Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
XIE Meiying (解美莹), LI Peipei (李沛沛), GUO Huifeng (郭惠锋), GAO Lixia (高丽霞) and YU Jiang (余江)**
Research Group of Environmental Catalysis and Separation Process, College of Chemical Engineering,
Beijing University of Chemical Technology, Beijing 100029, China
Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
XIE Meiying (解美莹), LI Peipei (李沛沛), GUO Huifeng (郭惠锋), GAO Lixia (高丽霞) and YU Jiang (余江)**
Research Group of Environmental Catalysis and Separation Process, College of Chemical Engineering,
Beijing University of Chemical Technology, Beijing 100029, China
Fe-based ionic liquid (Fe-IL) was synthesized by mixing FeCl3·6H2O and 1-butyl-3-methylimidazolium chloride [Bmim]Cl in this paper. The phase diagram of a ternary Fe-IL, ethanol and water system was investigated to construct a ternary desulfurization solution for wet flue gas desulfurization. The effects of flow rate and concentration of SO2, reaction temperature, pH and Fe-IL fraction in aqueous desulfurization solution on the desulfurization efficiency were investigated. The results shows that the best composition of ternary desulfurization solution of Fe-IL, ethanol and water is 1∶1.5∶3 by volume ratio, and pH should be controlled at 2.0. Under such conditions, a desulfurization rate greater than 90% could be obtained. The product of sulfuric acid had inhibition effect on the wet desulfurization process. With applying this new ternary desulfurization solution, not only the catalyst Fe-IL can be recycled and reused, but also the product sulfuric acid can be separated directly from the ternary desulfurization system.
Fe-based ionic liquid, ternary phase diagram, sulfide dioxide, wet flue gas desulfurization
Chinese Journal of Chemical Engineering,20(1) 140—145 (2012)
1 INTRODUCTION
The extensive uses of fossil fuels, e.g. combustion of oil and coal, lead to large amount of emission of acid gases including SO2to atmosphere and cause a serious danger to the human life and productive activities [1-3]. One of the most important approach is to intensify the adsorption of SO2by liquid to sweeten the flue gas [4]. The wet flue gas desulfurization catalyzed by metal ions has been widely used for iron ion or manganese ion with strong oxidation ability to convert S(IV) to S(VI) [5]. The removal of SO2by Fe3+ions catalyzed liquid-phase oxidation is described as the following chemical reactions [6-8]:

The overall reaction is quite simple:
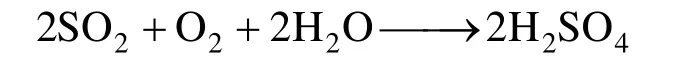
This desulfurization process has advantages of high desulfurization efficiency and fast reaction rate. But the removal of SO2can only be carried out in aqueous phase when SO2is in the forms of23SO−and HSO3−in aqueous solution, so that it is difficult to separate the desulfurization product and recycle the catalyst. The catalyst must be added continually [6-10]. Therefore, it is significant to explore a new wet flue gas desulfurization technology.
Ionic liquids (ILs) as promising functional materials have many advantages over the traditional absorbents, including extremely low vapor pressure, excellent gas solubility, high thermal and chemical stability. They present high potential to be used as good absorbents for sweetening SO2containing gas streams [11-13].
The hydrophobic Fe-based ionic liquids (Fe-IL) can be prepared by mixing BmimCl and FeCl3directly at room temperature in air. It was reported that organic sulfur in petroleum oil could be catalytically oxidized and removed by Fe-IL and H2O2[14]. Our work also indicated that H2S could be oxidized and converted to sulfur directly by Fe-IL, which can be regenerated by O2oxidation for reuse [15]. In this work, the high oxidation ability of Fe-IL is used as catalyst to oxidize SO2to H2SO4in water. We note that the hydrophobic Fe-IL can dissolve in water with addition of ethanol, and be retrieved after removing ethanol from their aqueous solution by heat. That is to say, a ternary system composed of Fe-IL, ethanol and water can be constructed to oxidize SO2to H2SO4, and the catalyst Fe-IL can be separated from the product H2SO4aqueous solution with removing ethanol from the ternary system by heat. As a result, it is a green wet flue gas desulfurization technology without loss of catalyst and product.
The schematic concept of desulfurization process by phase separation is shown in Fig. 1. In this desulfurization process, Fe-IL, ethanol and water are mixed to prepare the desulfurization system. Ethanol enhancesthe solubility of hydrophobic Fe-IL in water to form a homogenous solution. After desulfurization reaction, ethanol can be recovered by simple distillation of the desulfurization solution, and Fe-IL is separated from water and recycled to reuse, and the product H2SO4dissolved in desulfurization solution is retrieved. The influence factors of the desulfurization process were investigated to optimize the desulfurization conditions for improving the desulfurization performance.

Figure 1 Schematic concept of wet desulfurization by phase separation
2 EXPERIMENTAL
2.1 Materials
Methylimidazole (Mim, purity >99.5%) was provided by Changzhou Zhongkai Chemical Co. Chlorobutane (BuCl, AR) was provided by Beijing Yili Fine Chemical Co. Ethyl acetate (AR) was supplied by Beijing Beihua Fine Chemical Co. Hexahydrated ferric chloride (FeCl3·6H2O, AR) was purchased from Tianjin Fuchen Chemical Reagents Factory. Ethanol, hydrochloric acid and ammonia water (17%, by mass) were all AR reagent from the market. Karl-Fischer reagent was provided by Sinopharm Chemical Reagent Co. The pure nitrogen and oxygen, SO2with 0.25% and 0.47% (carried with N2) were provided by Beijing Zhongkehuijie Analysis Technology Co. Sulfuric acid (H2SO4) was supplied by Beijing Chemical Factory, AR. All chemical agents were used directly without any pretreatment.
2.2 Preparation of Fe-based ionic liquid
The Fe-based ionic liquid (Fe-IL, [Bmim]FeCl4) was synthesized by two steps as previously reported [15, 16]. The first step was to prepare 1-butyl-3-methylimidazolium chloride (BmimCl). Chlorobutane and methylimidazole were mixed and stirred at 70 °C for 72 h. After washed with isovolumetric ethyl acetate for 3-5 times and distillated in vacuum at 70 °C, a light yellow liquid of the product [Bmim]Cl was obtained. The second step was to prepare Fe-IL by mixing [Bmim]Cl and FeCl3·6H2O at molar ratio of 1∶2 in air for 12 h. A two-phase system was got after centrifugation, the dark green upper layer was so-called the Fe-based ionic liquid.
2.3 Construction of phase diagram of Fe-based ionic liquid-ethanol-water system
The ternary system of Fe-based ionic liquid, ethanol and water with different mass fractions was put into a thermostatic oscillation incubator and mixed adequately at 40 °C for 12 h. The accuracy of temperature was ±0.5 °C. Then, the equilibrium mixture was kept still for ca. 12 h to separate liquid-liquid two phases clearly.
The liquid-liquid equilibrium data for the ternary mixture were obtained by determining the concentrations of the three compounds in each phase. The water fractions were measured by Karl-Fischer moisture analyzer, and the Fe-based ionic liquid content was measured by ultraviolet/visible spectrophotometer at 192 nm, and then the content of ethanol was calculated according to the mass balance. The triangular phase diagram of the ternary system was drawn based on these measurements, with which the suitable composition of the desulfurization medium was to be determined.
2.4 Desulfurization process
The desulfurization experimental set-up was shown in Fig. 2 (a). The ternary desulfurization solution was prepared by mixing Fe-based ionic liquid, water and ethanol with different volume ratios. The pH of the ternary desulfurization solution was controlled by a buffer solution of HCl-NH4Cl. A 50 ml desulfurization solution was put into the reactor. The structure and the size of the reactor were respectively shown in Fig. 2. The reaction temperature was kept at 40 °C with a water jacket connected to a water-bath. The initial SO2concentration was 32.5 g·m−3, the O2fraction was 10% and the gas flow rate was 150 ml·min−1, respectively. After the temperature and the gas flow were stable, the gas stream was switched to the reactor to start the desulfurization process, and the performance was evaluated by the concentration ofSO2measured by iodometry as ctin the tail gas. The desulfurization efficiency η was calculated by original concentration of SO2, c0, refer to ct, as the following equation required:
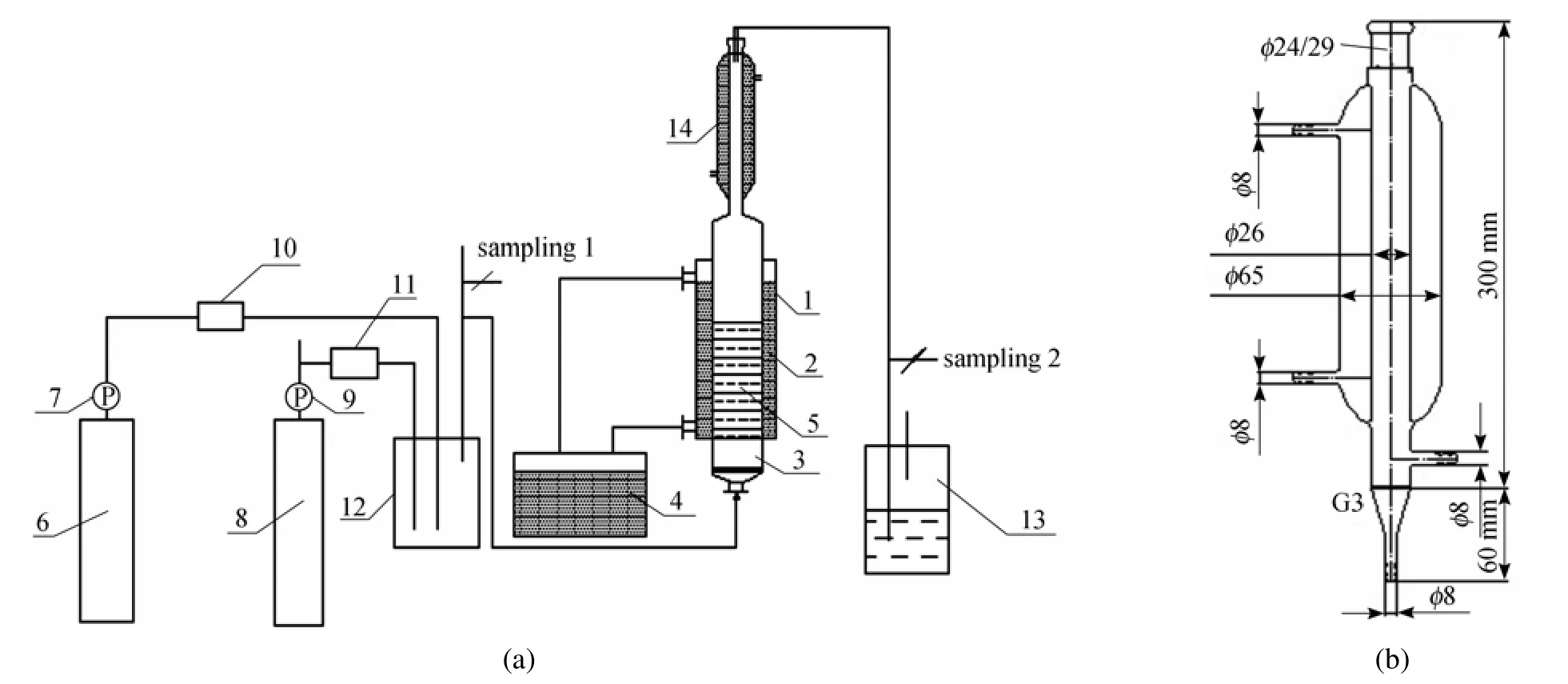
Figure 2 The set-up of desulfurization experiment (a) and the size of reactor 1 (b)
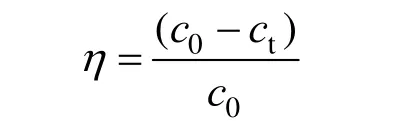
3 RESULTS AND DISCUSSION
3.1 The ternary phase diagram of Fe-based ionic liquid + ethanol + water atT=40 °C
Figure 3 is the ternary phase diagram of Fe-IL + ethanol + water at T=40 °C. It is clear that the Fe-IL is hydrophobic and immiscible with water, so that there existes a biphase sector in the phase diagram. With increase of ethanol in the biphasic mixture of Fe-IL and water, a homogenous solution of Fe-IL + ethanol + water can be obtained. Conversely, two phases composed of Fe-IL and water can be restored after ethanol is removed from the ternary system. All of these provide the base to implement the concept presented in Fig. 1. According to the wet desulfurization mechanism of SO2in aqueous solution [9, 10], it is thought that Fe(III) in Fe-IL can oxidize SO2to H2SO4with the presence of O2and H2O in the desulfurization solution. The complete miscibility of Fe-IL in water with the cosolvent of ethanol according to Fig. 3 ensures that not only the wet desulfurization can proceed efficiently in aqueous solution, but also Fe-IL as catalyst can be recovered and reused after ethanol is removed from the desulfurization solution. It completely meet the demand of the new concept of SO2desulfurization in Fig. 1.
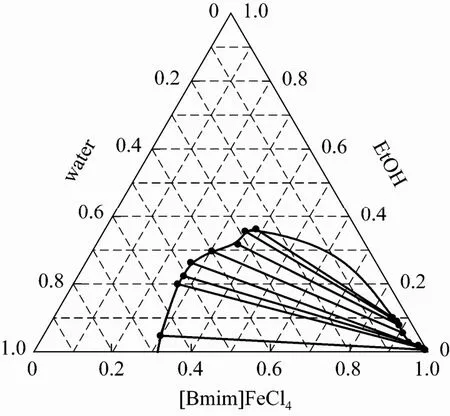
Figure 3 Ternary phase diagram for Fe-based ionic liquid + ethanol + water at T=40 °C
3.2 Wet desulfurization with ternary Fe-based ionic liquid, ethanol and water system
Wet SO2desulfurization in aqueous solution is a complex process, including absorption of SO2from gas phase into liquid phase, mass transfer in liquid phase, catalytic oxidization, and regeneration of catalyst, and even product separation, which involves many operation parameters including initial SO2concentration, gas flow rate, reaction temperature, pH and composition of desulfurization solution, etc.
3.2.1Effect of SO2concentration
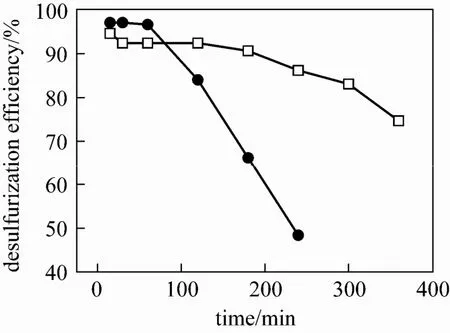
Figure 4 Desulfurization efficiency as a function of reaction time with different SO2concentration (VFe-IL∶Vwater∶Vethanol=1∶1.5∶3, SO2: 32.5 g·m−3, O2: 10%, pH=2.0, T=40 °C)□ 32.5 g·m−3; ● 60.5 g·m−3
The effect of SO2concentration on the desulfurization efficiency is shown in Fig. 4. The desulfurization efficiency went down with increase of reaction time. In fact, the SO2desulfurization efficiency is affected mutually by the SO2absorption and the regeneration rate of Fe(II) to Fe(III). If the SO2absorption rate is slow and the catalyst can be regenerated quickly, the whole desulfurization process is controlled by absorption. If the absorption is quick and the catalyst regeneration rate is slow, the whole desulfurization process is controlled by the catalyst regeneration. Whatever happens, the increase of SO2initial concentration can lead to a significant decrease in desulfurization efficiency. In Fig. 3, the desulfurization efficiency was more than 90% at the first hour even the SO2initial concentration was increased from 32.5 g·m−3to 60.5 g·m−3. It is due to the high concentration of the catalyst Fe(III) concentration at the initial desulfurization stage, which implies that the catalyst Fe(III) plays the active role in controlling the whole desulfurization process. The desulfurization efficiency decreased quickly after an hour of desulfurization when the concentration SO2was 60.5 g·m−3because the catalyst Fe(III) was consumed quickly and the its regeneration rate was too slow to match the demand of the SO2oxidation.
3.2.2Effect of reaction temperature
The dissolution process of SO2and O2from gas phase to liquid phase as well as the wet catalytic desulfurization was closely related with reaction temperature. Increasing temperature was not good for the solubility of O2and SO2in desulfurization solution, but could promote the catalytic chemical reaction rate. Fig. 5 showed that the desulfurization performances were similar at 30 °C, 40 °C and 50 °C, while the desulfurization efficiency went down quickly at 60 °C.
3.2.3Effect of flow rate
The relationships between desulfurization efficiency and time with different gas flow rate are shown in Fig. 6. Evidently, lower gas flow rate meant longer residence time, thus it was good for complete absorption of SO2into liquid phase and could keep the desulfurization efficiency at a high level for longer period. In this work, the optimal gas flow rate was 150 ml·min−1.
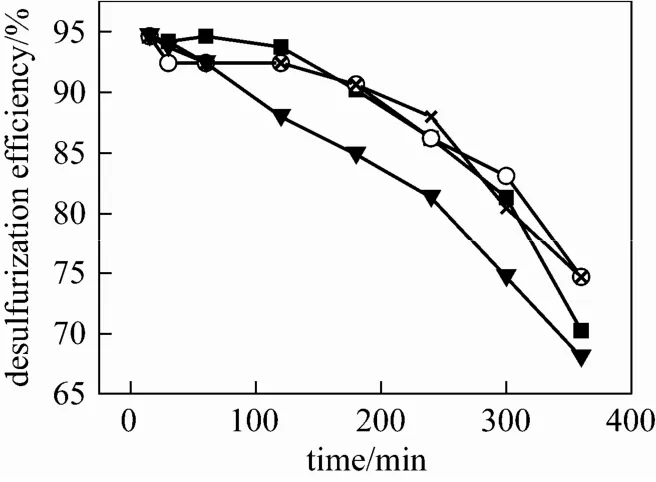
Figure 5 Desulfurization efficiency as a function of reaction time with different temperature (VFe-IL∶Vwater∶Vethanol= 1∶1.5∶3, SO2: 32.5 g·m−3, pH=2.0, O2: 10%)■ 30 °C; ○ 40 °C; × 50 °C; ▼ 60 °C

Figure 6 Desulfurization efficiency as a function of reaction time with different gas flow rate (VFe-IL∶Vwater∶Vethanol=1∶1.5∶3, SO2: 32.5 g·m−3, O2: 10%, pH=2.0, T= 40 °C)○ 150 ml·min−1; △ 200 ml·min−1; ▼ 300 ml·min−1
3.2.4Effect of O2loading
Figure 7 shows the effect of O2partial pressure on the desulfurization efficiency. There was a significant difference on the desulfurization performances with or without O2loading into the feed stream. It could be kept at approx. 100% for 250 min with O2loading, while it went down quickly (less than 90%) without O2loading after 120 min. Therefore, O2played an active role in the wet SO2desulfurization process. It was because of the regeneration of Fe-ILinduced by O2, and then the SO2desulfurization could be run continuously and effectively with higher concentration of Fe(III) ions. Without O2loading, Fe(III) was depleted gradually to result in decreasing desulfurization efficiency.

Figure 7 Desulfurization efficiency as a function of reaction time with/without O2loading (VFe-IL∶Vwater∶Vethanol= 1∶1.5∶3, SO2: 32.5 g·m−3, O2: 10% and 0, pH=2.0, T=40 °C)□ without O2; ● with O2
3.2.5Effect of pH
The pH value also is an important factor in the desulfurization process [4], because it affects the SO2solubility in desulfurization solution. When pH was greater than 2.5, Fe(III) would be hydrolyzed to produce precipitation, which reduced the content of active Fe(III) for desulphurization. The desulfurization performances were evaluated at pH 1.0, 1.5 and 2.0. As Fig. 8 shows, the desulfurization efficiency drops quickly with time at pH 1.0 and 1.5 because of low solubility of SO2in strongly acidic solution. The initial pH value was set at 2.0 in this experiment.

Figure 8 Desulfurization efficiency as a function of reaction time at different pH (VFe-IL∶Vwater∶Vethanol=1∶1.5∶3, SO2: 32.5 g·m−3, O2: 10%; T=40 °C)■ pH 2.0; ● pH 1.5; ● pH 1.0
3.2.6Effect of water in desulfurization solution

10%, pH=2, T=40 °C)▽ 1∶1.5∶3
SO2must dissolve in the water to form23SO−and HSO3−, and then can be catalytically oxidized by O2with Fe(III) catalyst. Therefore, water is necessary when conducting desulfurization with Fe-IL as catalyst. According to Fig. 9, the desulfurization efficiency went down sharply if there was not any water or a little water in desulfurization solution, e.g. VFe-IL∶Vwater∶Vethanolat 1∶0∶3 or 1∶0.5∶3. When the water fraction increased to 27.2%, namely, the desulfurization solution composed of Fe-IL, water and ethanol was 1∶1.5∶3 by volume ratio, the desulfurization efficiency greater than 90% could be obtained and kept for 360 min.
3.2.7Effect of product sulfuric acid
Sulfuric acid is the only product in this desulfurization process. After ethanol is removed from the ternary desulfurization solution, Fe-IL can automatically separate from the water phase. As the desulfurization reaction run, the pH values of the desulfurization solution went down from 2.0 to 1.2 [Fig. 10 (a)], which implied that SO2had been oxidized to sulfuric acid [Fig. 10 (b)]. The concentration of sulfuric acid gradually went up to ca. 4.2% after 240 min, while the desulfurization efficiency went down to from 98.8% to ca. 95.8%. Therefore, the product of sulfuric acid had an inhibitory effect on the desulfurization performance.
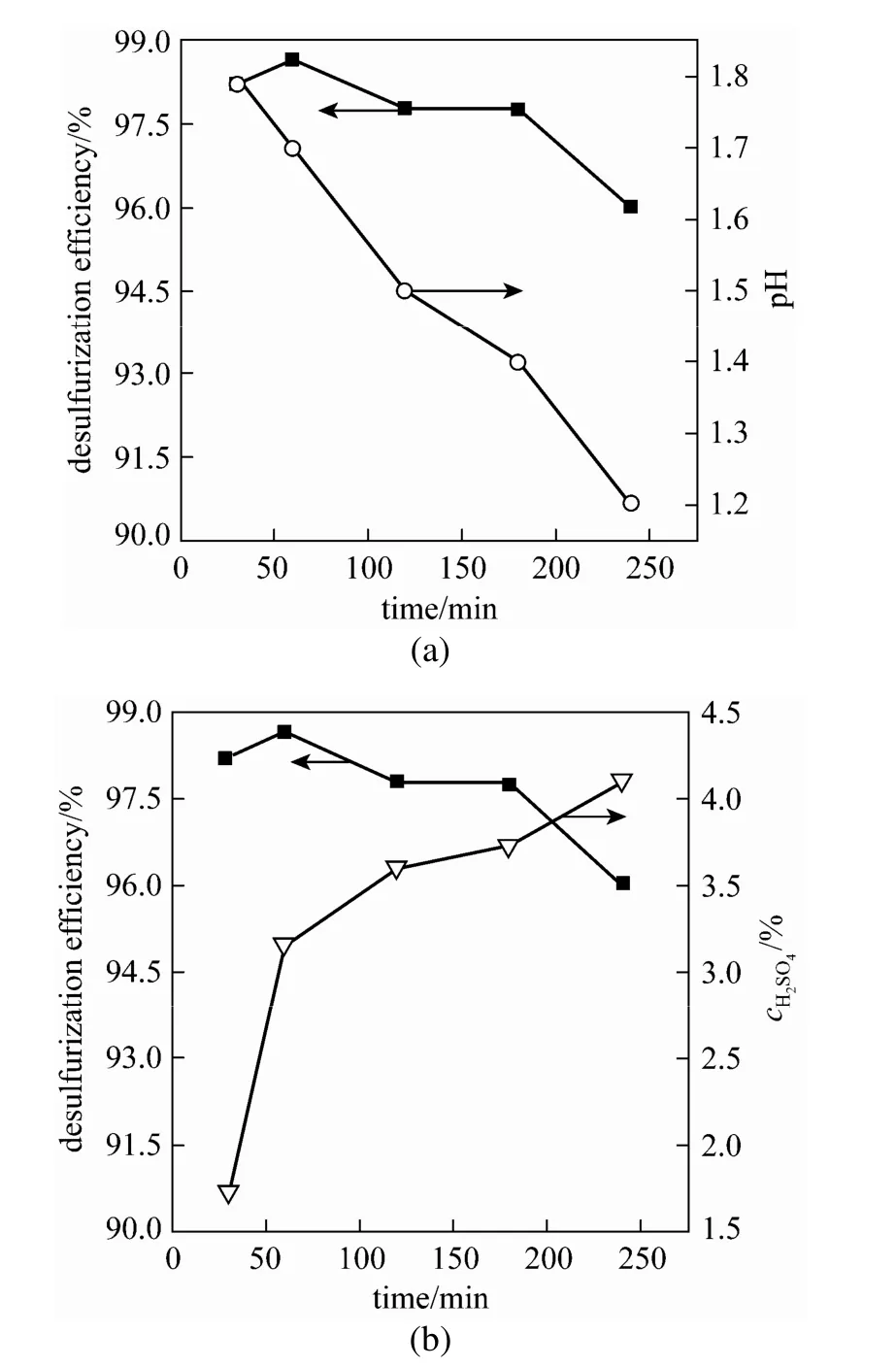
Figure 10 Effect of product sulfuric acid on desulfurization efficiency (VFe-IL∶Vwater∶Vethanol=1∶1.5∶3, SO2: 32.5 g·m−3, O2: 10%, T=40 °C)
4 CONCLUSIONS
In this paper, BmimFeCl4(Fe-IL) is used ascatalyst and mixed with ethanol and water to prepare a ternary desulfurization solution for wet flue gas desulfurization:
(1) The ternary phase diagram of Fe-IL, ethanol and water was investigated and the suitable ternary wet desulfurization solution composed of Fe-IL, water and ethanol by volume ratio at 1∶1.5∶3 was proposed.
(2) The effects of flow rate and concentration of SO2, reaction temperature, pH and the water fraction in the desulfurization solution on the desulfurization efficiency were investigated. A ternary desulfurization solution at 1∶1.5∶3 (water volume fraction, 27.2%) by volume ratio was applied to obtain the desulfurization rate greater than 90% at 40 °C with initial pH 2.0. The product of sulfuric acid had inhibition effect on wet desulfurization performance.
With application of this new ternary desulfurization solution, not only the catalyst of Fe-based ionic liquid can be recycled and reused, but also the product of sulfuric acid can be separated directly from the ternary desulfurization solution by removing ethanol.
NOMENCLATURE
ctSO2concentration in tail gas, g·m−3
c0SO2initial concentration, g·m−3
T reaction temperature, °C
t reaction time, min
V desulfurization solution volume, ml
η the desulfurization efficiency, %
REFERENCES
1 Jiang, X.C., Nie, Y., Li, C.X., Wang, Z.H., “Imidazolium-based alkylphosphate ionic liquids—a potential solvent for extractive desulfurization of fuel”, Fuel,87, 79-84 (2008).
2 Jiang, J.H., Li, Y.H., Cai, W.M., “Experimental and mechanism research of SO2removal by cast iron scraps in a magnetically fixed bed”, J. Hazard. Mater.,153, 508-513 (2008).
3 Slimane, R.B., Abbasian, J., “Copper-based sorbents for coal gas desulfurization at moderate temperatures”, Ind. Eng. Chem. Res.,39, 1338-1344 (1997).
4 Fan, M.H., Zhuang, Y.H., “Research on a new desulphurization process with FeSO4solution as absorbent”, Environ. Sci.,19(1), 5-8 (1995). (in Chinese)
建设农残检测室,提升市场监管水平。建立农残检测室是海南省委2015年十大“为民办实事”项目之一,福山食品药品监管所积极响应号召,全力投入到福山农贸市场农残免费检测室的建设工作中。该所加强沟通,多次赶赴现场了解情况,解决存在的问题,保障了农残检测室的建设。目前该检测室已投入使用,配备两名专业检测人员,一台农残快速检测仪,其他配套用具齐全。检测室每日固定抽样量10个,消费者可通过LED屏了解所购买的蔬菜农药残留情况。
5 Kraft, J., van Eldik R., “Kinetics and mechanism of the iron(III) -catalyzed autoxidation of sulfur(IV) oxides in aqueous solution. 1. Formation of transient iron(III) -sulfur(Pc) complexes”, Inorg. Chem.,28, 2297-2305 (1989).
6 Conklin, M.H., Hoffmann, M.R., “Metal ion-sulfur(IV) chemistry. 3. Thermodynamics and kinetics of transient iron(HI)-sulfur(IV) complexes”, Environ. Sci. Technol.,22(8), 899-907 (1988).
7 Olywen, G.H., Olywen, S., Coordination and Catalysis, Verlag Chemie, Weinheim-New York, 88-95 (1977).
8 Brandt, C., van Edik. R., “Transition metal-catalyzed oxidation of sulfur(IV) oxides. atmospheric-relevant processes and mechanisms”, Chem. Rev.,95(1), 119-190 (1995).
9 Freiberg, J., “The mechanism of iron catalyzed oxidation of SO2in oxygenated solutions”, Atmos. Environ.,9(6-7), 661-672 (1975).
10 Butler, A.D., Fan, M.H., Brown, R.C., Cooper, A.T., van Leeuwen, J.H., Sung, S., “Absorption of dilute SO2gas stream with conversion to polymeric ferric sulfate for use in water treatment”, Chem. Eng. J.,98, 265-273 (2004).
12 Anderson , J.L., Dixon, J.K., Maginn, E.J., Brennecke, J.F., “Measurement of SO2solubility in ionic liquids”, J. Phys. Chem. B,110(31), 15095-15062 (2006).
13 Rogers, R.D., Seddon, K.R., “Ionic liquids-solvents of the future?”Science,302, 792-793 (2003).
14 Jiang, Y.Q., Zhu, W.S., Li, H.M., Yin, S, Liu, H, Xie, Q.J., “Oxidative Desulfurization of Fuels Catalyzed by Fenton-Like Ionic Liquids at Room Temperature”, CHEMSUSCHEM,4(3), 399-403 (2011).
15 He, Y., Yu, J., Chen, L.B., “Wet oxidation desulfurization of hydrogen sulfide with application of Fe-based ionic liquid”, CIESC J.,61(4), 963-968 (2010). (in Chinese)
16 Yao, R.S., Li, P.P., Sun L.L., He, Y., Chen, L.B., Yu, Y., Mu, R., Yu, J., “Physicochemical properties of iron-based chloride imidazole ionic liquid and wet desulfurization mechanism of hydrogen sulfide”, J. China Coal Soc.,36(1), 135-139 (2011) . (in Chinese)
2011-08-14, accepted 2011-11-16.
* Supported by the National Natural Science Foundation of China (21076019, 90610007), the National High Technology Research and Development Program of China (2007AA06Z115), the Ph.D. Programs Foundation of Ministry of Education of China (20090010110003), the Fundamental Research Funds for the Central Universities (ZD1001).
** To whom correspondence should be addressed. E-mail: yujiang@mail.buct.edu.cn
——电影《郭福山》主题歌(男中音独唱)
 Chinese Journal of Chemical Engineering2012年1期
Chinese Journal of Chemical Engineering2012年1期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Simultaneous Removal of Thiophene and Dibenzothiophene by Immobilized Pseudomonas delafieldii R-8 cells*
- Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- The Research Progress of CO2Capture with Ionic Liquids*
