remove thiophene and DBT when they coexist in model oil, and the residual sulfur concentration is 7.5 mmol·L−1. Therefore, it can be concluded that resting R-8 cells utilize preferentially thiophene when DBT is present, and the existing DBT has a negative effect on the sulfur removal rate by R-8 cells. In our previous work [18], it is found that the product 2-HBP from the degradation of DBT can inhibit the desulfurization activity of cells. The speed of sulfur utilization by R-8 cells is as follows:
thiophene > thiophene and DBT ≈ DBT.
3.2 Biodesulfurization in model oil by immobilized R-8 cells
The influence of immobilization on desulfurization activity is investigated by comparing the removal of sulfur compounds with immobilized cells and free cells. The model oil contains 3 mmol·L−1sulfur compounds in n-octane (thiophene∶DBT=1∶1). The reaction solution is composed of alginate immobilized cells or free cells, 10 ml model oil and 20 ml saline. Biodesulfurization was carried out in 100 ml flasks at 30 °C and 180 r·min−1.
The BDS activities of immobilized cells and free cells are compared in Fig. 2. It can be concluded that immobilization decreases about 17% of the sulfur removal activity. However, immobilization provides some advantages such as improving the cells’ stability, increasing batch or continuous use, easy separating from the reaction mixture and possible modulation of catalytic environment by changing immobilizing reagent. The ratio of oil to water is 1∶2 in this experiment, which is optimized by the previous results for free resting cells [18]. Actually, the ratio can be increased for immobilized cells, which will greatly enhance the BDS capacity of R-8 cells. By comparison, the same amount of free resting cells could only be used once, and the recycled ones retained only 50% density and 15% activity. Therefore, cell immobilization is considered to be a promising approach to meet the requirement of industrialization.
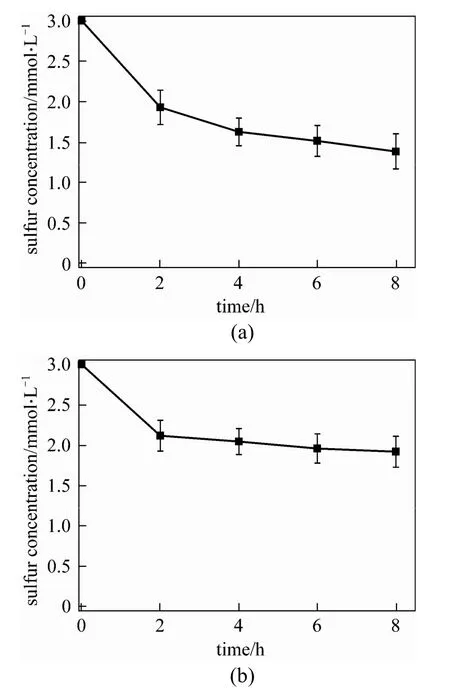
Figure 2 Biodesulfurization of DBT by (a) resting and (b) immobilized R-8 cells■ thiophene and DBT
3.3 Effect of oil/water ratio on immobilized cells’BDS activity
Under different oil/water ratio, R-8 cells exhibitdifferent BDS ability. In biocatalytical process, water is not only important but also necessary for biocatalysts. The reduce the water volume in the bioreactor allows more oil to be processed by immobilized cells. Therefore, the proper oil/water ratio should be investigated. The reaction mixture is composed of 8 g alginate immobilized cells, 10 ml model oil and saline of different volume. The experimental results are showed in Fig. 3.
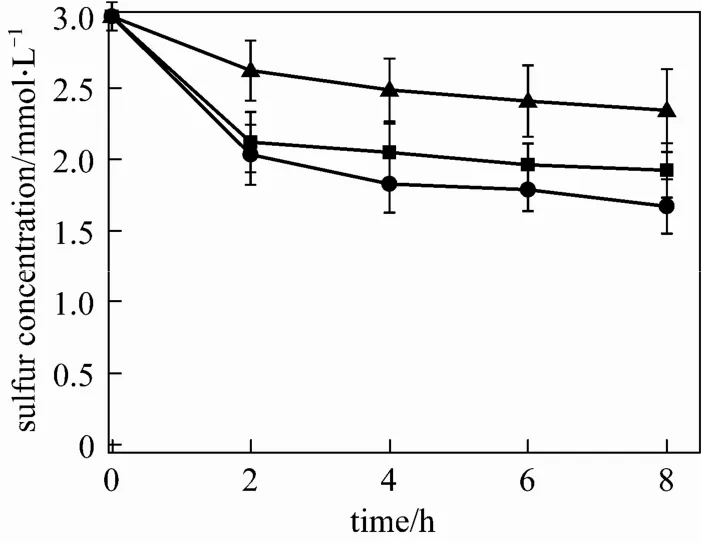
Figure 3 Effect of oil/water ratio on desulfurization activity of alginate immobilized R-8 cellsoil/water ratio: ■ 1∶2; ● 5∶1; ▲ 10∶0
Free cells cannot be separated from water easily after BDS. However, immobilized R-8 cells at the ratio of 10∶0 still have desulfurization activity, as shown in Fig. 3. This is because there is a certain amount of water in the calcium alginate beads. Due to the poor dispersion and adhesion of calcium alginate particles, the desulfurization activity is low in 100% oil phase. The addition of water can improve the dispersion of immobilized cells in the oil and increase the mass transfer rate. However, the addition of excessive aqueous medium should be avoided in order to improve the efficiency of bioreactor.
3.4 Recycling BDS by alginate immobilized cells
Alginate immobilized P. delafieldii R-8 cells are used for repeated BDS in the biphasic systems. The reaction mixture contains 8 g alginate immobilized cells, 10 ml model oil and 2 ml saline. If the desulfurization activity of cells decreased to 90% or less after one batch, an activation process was introduced. The cells are reactivated for about 4 h in MBSM medium to recover their activity. The initial concentration of sulfur compounds in model oil is 2 mmol·L−1(thiophene∶DBT=1∶1). Each cycle of desulfurization is carried out for 24 h. After reaction, the beads are separated from the model oil by a stainless steel sieve and washed by saline as regeneration. Normally, the activation process is carried out at the intervals of 4 days of BDS in MBSM solutions, after which the desulfurization ability of alginate immobilized cells is recovered.
As shown in Fig. 4, the desulfurization time is about 360 h, accounts for 80% of the total time. The results show that immobilized cells were successfully reused for BDS over fifteen batch cycles, retaining at least 75% initial desulfurization activity. We can improve the desulfurization ability of immobilized cells by reducing the time of each desulfurization cycle and disperse the activation time. Therefore, the life of the immobilized beads can be extended greatly and the time of reused batch can be increased.

Figure 4 Recycling removal sulfur using alginate immobilized R-8 cells. The intervals of BDS are the activation processes which are carried out in BSM solution■ sulfur compounds
4 CONCLUSIONS
Thiophene and dibenzothiophene (DBT) are simultaneously removed by Pseudomonas delafieldii R-8 cells. Alginate immobilization is used to improve biodesulfurization activity. Immobilized cells can be applied for long time recycles for BDS. The oil/water ratio is increased from 1∶2 to 5∶1, which greatly improve the efficiency of bioreactor. It is demonstrated successfully that immobilized cells are reused for BDS over fifteen batch cycles, retaining at least 75% specific desulfurization activity. Total desulfurization time of the immobilized beads reaches 450 h. The encouraging results of this work indicate that immobilization R-8 cells meet the demands of industrial biocatalyst. Industrialize biodesulfurization using immobilized R-8 cells have a good application prospect, which may be generally applied for other microorganism in BDS research. Further development and optimization of the BDS process to reduce the economic costs is one of the key steps to realize the scale-up industrial desulfurization.
REFERENCES
1 Song, C., Ma, X.L., “New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization”, Appl. Catal. B Environ.,41, 207-238 (2003).
2 Nandi, S., “Biodesulfurization of hydro-desulfurized diesel in airlift reactor”, J. Sci. Ind. Res.,69, 543-547 (2010).
3 Gupta, N., Roychoudhury, P.K., Deb, J.K., “Biotechnology of desulfurization of diesel: prospects and challenges”, Appl. Microbiol. Biotechnol.,66, 356-366 (2005).
4 Kilbane, J.J., “Microbial biocatalyst developments to upgrade fossilfuels”, Curr. Opin. Biotechnol.,17, 305-314 (2006).
5 Bhatia, S., Sharma, D.K., “Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3”, Biochem. Eng. J.,50, 104-109 (2010).
6 Li, F.L., Zhang, Z.Z., Feng, J.H., Cai, X.F., Xu, P., “Biodesulfurization of DBT in tetradecane and crude oil by a facultative thermophilic bacterium Mycobacterium goodii X7B”, J. Biotechnol.,127, 222-228 (2007).
7 Maghsoudi, S., Vossoughi, M., Kheirolomoom, A., Tanaka, E., Katoh, S., “Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp strain P32C1”, Biochem. Eng. J.,8, 151-156 (2001).
8 Lee, I.S., Bae, H.S., Ryu, H.W., Cho, K.S., Chang, Y.K., “Biocatalytic desulfurization of diesel oil in an air-lift reactor with immobilized Gordonia nitida CYKS1 cells”, Biotechnol. Prog.,21,781-785 (2005).
9 Yu, B., Xu, P., Shi, Q., Ma, C.Q., “Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain”, Appl. Environ. Microbiol.,72, 54-58 (2006).
10 Choi, O.K., Cho, K.S., Ryu, H.W., Chang, Y.K., “Enhancement of phase separation by the addition of de-emulsifiers to three-phase (diesel oil/biocatalyst/aqueous phase) emulsion in diesel biodesulfurization”, Biotechnol. Lett.,25, 73-77 (2003).
11 Konishi, M., Kishimoto, M., Tamesui, N., Omasa, I., Shioya, S., Ohtake, H., “The separation of oil from an oil-water-bacteria mixture using a hydrophobic tubular membrane”, Biochem. Eng. J.,24, 49-54 (2005).
12 Mukhopadhyaya, M., Chowdhury, R., Bhattacharya, P., “Trickle bed biodesulfurizer of diesel with backwash and recycle”, AIChE J.,53, 2188-2197 (2007).
13 Chang, J.H., Chang, Y.K., Ryu, H.W., Chang, H.N., “Desulfurization of light gas oil in immobilized-cell systems of Gordona sp. CYKS1 and Nocardia sp. CYKS2”, FEMS Microbiol. Lett.,182, 309-312 (2000).
14 Shan, G.B., Zhang, H.Y., Cai, W.Q., Xing, J.M., Liu, H.Z., “Improvement of biodesulfurization rate by assembling nanosorbents on the surfaces of microbial cells”, Biophys. J.,89, 58-60 (2005).
15 Hou, Y.F., Kong, Y., Yang, J.R., Zhang, J.H., Shi, D.Q., Xin, W.,“Biodesulfurization of dibenzothiophene by immobilized cells of Pseudomonas stutzeri UP-1”, Fuel,84, 1975-1979 (2005).
16 Houng, J.Y., Chiang, W.P., Chen, K.C., Tiu, C., “Hydroxylation of progesterone in biphasic media using alginate entrapped Aspergillus ochraceus gel beads coated with polyurea”, Enzyme Microb. Technol.,16, 485-491 (1994).
17 Leon, R., Fernandes, P., Pinheiro, H.M., Cabral, J.M., “Whole cell biocatalysis in organic media”, Enzyme Microb. Technol.,23, 483-500 (1998).
18 Li, Y.G., Xing, J.M., Xiong, X.C., Li, W.L., Gao, H.S., Liu, H.Z.,“Improvement of biodesulfurization activity of alginate immobilized cells in biphasic systems”, J. Ind. Microbiol. Biotechnol.,35, 145-150 (2008). (in Chinese)
19 Xiong, X.C., Xing, J.M., Li, X., Bai, X.J., Li, W.L., Li, Y.G., Liu, H.Z., “Enhancement of biodesulfurization in two-liquid systems by heterogeneous expression of vitreoscilla hemoglobin”, Appl. Microbiol. Biotechnol.,73, 2394-2397 (2007).
20 Luo, M.F., Gou, Z.X., Xing, J.M., Liu, H.Z., Chen, J.Y., “Microbial desulfurization of modeland straight-run diesel oils”, J. Chem. Technol. Biotechnol.,78, 873-876 (2003).
2011-05-09, accepted 2011-11-16.
* Supported by the National Natural Science Foundation of China (21076216, 30970046).
** To whom correspondence should be addressed. E-mail: jmxing@home.ipe.ac.cn
 Chinese Journal of Chemical Engineering2012年1期
Chinese Journal of Chemical Engineering2012年1期
- Chinese Journal of Chemical Engineering的其它文章
- The Research Progress of CO2Capture with Ionic Liquids*
- Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
