Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
Chun Heng Lohand Rong Wang,*
1School of Civil and Environmental Engineering, Nanyang Technological University, 639798 Singapore, Singapore
2Singapore Membrane Technology Centre, Nanyang Technological University, 639798 Singapore, Singapore
Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
Chun Heng Loh1,2and Rong Wang1,2,*
1School of Civil and Environmental Engineering, Nanyang Technological University, 639798 Singapore, Singapore
2Singapore Membrane Technology Centre, Nanyang Technological University, 639798 Singapore, Singapore
Poly(vinylidene fluoride) (PVDF) has become one of the most popular materials for membrane preparation via nonsolvent induced phase separation (NIPS) process. In this study, an amphiphilic block copolymer, Pluronic F127, has been used as both a pore-former and a surface-modifier in the fabrication of PVDF hollow fiber membranes to enhance the membrane permeability and hydrophilicity. The effects of 2nd additive and coagulant temperature on the formation of PVDF/Pluronic F127 membranes have also been investigated. The as-spun hollow fibers were characterized in terms of cross-sectional morphology, pure water permeation (PWP), relative molecular mass cut-off (MWCO), membrane chemistry, and hydrophilicity. It was observed that the addition of Pluronic F127 significantly increased the PWP of as-spun fibers, while the membrane contact angle was reduced. However, the size of macrovoids in the membranes was undesirably large. The addition of a 2nd additive, including lithium chloride (LiCl) and water, or an increase in coagulant temperature was found to effectively suppress the macrovoid formation in the Pluronic-containing membranes. In addition, the use of LiCl as a 2nd additive also further enhanced the PWP and hydrophilicity of the membranes, while the surface pore size became smaller. PVDF hollow fiber with a PWP as high as 2530 L·m−2·h−1·MPa−1, a MWCO of 53000 and a contact angle of 71° was successfully fabricated with 3% (by mass) of Pluronic F127 and 3% (by mass) of LiCl at a coagulant temperature of 25 °C, which shows better performance as compared with most of PVDF hollow fiber membranes made by NIPS method.
amphiphilic block copolymer, pore forming, surface modifying, additive, poly(vinylidene fluoride), hollow fiber membrane
Chinese Journal of Chemical Engineering,20(1) 71—79 (2012)
1 INTRODUCTION
The first asymmetric cellulose acetate membrane via nonsolvent induced phase separation (NIPS) technique was developed by Loeb and Souriajan in 1960’s [1]. Since then, much effort has been devoted to prepare high-performance membrane by this method for various applications. The use of NIPS for membrane preparation has been extended from cellulose acetate to many other polymer materials including poly(vinylidene fluoride) (PVDF). PVDF is a semi-crystalline polymer with good thermal stability, chemical resistance, and mechanical properties [2-4]. In addition, among the common hydrophobic polymers, PVDF has a higher processing ability compared to polytetrafluoroethylene (PTFE) and polypropylene (PP) as the former can be easily dissolved in common solvents [5]. All the above advantages make PVDF one of the most popular choices as a membrane material for various applications including microfiltration [6], ultrafiltration [7], pervaporation [8], membrane distillation [9, 10], and gas-liquid membrane contactor [11].
Due to the hydrophobic nature of PVDF material, however, it has been found that the penetration of coagulant (water) into the polymer dope solution is restricted during the phase inversion process [12]. As a result, the slow coagulation rate of PVDF might cause difficulty in the preparation of highly porous membranes. One efficient method to improve the porosity of PVDF membranes is to introduce suitable hydrophilic additives as a pore former into the polymer dope solution. As PVDF is usually thermodynamically incompatible with the additives, the dope solution may completely separates into two phases during demixing process and large pores can be formed after the additive-rich phase leached out into the water due to their hydrophilic nature [13]. A wide range of additives has been used as pore-former in membrane fabrication, which includes hydrophilic polymers, inorganic salts or acids, weak co-solvents, and strong non-solvents [14].
On the other hand, a membrane with hydrophobic surface may also face severe organic fouling due to the hydrophobic interaction between organic foulants and membrane surface in water or wastewater treatments [15, 16]. Therefore it is desirable to improve the hydrophilicity of PVDF membranes in order to enhance the membrane performance and reduce the operating cost. Among various techniques for hydrophilic modification of PVDF membranes, the blending of hydrophilic modifiers with PVDF contributes a more convenient method as it does not require additional processing steps [17]. Various hydrophilic polymers, amphiphilic block copolymers, and inorganic nanoparticles have been reported in the preparation of hydrophilic PVDF membranes via blending method [18, 21]. In the case of blending amphiphilic block copolymers with PVDF, the copolymers tended to segregate to the polymer/water interface due to the low interfacialenergy between the hydrophilic segment and water, while the hydrophobic, water-insoluble segment of the copolymer firmly anchored in the polymer matrix [17, 22]. Consequently, a hydrophilic layer was formed on the membrane surfaces including the surface of internal pores [22]. Due to the presence of hydrophobic chains in the molecules, amphiphilic copolymers as the hydrophilic modifier are expected to have a higher stability in membrane matrix compared to the hydrophilic polymers.
It is interesting to note that the blending of amphiphilic block copolymers for the preparation of PVDF membranes not only results in the improved hydrophilicity, but also increases the membrane porosity due to the presence of hydrophilic segments in the copolymers [23]. Hashim et al. [24] has also reported an improved hydrophilicity and a significant flux enhancement of PVDF membranes prepared from the dope containing PVDF grafted with poly(ethylene glycol) methyl ether methacrylate (PEGMA) compared to the original PVDF membrane. These results indicate that the amphiphilic block copolymers can act as both surface-modifying and pore-forming agents in the preparation of PVDF membranes.
To date, most of the amphiphilic block copolymers used as additives in PVDF membrane fabrication via NIPS is self-synthesized while the report on the use of commercially available ones is limited [17, 23-25]. Zhao et al. [23] has reported the preparation of PVDF membrane using a commercial amphiphilic block copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO). A series of similar commercial products with a trade name of Pluronic, which are often denoted as PEO-PPO-PEO and PPO-PEOPPO, has also been successfully used to prepare hydrophilic polyethersulfone (PES) membranes [26-31]. Pluronic has been considered as a good alternative to the self-synthesized amphiphilic copolymers as it is less costly and does not require sophisticated techniques for polymerization. In our previous study, the pore-forming ability of different types of Pluronic in the fabrication of PES hollow fiber membranes was also observed and the membranes prepared from Pluronic F127 and F108 exhibited the best performance [32]. However, to the best of our knowledge, the preparation of hydrophilic PVDF hollow fiber membranes using Pluronic via NIPS has not yet been reported.
In present study, an attempt was made to investigate the effects of Pluronic F127 as both a pore-former and a surface modifier on the fabrication of PVDF hollow fiber membranes via NIPS. To further improve the membrane performance, lithium chloride and water as a second additive have been added into the Pluronic-containing PVDF dope, respectively. The effect of coagulant temperature on the formation of PVDF hollow fibers prepared using Pluronic F127 has also been studied. It is anticipated that this study can provide guidance for the preparation of high performance PVDF hollow fiber membranes via NIPS process using amphiphilic block copolymers as an additive.
2 EXPERIMENTAL
2.1 Membrane material and chemicals
PVDF (Kynar 761, relative molecular mass 444000, Arkema) was dried at 50 °C under vacuum for at least 1 day before use. N-methyl-2-pyrrolidone (NMP) purchased from Merck was used as the solvent. Pluronic F127 (relative molecular mass 12600, PEO100-PPO65-PEO100, Sigma), lithium chloride (LiCl, anhydrous, MP Biomed), and Mili-Q water were used as the non-solvent additives in membrane preparation. Dextran with different relative molecular mass ranging from 6000 to 500000 (Sigma) was used to determine the relative molecular mass cut-off (MWCO) of the hollow fiber membranes. Hexane and 2-propanol (Merck) were used for membrane drying by solvent-exchange method. All the reagents were used as received.
2.2 Spinning dope preparation and dope viscosity measurements
A polymer dope solution for spinning was prepared by simultaneously dissolving desired amount of pre-dried PVDF and the additives which consist of Pluronic F127, LiCl, or water in NMP using a jacket flask. The dope solution was mechanically stirred for at least 2 d at 60 °C. The homogenous dope prepared was then cooled down to room temperature and subsequently degassed under vacuum for overnight before spinning.
Rheological characteristic of the spinning dope solutions was measured by a rheometer (Physica MCR 101, Anton Paar). The measurements were carried out using a 25 mm measuring plate (CP25-1) under steady-state shear rate ranging from 0.01 to 1000 s−1at 25 °C. The viscosity of dope solutions was recorded at the shear rate of 10 s−1.
2.3 Spinning of PVDF hollow fiber membranes and post-treatment
The PVDF hollow fiber membranes were fabricated via dry-jet wet spinning process. For the spinning of different dopes, the dope flow rate was kept constant while different bore fluid flow rates were employed in order to achieve a reasonable thickness of as-spun membranes. The nascent hollow fiber was collected at free-falling take-up speed and subsequently stored in a water bath for at least 2 d to remove residual solvent. The dope compositions and detailed spinning conditions are listed in Tables 1 and 2, respectively.
For some characterizations which required the hollow fibers to be dried, post-treatment was performed to alleviate membrane shrinkage during drying process. The membranes were immersed in 2-propanol followed by hexane for 4 h each. The membranes were then air-dried at room temperature before use for characterizations.
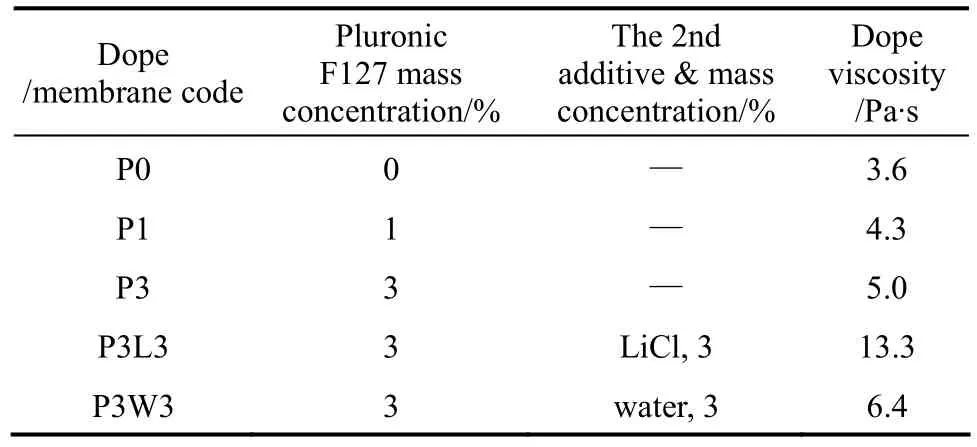
Table 1 Additive concentration in dope solutions and viscosity of dopes containing 18% of PVDF
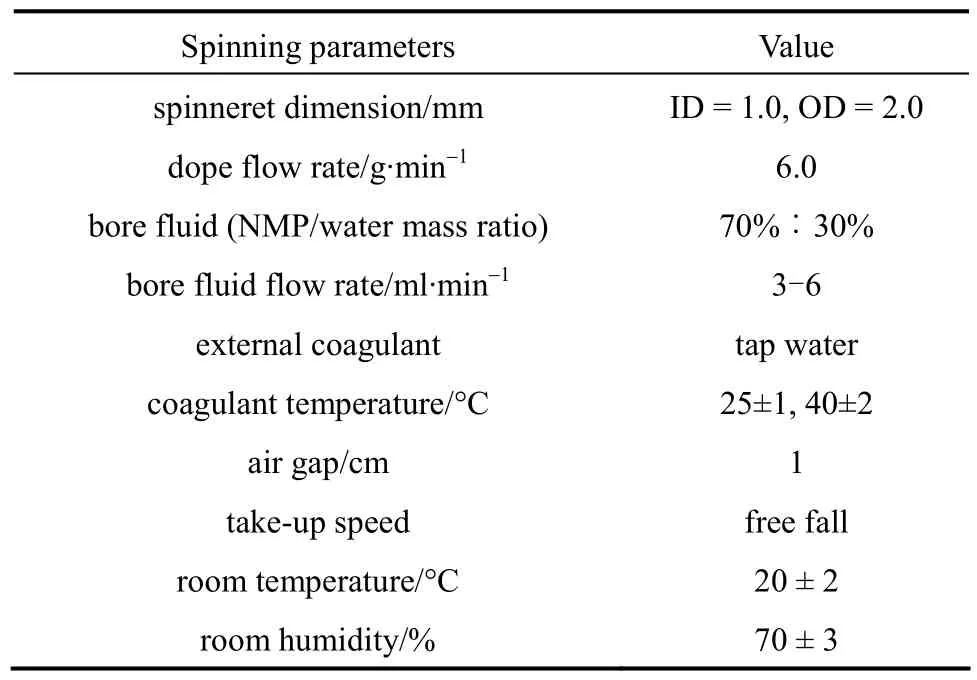
Table 2 Spinning parameters for PVDF hollow fiber membranes
2.4 Pure water permeation and MWCO measurement of PVDF hollow fiber membranes
Hollow fiber membranes were kept wet in water before use for pure water permeation (PWP) and MWCO characterization. Lab-scale hollow fiber module was made by sealing 10 pieces of hollow fibers in a glass tube. The effective length of the hollow fibers was 26 cm and Mili-Q water as feed was circulated through the shell side of the hollow fibers. Compaction on the membranes was done under the pressure of at least 34 kPa for 1 h and the permeate was subsequently collected for 5 min under 34 kPa. The PWP of the hollow fibers (L·m−2·h−1·MPa−1) was calculated using the formula:

where Q is the amount of permeate collected over a duration of time for the hollow fibers; ΔP the pressure difference between the feed and permeate side of hollow fibers; A the outer surface area of hollow fibers in the module.
The MWCO of a hollow fiber membrane is defined as the relative molecular mass at which 90% of the solute is retained by membranes. The 1-hour compacted hollow fibers were used for MWCO test and 1500 mg·L−1of a dextran aqueous solution with a relative molecular mass distribution ranging from 6000 to 500000 was used as the feed. The permeate was collected after 30 min of feed circulation under 34 kPa to make sure that the system was stabilized. Gel permeation chromatography (GPC) (PL-GPC50 Plus, A Varian, Inc.) was used to characterize the dextran relative molecular mass distribution in the feed and permeate. Rejection of hollow fibers was then calculated according to the formula expressed as
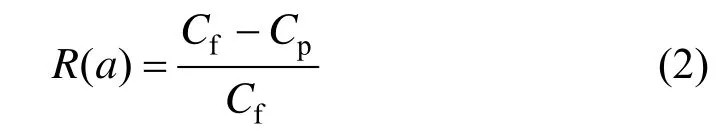
where R is the rejection coefficient for dextran molecule with a certain relative molecular mass a; Cfand Cpthe concentrations of dextran with a relative molecular mass of a in the feed and permeate solutions, respectively.
2.5 Other characterizations of PVDF hollow fiber membranes
The cross-sectional morphology of hollow fiber membranes was observed using a scanning electron microscope (SEM) (EVO 50, Carl Zeiss AG). The hollow fibers were broken in liquid nitrogen and sputtered with gold prior to the test.
In order to examine the presence of Pluronic in PVDF hollow fibers, attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) was carried out by IRPrestige-21 spectrophotometer from Shimadzu. The outer surface of dried membranes was directly analyzed and the IR spectra were obtained by 45 scans at a resolution of 4 cm−1.
The hydrophilicity of PVDF hollow fibers was examined by both dynamic contact angle and liquid entry pressure of water (LEPw). Dynamic contact angle of PVDF hollow fibers was measured by a tensiometer (DCAT11 Dataphysics, Germany) according to Wilhelmy method. Five immersion-emersion cycles in Mili-Q water were carried out for each specimen with a repetition of 5 times for all the samples. The advancing contact angle of the fifth cycle was recorded. LEPw was measured using dead-end hollow fiber modules containing a single fiber. Pressure applied to the lumen of the fibers was gradually increased until a continuous flow of water was observed at the shell side of the fibers, and the pressure was recorded as the membrane LEPw. The detailed methodology has been described elsewhere [33].
3 RESULTS AND DISCUSSION
3.1 Viscosity of PVDF dope solutions
The kinetics of phase inversion, which always refers to the out-diffusion rate of the solvent and the in-diffusion rate of the nonsolvent during membrane formation, is highly related to the viscosity of the polymer dope solution: a high viscosity hinders the exchange of solvent/non-solvent during the phase inversion process and vice versa. The viscosity of thedope prepared for spinning is presented in Table 1. The results revealed that an increase in Pluronic F127 mass concentration from 0 to 3% in PVDF dopes caused an increase in the dope viscosity from 3.6 to 5.0 Pa·s. The reason for this increase is that the entanglement between the polymer chains of PVDF and Pluronic F127 becomes more significant with increasing Pluronic F127 concentration [34].
When comparing the dope with and without the addition of 2nd additives in Table 1, it can be observed that the dope viscosity increased with the addition of 2nd additives into the polymer dopes. This dope viscosity enhancement effect was more evident for LiCl than that for water. For instance, the viscosity increased significantly from 5.0 Pa·s for dope P3 to 13.3 Pa·s for dope P3L3, which was the case with the addition of LiCl as a 2nd additive; whereas, a slight increase in viscosity from 5.0 Pa·s for dope P3 to 6.4 Pa·s for dope P3W3, which showed the case with the addition of water as a 2nd additive, was observed. This phenomenon has also been reported by other studies [10, 14, 35]. The significant enhancement of viscosity caused by the addition of LiCl can be attributed to the formation of acid-base complexes between LiCl and the solvent, as well as the interaction between Li+cation and electron donor in the polymer molecule [14].
3.2 Morphology of PVDF hollow fiber membranes
3.2.1Effect of Pluronic as additive
Figure 1 (a-c) show the cross-sectional morphology of PVDF hollow fibers prepared with/without the addition of Pluronic F127 at a coagulant temperature of 25 °C. Membrane P0, which was prepared without any additive, exhibited an asymmetric structure consisting of finger-like macrovoids developed underneath the outer surface of the fibers, and a sponge-like structure near the lumen of the fibers. With the addition of 1% and 3% (by mass) of Pluronic F127 (membrane P1 and P3), the macrovoids almost fully developed across the cross-section and the size of macrovoids was significantly increased compared with the morphology of membrane P0. It was also noticed that irregular contour was formed at the lumen of the fibers with the addition of Pluronic F127.
The formation of finger-like macrovoids developed from the outer surface of the fibers was caused by the instantaneous demixing promoted by the rapid solvent/nonsolvent exchange since water was used as the external coagulant. On the other hand, due to the presence of large amount of NMP [70% (by mass)] in the bore fluid, the solvent/nonsolvent exchange rate was reduced. Consequently, delayed demixing occurred which was accompanied with the formation of sponge-like structure and the elimination of skin layer near the lumen side [36, 37].
The observation on the formation of larger macrovoids in Figs. 1 (b) and 1 (c) is believed to be associated with thermodynamic and kinetic effects caused by the addition of Pluronic F127 into the dope system. It has been reported in our previous paper that the addition of Pluronic into polymer dopes resulted in both the reduction in thermodynamic stability and increase in non-solvent in-diffusion rate due to the nonsolvent nature and hydrophilic properties of the Pluronic F127, respectively [32]. Both factors can promote instantaneous demixing during the phase inversion process and in turn enhance macrovoid formation. On the other hand, the slight enhancement in dope viscosity with the addition of Pluronic F127 may cause a slower solvent-nonsolvent exchange in the nascent fibers and suppress macrovoid formation, but it is likely that this kinetic effect is not significant compared to the thermodynamic effect induced.
These big macrovoids formed in membrane P1 and P3 are not desirable as the mechanical strength of the membranes will be strongly deteriorated. Therefore, an attempt was made to suppress the macrovoid formation by the addition of 2nd additives, including LiCl and water, into the dope containing 3% (by mass)Pluronic F127, as discussed in the following section.
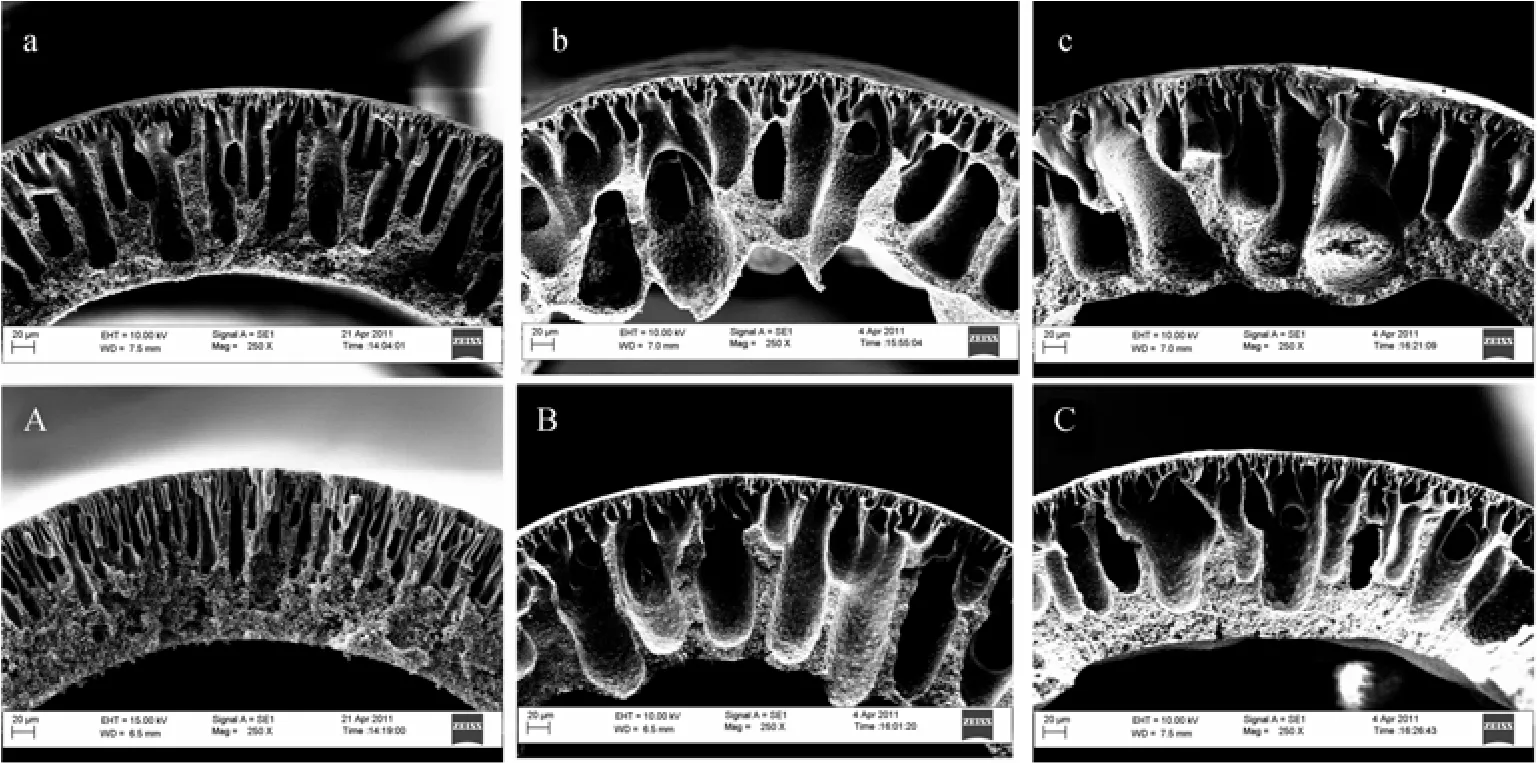
Figure 1 Cross-sectional morphology of hollow fiber membranes P0 (a, A) , P1 (b, B), P3 (c, C) spun with different concentration of Pluronic F127 as additives at coagulant temperature of 25 °C (a to c) and 40 °C (A to C)
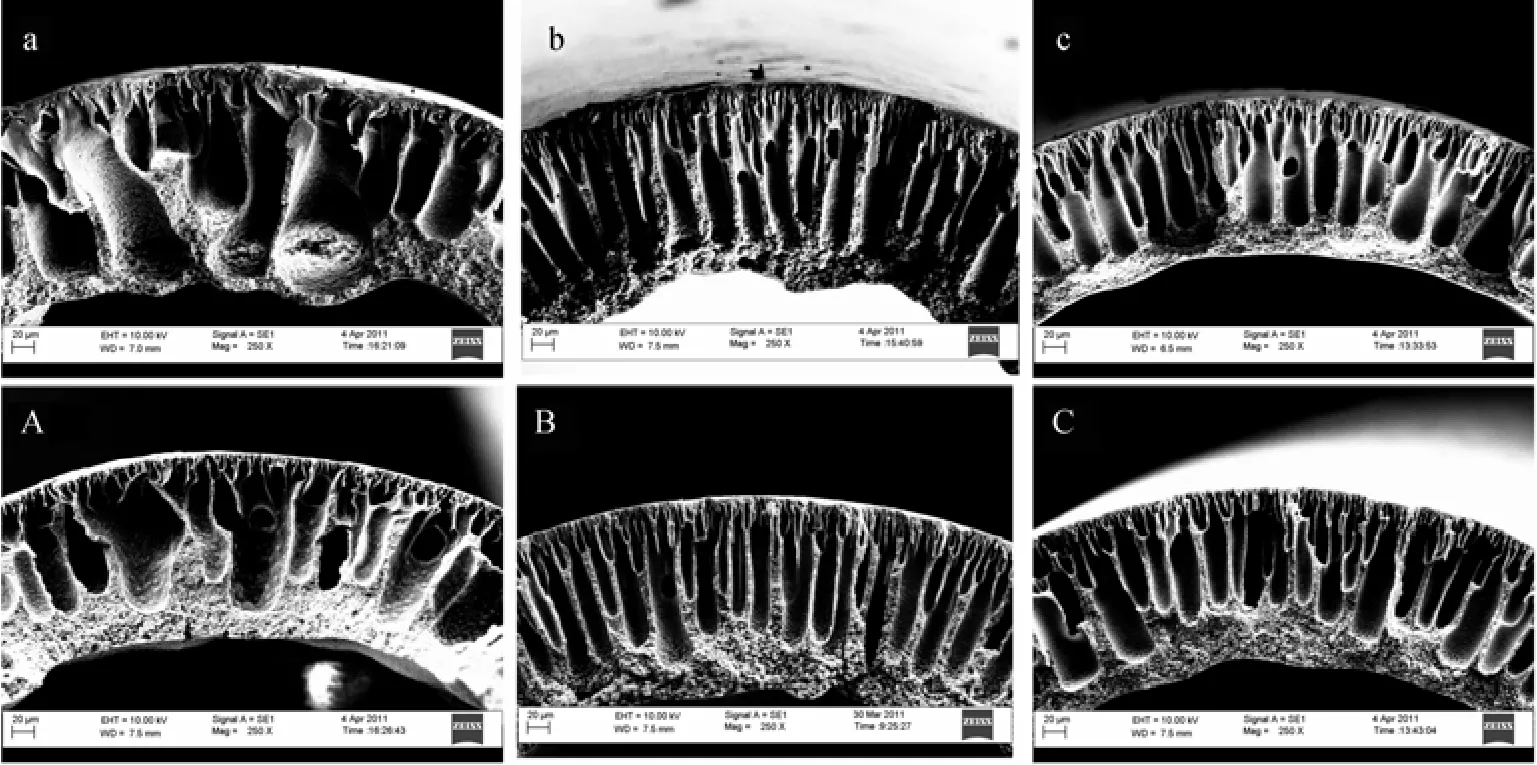
Figure 2 Cross-sectional morphology of hollow fiber membranes P3 (a, A) , P3L3 (b, B) , P3W3 (c, C) spun with/without 2nd additives at coagulant temperature of 25 °C (a to c) and 40 °C (A to C)
3.2.2Effect of second additives
Figures 2 (a-c) present the cross-sectional morphology of PVDF hollow fiber membranes prepared with/without the addition of 2nd additives at a coagulant temperature of 25 °C, while the concentration of Pluronic F127 was kept constant at 3% (by mass). The morphology was observed to change from big macrovoids to narrower finger-like structure when LiCl and water were added into the Pluronic-containing dopes, respectively. Similar macrovoid suppression during membrane formation has also been observed with the addition of LiCl into a poly(ethylene glycol) (PEG)-containing PVDF dope [38] and the addition of water into PEG-containing PES dope [39]. With the presence of LiCl in the dope, the dope viscosity enhances significantly, as mentioned in Section 3.1. The high viscosity in turn hindered the exchange of solvent/nonsolvent during the phase inversion process and hence the size of macrovoids was reduced due to the delayed precipitation [38]. On the other hand, the macrovoid suppression reported by Liu et al. [39] was attributed to the presence of high water content in the dope but the mechanism was unclear.
3.2.3Effect of coagulant temperature
Comparing Figs. 1 (a-c) with (A-C) as well as Figs. 2 (a-c) with (A-C) which shows the crosssectional morphology of fibers spun at a coagulant temperature of 25 °C and 40 °C, respectively, the effect of coagulant temperature on macrovoid formation can be observed. The size of macrovoids developed underneath the outer surface of the fibers became smaller when a higher coagulant temperature was employed during the spinning process. In addition, the area of sponge-like structure near the lumen of the fibers was increased at a coagulant temperature of 40 °C compared to that at 25 °C.
It has been reported that the thermodynamic stability of a polymer dope system is enhanced at a higher temperature, which in turn reduced the polymer precipitation rate [38, 40, 41]. When the nascent fibers are immersed into the coagulant bath with a temperature of 40 °C, the temperature of the dope in contact with the water is increased. This higher temperature results in a delayed demixing process due to the enhanced thermodynamic stability of the dope. Therefore, smaller and shorter macrovoids has been developed from the shell of fibers compared to the case with a lower coagulant temperature.
3.3 Effect of additives and coagulant temperatures on membrane performance
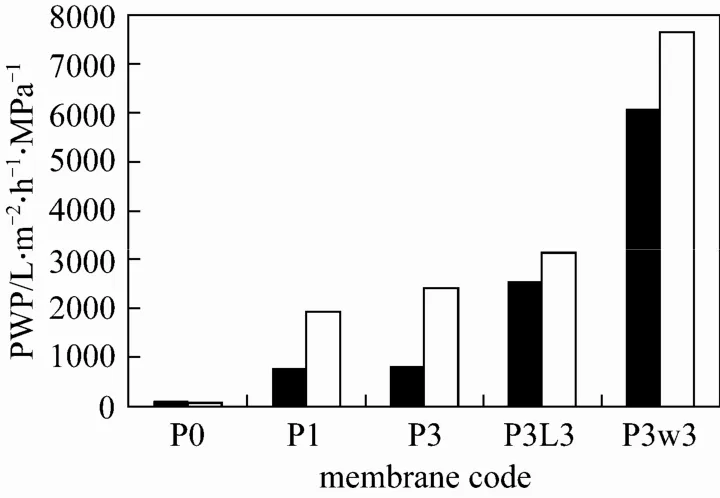
Figure 3 PWP of hollow fibers spun with different additives and different coagulant temperature■ 25 °C; □ 40 °C
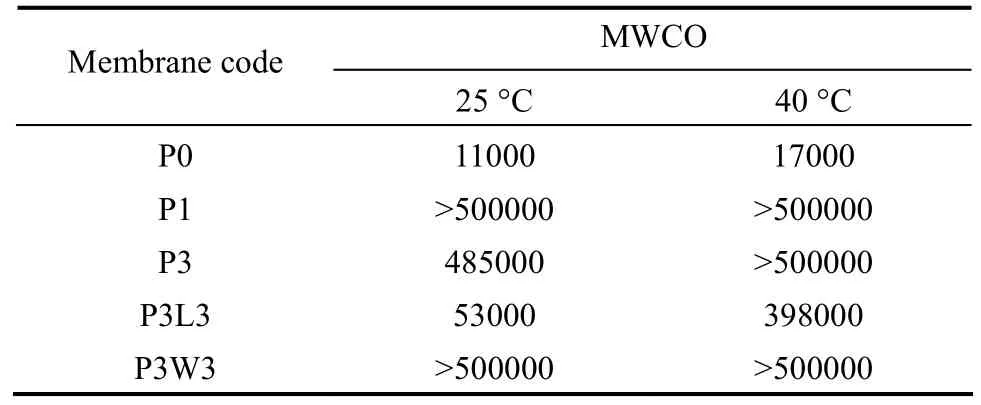
Table 3 MWCO of hollow fibers spun with different additives and different coagulant temperatures
The PWP and MWCO of hollow fiber membranes prepared with different conditions, including Pluronic F127 concentration, types of 2nd additive, and coagulant temperature, are presented in Fig. 3 and Table 3, respectively. It was observed that membrane P0, which was prepared without any additive, exhibited a very low PWP of 60 L·m−2·h−1·MPa−1when a coagulant temperature of 25 °C was used. With the addition of 1% and 3% (by mass) of Pluronic F127, the PWP of membrane P1 and P3 increased sharply to 760 and 780 L·m−2·h−1·MPa−1, respectively while their MWCO was close to or larger than 500000. Similar trend has been observed for the case with a coagulant temperature of 40 °C. The significant improvement of PWP might be attributed to the enhanced surface porosity while the large MWCO indicates the formation of large surface pores when Pluronic F127 was added as an additive to the polymer dopes. These phenomena provide an evidence of the good pore-forming ability of Pluronic F127 in the formation of PVDF hollow fiber membranes.
The effect of addition of 2nd additives into the Pluronic-containing dope on the membrane performance can be examined by comparing the experimental results (at coagulant temperature of 25 °C) of P3, P3L3, and P3W3 in Figs. 3 (a-c) and Table 3. With the addition of LiCl as a 2nd additive into the dope containing 3% (by mass) of Pluronic F127, the PWP of membrane P3L3 increased largely to 2530 L·m−2·h−1·MPa−1compared to membrane P3 with a PWP of 780 L·m−2·h−1·MPa−1. Surprisingly, a significant reduction of MWCO from 485000 to 53000 was also observed with the addition of LiCl. On the other hand, an even sharp increase in PWP to more than 6000 L·m−2·h−1·MPa−1was observed on membrane P3W3 with the addition of water as a 2nd additive while its MWCO was larger than 500000.
The large enhancement of PWP might be attributed to the higher membrane surface porosity or thinner skin layer formed due to the change of precipitation path, since the addition of LiCl and water brings the dope system closer to the cloud point due to the reduction of thermodynamic stability. Similar enhancement in surface porosity has also been reported when LiCl was added into a PEG-containing dope [10, 42]. Although the PWP of both membrane P3L3 and P3W3 was enhanced significantly, the results of MWCO reveal that the addition of LiCl causes a reduction in surface pore size while this effect cannot be observed with the addition of water. This indicates that LiCl not only acts as a flux enhancer but also effectively controls the pore size.
As shown in Fig. 3, the PWP of PVDF hollow fibers spun with the addition of Pluronic F127, including membrane P1, P3, P3L3, and P3W3, was significantly increased when a higher coagulant temperature was employed. The hotter external coagulant also caused an increase in the MWCO of membrane P3L3, indicating that the surface pore size was enlarged. This phenomenon might be related to the temperaturedependent aggregation number of Pluronic F127 micelle. The aggregation number of Pluronic F127, indicating the number of molecules present in the Pluronic F127 micelle, has been reported to be increased with increasing temperature [43, 44]. It is believed that the higher aggregation number of Pluronic F127, which means the larger size of micelles, leads to the formation of bigger pore size near the outer surface of the nascent fibers when it is in direct contact with the external coagulant with a temperature of 40 °C [30, 45].
3.4 ATR-FTIR analysis

Figure 4 ATR-FTIR spectra of PVDF hollow fibers spun with different additives at a coagulant temperature of 25 °C
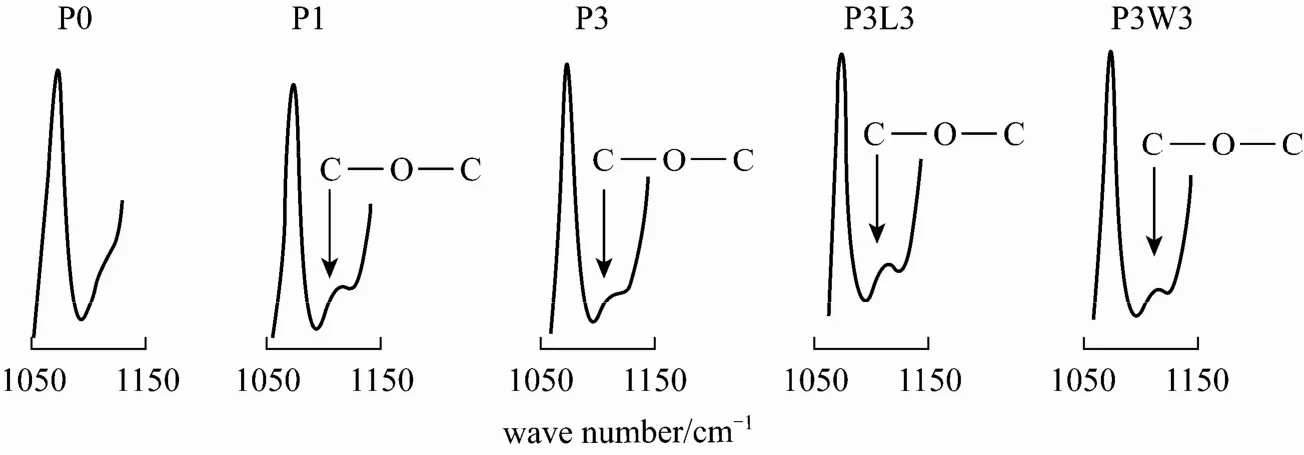
Figure 5 Enlarged ATR-FTIR spectra (1050-1150 cm−1) of PVDF hollow fibers spun with different additives at a coagulant temperature of 25 °C
To assess the presence of Pluronic F127 in the as-spun hollow fibers, ATR-FTIR analysis has been carried out. Figs. 4 and 5 present the ATR-FTIR spectra and its enlarged spectra at 1050-1150 cm−1, respectively, for PVDF hollow fibers prepared with different additives at a coagulant temperature of 25 °C. No significant difference can be observed between the spectra for different membranes in Fig. 4. When comparing the enlarged spectra in Fig. 5, small new peaks at around 1115 cm−1were observed for membranes prepared with the addition of Pluronic F127, regardless of the addition of 2nd additives. Similarly, the new peak was also observed from the spectra of membranes prepared with the addition of Pluronic F127 at a coagulant temperature of 40 °C (figure not shown). This absorbance peak represents the characteristic band for C OC stretching corresponding to the ether group, which confirms the presence of Pluronic F127 molecules in the membrane matrix [24]. The results suggest that a portion of Pluronic F127 might have washed out by water or 2-propanol/hexane during the membrane drying process, while some portions of Pluronic F127 still stay in the membrane matrix as the PPO block in the Pluronic F127 molecules provides an anchorage in the membrane despite the highly water-soluble nature of Pluronic F127 [27, 46].
3.5 Membrane contact angle and LEPw
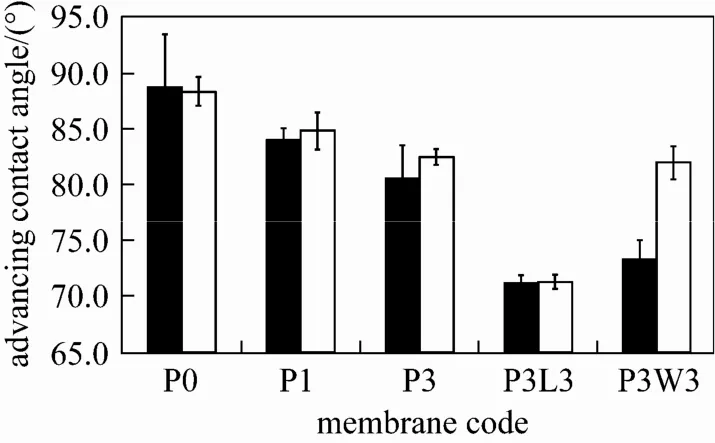
Figure 6 Advancing contact angle of PVDF hollow fibers spun with different additives at coagulant temperatures of 25 and 40 °C■ 25 °C; □ 40 °C
The hydrophilicity of PVDF hollow fiber membranes can be examined by dynamic contact angle as shown in Fig. 6. At a coagulant temperature of 25 °C, the advancing contact angle was reduced from 89° to 81° with increasing Pluronic mass concentration from 0 to 3% (membrane P0 to P3). Similar trend was also observed for the case of 40 °C coagulant. This can be attributed to the presence of the hydrophilic PEO chains on the membrane surface due to the surface segregation of Pluronic F127 molecules during the phase inversion process. The results are in accordance with the ATR-FTIR analysis in the previous section, which indicates the presence of ether group in the membranes prepared with the addition of Pluronic F127.
At a coagulant temperature of 25 °C, the addition of LiCl and water as a 2nd additive resulted in a further decrease in advancing contact angle of membrane P3L3 and P3W3 to 71° and 73°, respectively. When a higher coagulant temperature was employed, the significant decrease in contact angle was also observed for membrane P3L3 while the contact angle of membrane P3W3 did not differ much from that of membrane P3. The decrease in advancing contact angle has also been reported on poly(vinylidene-fluoride-cohexafluoropropylene) hollow fiber membranes prepared with the addition of LiCl as an additive [14]. This might be because of the presence of LiCl in the membrane matrix as it does not completely diffuse out during the membrane post-treatment. On the other hand, the significant decrease in contact angle caused by the addition of water has not been reported in literature to the best of our knowledge, and more investigation is required to carry out in the future to understand this phenomenon.
The advancing contact angle of membrane P0 to P3 prepared at a coagulant temperature of 40 °C wasobserved to be similar with their counterparts prepared at a lower temperature, indicating that the change in coagulant temperature exhibits no obvious influence on the hydrophilicity of the membranes prepared with/without Pluronic F127 as a single additive. This observation is also valid for membrane P3L3 which was prepared with the addition of LiCl as a 2nd additive.
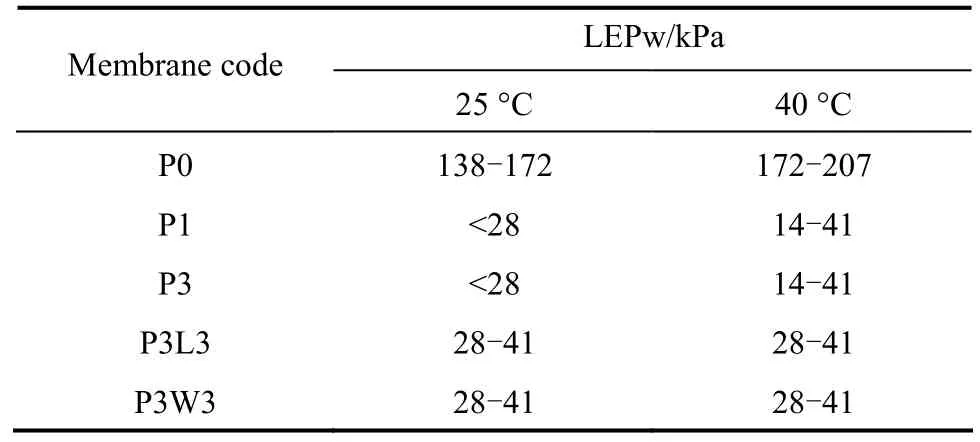
Table 4 LEPw of PVDF hollow fiber membranes spun with different additives at coagulant temperatures of 25 and 40 °C
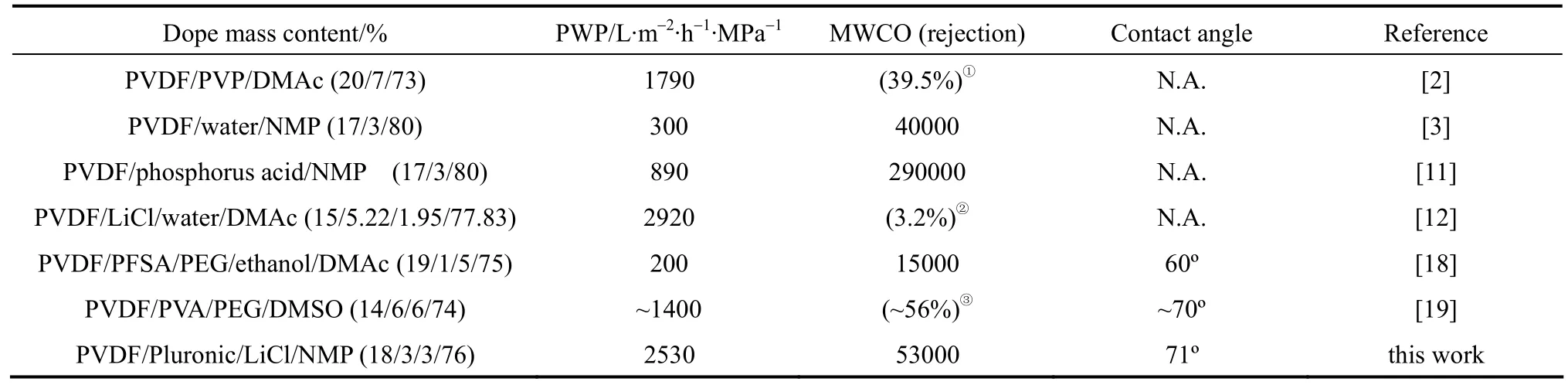
Table 5 Comparison of various PVDF hollow fiber membranes prepared via NIPS
The LEPw of PVDF hollow fibers prepared with/without the addition of Pluronic F127 and 2nd additives at different coagulant temperature is listed in Table 4. It was observed that all the membranes prepared with Pluronic F127 exhibited LEPw of less than 50 kPa, which is much lower than that of membrane P0 with a LEPw of more than 0.13 MPa. The coagulation temperature seems to have no much influence on the LEPw of membranes prepared with the addition of Pluronic F127. The lower LEPw reveals that the membranes become wetted easily with the addition of Pluronic F127 and with/without the 2nd additives, due to the formation of bigger pores and the enhanced hydrophilicity as discussed previously. This feature is favorable for the membrane used in water or wastewater treatments.
Table 5 lists the PWP, MWCO, and contact angle of PVDF hollow fiber membranes previously made by blending of pore-former or surface modifier via NIPS. It can be seen that the membrane prepared in this study has a relatively high PWP compared to most other self-fabricated membranes while the MWCO is reasonably low, which means smaller pore sizes. However, the membrane prepared with Pluronic F127 is not as hydrophilic as that prepared with the addition of Perfluorosulfonic acid (PFSA) [18]. This might be attributed to the higher stability of PFSA in the PVDF membrane matrix due to the presence of fluorocarbons. Nevertheless, Pluronic as an additive is still competitive considering its lower cost and higher permeability of resultant membrane compared to other additives.
4 CONCLUSIONS
The amphiphilic block copolymer, Pluronic F127, was used as an additive in the PVDF hollow fiber membranes fabrication in an attempt to increase the membrane permeability and enhance the membrane hydrophilicity. With the addition of Pluronic F127 into the polymer dope, the as-spun membranes exhibited higher PWP and slightly enhanced hydrophilicity. It was found that the macrovoid size, surface pore size, water permeability, and hydrophilicity of the membranes was affected when LiCl or water were added as a 2nd additive into the dopes containing Pluronic F127. A higher coagulant temperature was also found to suppress the macrovoids formation, enhance the water permeability, and increase the surface pore size.
From this study, it can be concluded that PVDF hollow fiber membrane with high performance can be prepared with Pluronic F127 as both the pore-former and the surface modifier. By using a coagulant temperature of 25 °C and a combination of Pluronic F127 and LiCl as the additive, PVDF hollow fiber with PWP as high as 2530 L·m−2·h−1·MPa−1, MWCO of 53000, and contact angle of 71° was successfully fabricated, which is better than most of PVDF hollow fiber membranes made by NIPS method.
ACKNOWLEDGEMENTS
We would like to thank the Environment and Water Industrial Program Office of Singapore for funding support under the project #0901-IRIS-02-03. We are also grateful to Singapore Economic Development Board for funding Singapore Membrane Technology Centre. Ms. Kuah Hui Mun is acknowledged for her kind assistance.
REFERENCES
1 Loeb, S., Sourirajan, S., “Sea water demineralization by means of an osmotic membrane”, In: Saline Water Conversion-II, American Chemical Society, Washington, D.C., 117-132 (1963).
2 Wang, D., Li, K., Teo, W.K., “Preparation and characterization of polyvinylidene fluoride (PVDF) hollow fiber membranes”, J. Membr. Sci.,163, 211-220 (1999).
3 Feng, C., Wang, R., Zhang, H., Shi, L., “Diverse morphologies of PVDF hollow fiber membranes and their performance analysis as gas/liquid contactors”, J. Appl. Polym. Sci.,119, 1259-1267 (2011).
4 Awanis Hashim, N., Liu, Y., Li, K., “Stability of PVDF hollow fibre membranes in sodium hydroxide aqueous solution”, Chem. Eng. Sci.,66, 1565-1575 (2011).
5 Shi, L., Wang, R., Cao, Y., Feng, C., Liang, D.T., Tay, J.H., “Fabrication of poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP)asymmetric microporous hollow fiber membranes”, J. Membr. Sci.,305, 215-225 (2007).
6 Baroña, G.N.B., Cha, B.J., Jung, B., “Negatively charged poly(vinylidene fluoride) microfiltration membranes by sulfonation”, J. Membr. Sci.,290, 46-54 (2007).
7 Ma, J., Wang, Z., Pan, M., Guo, Y., “A study on the multifunction of ferrous chloride in the formation of poly(vinylidene fluoride) ultrafiltration membranes”, J. Membr. Sci.,341, 214-224 (2009).
8 Ong, Y.K., Widjojo, N., Chung, T.S., “Fundamentals of semi-crystalline poly(vinylidene fluoride) membrane formation and its prospects for biofuel (ethanol and acetone) separation via pervaporation”, J. Membr. Sci.,378, 149-162 (2011).
9 Yang, X., Wang, R., Shi, L., Fane, A.G., Debowski, M., “Performance improvement of PVDF hollow fiber-based membrane distillation process”, J. Membr. Sci.,369, 437-447 (2011).
10 Hou, D., Wang, J., Qu, D., Luan, Z., Ren, X., “Fabrication and characterization of hydrophobic PVDF hollow fiber membranes for desalination through direct contact membrane distillation”, Sep. Purif. Technol.,69, 78-86 (2009).
11 Atchariyawut, S., Feng, C., Wang, R., Jiraratananon, R., Liang, D.T.,“Effect of membrane structure on mass-transfer in the membrane gas-liquid contacting process using microporous PVDF hollow fibers”, J. Membr. Sci.,285, 272-281 (2006).
12 Wang, D., Li, K., Teo, W.K., “Porous PVDF asymmetric hollow fiber membranes prepared with the use of small molecular additives”, J. Membr. Sci.,178, 13-23 (2000).
13 Feng, C., Wang, R., Shi, B., Li, G., Wu, Y., “Factors affecting pore structure and performance of poly(vinylidene fluoride-co-hexafluoro propylene) asymmetric porous membrane”, J. Membr. Sci.,277, 55-64 (2006).
14 Shi, L., Wang, R., Cao, Y., Liang, D.T., Tay, J.H., “Effect of additives on the fabrication of poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) asymmetric microporous hollow fiber membranes”, J. Membr. Sci.,315, 195-204 (2008).
15 Larsson, K., “Interfacial phenomena—bioadhesion and biocompatibility”, Desalination,35, 105-114 (1980).
16 Khulbe, K.C., Feng, C., Matsuura, T., “The art of surface modification of synthetic polymeric membranes”, J. Appl. Polym. Sci.,115, 855-895 (2010).
17 Hester, J.F., Banerjee, P., Mayes, A.M., “Preparation of protein-resistant surfaces on poly(vinylidene fluoride) membranes via surface segregation”, Macromolecules,32, 1643-1650 (1999).
18 Yuan, G.L., Xu, Z.L., Wei, Y.M., “Characterization of PVDF-PFSA hollow fiber UF blend membrane with low-molecular weight cut-off”, Sep. Purif. Technol.,69, 141-148 (2009).
19 Li, N., Xiao, C., An, S., Hu, X., “Preparation and properties of PVDF/PVA hollow fiber membranes”, Desalination,250, 530-537 (2010).
20 Liu, F., Xu, Y.Y., Zhu, B.K., Zhang, F., Zhu, L.P., “Preparation of hydrophilic and fouling resistant poly(vinylidene fluoride) hollow fiber membranes”, J. Membr. Sci.,345, 331-339 (2009).
21 Liu, F., Hashim, N.A., Liu, Y., Abed, M.R.M., Li, K., “Progress in the production and modification of PVDF membranes”, J. Membr. Sci.,375, 1-27 (2011).
22 Asatekin, A., Kang, S., Elimelech, M., Mayes, A.M., “Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft-poly(ethylene oxide) comb copolymer additives”, J. Membr. Sci.,298, 136-146 (2007).
23 Zhao, Y.H., Qian, Y.L., Zhu, B.K., Xu, Y.Y., “Modification of porous poly(vinylidene fluoride) membrane using amphiphilic polymers with different structures in phase inversion process”, J. Membr. Sci.,310, 567-576 (2008).
24 Hashim, N.A., Liu, F., Li, K., “A simplified method for preparation of hydrophilic PVDF membranes from an amphiphilic graft copolymer”, J. Membr. Sci.,345, 134-141 (2009).
25 Zhao, Y.H., Xu, Y.Y., Zhu, B.K., “In situ crosslinking of hyperbranched polyglycerol in casting solutions and its effect on the structure and properties of porous PVDF membranes”, J. Appl. Polym. Sci.,117, 548-556 (2010).
26 Alexandridis, P., Holzwarth, J.F., Hatton, T.A., “Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association”, Macromolecules,27, 2414-2425 (1994).
27 Wang, Y.Q., Wang, T., Su, Y.L., Peng, F.B., Wu, H., Jiang, Z.Y.,“Remarkable reduction of irreversible fouling and improvement of the permeation properties of poly(ether sulfone) ultrafiltration membranes by blending with pluronic F127”, Langmuir,21, 11856-11862 (2005).
28 Wang, Y.Q., Su, Y.L., Ma, X.L., Sun, Q., Jiang, Z.Y., “Pluronic polymers and polyethersulfone blend membranes with improved fouling-resistant ability and ultrafiltration performance”, J. Membr. Sci.,283, 440-447 (2006).
29 Wang, Y., Su, Y., Sun, Q., Ma, X., Jiang, Z., “Improved permeation performance of Pluronic F127-polyethersulfone blend ultrafiltration membranes”, J. Membr. Sci.,282, 44-51 (2006).
30 Zhao, W., Su, Y., Li, C., Shi, Q., Ning, X., Jiang, Z., “Fabrication of antifouling polyethersulfone ultrafiltration membranes using Pluronic F127 as both surface modifier and pore-forming agent”, J. Membr. Sci.,318, 405-412 (2008).
31 Susanto, H., Ulbricht, M., “Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives”, J. Membr. Sci.,327, 125-135 (2009).
32 Loh, C.H., Wang, R., Shi, L., Fane, A.G., “Fabrication of high performance polyethersulfone UF hollow fiber membranes using amphiphilic Pluronic block copolymers as pore-forming additives”, J. Membr. Sci.,380, 114-123 (2011).
33 Smolders, K., Franken, A.C.M., “Terminology for membrane distillation”, Desalination,72, 249-262 (1989).
34 Ohya, H., Shiki, S., Kawakami, H., “Fabrication study of polysulfone hollow-fiber microfiltration membranes: Optimal dope viscosity for nucleation and growth”, J. Membr. Sci.,326, 293-302 (2009). 35 Kong, J., Li, K., “Preparation of PVDF hollow-fiber membranes via immersion precipitation”, J. Appl. Polym. Sci.,81, 1643-1653 (2001).
36 Mulder, M., Basic Principles of Membrane Technology, 2nd edition, Kluwer Academic, Dordrecht, Boston (1996).
37 Madaeni, S.S., Rahimpour, A., “Effect of type of solvent and non-solvents on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration”, Polym. Adv. Technol.,16, 717-724 (2005).
38 Wongchitphimon, S., Wang, R., Jiraratananon, R., Shi, L., Loh, C.H.,“Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes”, J. Membr. Sci.,369, 329-338 (2011).
39 Liu, Y., Koops, G.H., Strathmann, H., “Characterization of morphology controlled polyethersulfone hollow fiber membranes by the addition of polyethylene glycol to the dope and bore liquid solution”, J. Membr. Sci.,223, 187-199 (2003).
40 Yeow, M.L., Liu, Y.T., Li, K., “Isothermal phase diagrams and phase-inversion behavior of poly(vinylidene fluoride)/solvents/ additives/water systems”, J. Appl. Polym. Sci.,90, 2150-2155 (2003).
41 Khayet, M., Cojocaru, C., García-Payo, M.C., “Experimental design and optimization of asymmetric flat-sheet membranes prepared for direct contact membrane distillation”, J. Membr. Sci.,351, 234-245 (2010).
42 Yu Wang, K., Chung, T.S., Gryta, M., “Hydrophobic PVDF hollow fiber membranes with narrow pore size distribution and ultra-thin skin for the fresh water production through membrane distillation”, Chem. Eng. Sci.,63, 2587-2594 (2008).
43 Alexandridis, P., Alan Hatton, T., “Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling”, Colloids Surf., A,96, 1-46 (1995).
44 Su, Y.L., Liu, H.Z., “Temperature-dependent solubilization of PEO-PPO-PEO block copolymers and their application for extraction trace organics from aqueous solutions”, Korean J. Chem. Eng.,20, 343-346 (2003).
45 Peng, J., Su, Y., Chen, W., Shi, Q., Jiang, Z., “Effects of coagulation bath temperature on the separation performance and antifouling property of poly(ether sulfone) ultrafiltration membranes”, Ind. Eng. Chem. Res.,49, 4858-4864 (2010).
46 BASF, “Pluronic PE Types, Technical Information”, http://www.basf-korea.co.kr/02_products/03_chemicals/specialty_ch emicals/data/TI_Pluronic%20PE_ES1026e_Mar2005.pdf (2005).
2011-08-22, accepted 2011-11-16.
* To whom correspondence should be addressed. E-mail: rwang@ntu.edu.sg
 Chinese Journal of Chemical Engineering2012年1期
Chinese Journal of Chemical Engineering2012年1期
- Chinese Journal of Chemical Engineering的其它文章
- The Research Progress of CO2Capture with Ionic Liquids*
- Simultaneous Removal of Thiophene and Dibenzothiophene by Immobilized Pseudomonas delafieldii R-8 cells*
- Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
