TIME-DEPENDENT FTIR SPECTRAL CHANGES IN RATS OF MASSIVE HEMORRHAGE DEATH DURING THE LATER POSTMORTEM
LI Shi-ying,SHAO Yu,LI Zheng-dong,LI Li,CHEN Yuan-yuan,CHEN Yi-jiu,HUANG Ping
(1.Shanghai Key Laboratory of Forensic Medicine,Institute of Forensic Science,Ministry of Justice,P.R.China, Shanghai 200063,China;2.Department of Forensic Medicine,Shanghai Medical College,Fudan University, Shanghai 200032,China)
TIME-DEPENDENT FTIR SPECTRAL CHANGES IN RATS OF MASSIVE HEMORRHAGE DEATH DURING THE LATER POSTMORTEM
LI Shi-ying1,2,SHAO Yu1,2,LI Zheng-dong1,LI Li1,CHEN Yuan-yuan1,CHEN Yi-jiu1,HUANG Ping1
(1.Shanghai Key Laboratory of Forensic Medicine,Institute of Forensic Science,Ministry of Justice,P.R.China, Shanghai 200063,China;2.Department of Forensic Medicine,Shanghai Medical College,Fudan University, Shanghai 200032,China)
The aim of the current study was to investigate the spectra in the different organs of the rats which died of massive hemorrhage;to explore their spectral changes 15 days postmortem and the best mathematical model with different band absorption ratio changes to postmortem interval(PMI);and to compare the spectral changes of different temperature.Thirty male Sprague-Dawley rats were sacrificed by cutting abdominal aorta,and the cadavers were divided equally and kept at 4℃,20℃ and 30℃ in the control chamber.From the same rat,seven different organs were sampled at intervals of 1-15 days postmortem,and then measured by Fourier transfom infrared(FTIR)spectrometer.Six mathematical model functions were explored.The absorbance of bands and band absorbance ratios of absorption peak in each organ showed a time-dependent increase or decrease,most band absorbance ratios remaining stable for 7-15 days postmortem.Cubic model functions of the various bands absorbance ratios against PMI showed a stronger related coefficient.The absorbance bands with obvious changes at 20℃ showed stabilized tendencies at 4℃ and significant changes at 30℃ within 15 days postmortem.In addition,FTIR spectroscopy revealed a time-dependent metabolic process,with potential of being used to estimate PMI during 7 days postmortem,which merits further investigation.
forensic pathology;spectroscopy,Fourier transform infrared;hemorrhage;postmortem interval; rats
1 Introduction
For several decades,forensic pathologists have been seeking for a quantitative,objective method to estimate postmortem interval(PMI),especially in the cases of the crime elucidation.To date,many techniques have been employed to determine PMI,but most of them are time-consuming and cannot be performed in situ.Some methods,therefore,are based on subjective evaluation,such as livor mortis,rigor mortis and putrefaction.If the postmortem phenomenon does not change significantly,the estimation of PMI in the later postmortem period mainly relies on the subjective evaluation.In addition,these methods determine PMI with a few or just one specific parameter such as a decrease in body temperature,metabolites of fats,proteins and nucleic acids[1-3].Therefore,the major advantage of attenuated total reflection(ATR)Fourier transform infrared(FTIR)spectroscopy technique is the possibility of real time detection through a non-invasive and non-destructive investigation[4-5].
Our previous studies involving the use of KBr disk and ATR FTIR spectroscopy revealed time-dependentpostmortem changesin the liver,spleen, kidney,and other different organs in rats and humans.The absorbance ratios of the various bands were investigated to find the best fit with the cubic model function in the rats,and the comparison of their spectra with selected human postmortem cases showed similar metabolic changes[6-9],which were reported to extend only to 144 or 168 hours in the postmortem evaluation.However,the longer term postmortem changes need to be quantitatively extrapolated over 7 days after death.In the current study, the postmortem FTIR spectral changes extended to 15 days involving the brain,heart,lung,liver, spleen,kidney cortex and muscle.
The violent cause of death can be caused by any injury and intoxication,and the most common ones are found to be craniocerebral trauma,asphyxia, hemorrhage in forensic practice.Our previous study reported the time-dependent FTIR spectral changes in rat occipito-cervical injury model[6,7,9-10].Massive hemorrhage is due to the loss of a large amount of blood,ultimately affecting the multiple organs and biochemical components in tissues.Yet it remains unclear whether FTIR spectra changes in the rats of hemorrhagic death are associated with those in occipito-cervical trauma death.
The objective of the current study was to investigate the FTIR spectral changes in 7 different organs during 15 days postmortem in the rat cadavers of hemorrhagic shock death;to relate these changes to PMI and explore the best mathematical model with different band absorption ratio changes to PMI; and tocomparethespectral changes ofdifferent temperature.
2 Materials and methods
2.1 Animal specimens
Ten male Sprague-Dawley rats weighing 240-260 g,provided by the Animal Center of Shanghai Jiao Tong University,were sacrificed by cutting the abdominal aorta so that cardiorespiratory failure occurred,and the cadavers were kept at (20±2)℃ in a controlled environment chamber.From the rats,the brain,heart,lung,liver,spleen,kidney cortex and muscle samples were derived at time zero,1 d,2 d, 3 d,5 d,6 d,7 d,9 d,11 d,13 d and 15 d.Twenty male Sprague-Dawley rats weighing 240-260 g were sacrificed using the same method and kept at 4℃ and 30℃ chambers for the following experiment, each group consisting of 10 rats.All the animal experiments wereperformed in accordancewith the principles for the Care and Use of Laboratory Animal Committee of IFS.
2.2 Sample preparation
The specimens 1 mm×1 mm×1 mm in size were washed with phosphate-buffered saline thrice before placed into cylindrical tubes to be frozen immediately in liquid nitrogen.
2.3 FTIR spectral measurements
ATR accessory(Pike Technologies,Madison,WI, USA)was linked to a Shimadzu 8400S FTIR spectrometer(Shimadzu Inc.,Tokyo,Japan).ZnSe crystal plate was a single-bounce ATR accessory with IR throughput.The angle of incidence was 45°.Hardness was 1 150 kg/mm2and the pH range of sample was 1-12.The MIRacle as the ATR accessory was used for pressuring clamp.The spectrometer was kept in a warm state prior to use minimize instability, and was continuously purged with dry air to eliminate atmospheric water vapor.For a measurement of IR absorbance 4 000-400 cm-1,100 scans were coadded to attain a frequency resolution of 4 cm-1,in which the acquisition and processing time was approximately 2 min.IR solution 1.10 software(Shimadzu Inc.,Tokyo,Japan)was used to analyze the spectra and record the data including the calculations of peak position,band intensity and area.Baseline correction was done for all the original spectra.Axrepresents the infrared absorbance at wave-number x cm-1.The baseline points were set at minima near 4 000,3 700, 2700,1800 and 900cm-1.The main band absorbances were observed(Table 1).

Table 1 Identification of main FTIR absorption peaks
2.4 Data analysis
Statistical analysis was conducted by evaluating the mean value of band absorbance ratio.The experimental replicates were averaged and the±s were calculated foreach time point.Curve estimation analysis between band absorbance ratios and PMI were performed to gain the best mathematical model function.Six mathematical model functions were explored (linear,quadratic,cubic,compound,growth, exponential),and P values of less than 0.05 were regarded as being statistical significant.All the statisticalanalysisofthedatawasperformed using SPSS 17.0 software(SPSS Inc.,USA).
3 Results
The spectral changes of the brain,heart,lung, liver,spleen,kidney cortex and muscle samples were similar.Each of them showed three different spectral band absorbance tendencies during the postmortem period from time zero to 15d at 20℃ in the rats of massive hemorrhage death,an increase in A3012,A2958, A2925,A2871,A2852,A1541,A1396;a decrease in A1238,A1080, A1040(liver);and maintained stability in A3303,A1652, A1456,A1338,A1313.As an example,the spectral changes of liver showed in detail.The FTIR spectra absorption peaks were observed in the liver(Fig.1).The band absorbance ratios showed an increase:A3012/A3303, A2958/A3303,A2925/A3303,A2871/A3303,A2852/A3303,and a decrease:A1652/A1541,A1652/A1396,A1541/A1396,A1456/A1396, A1238/A1396,A1080/A1396(Fig.2).
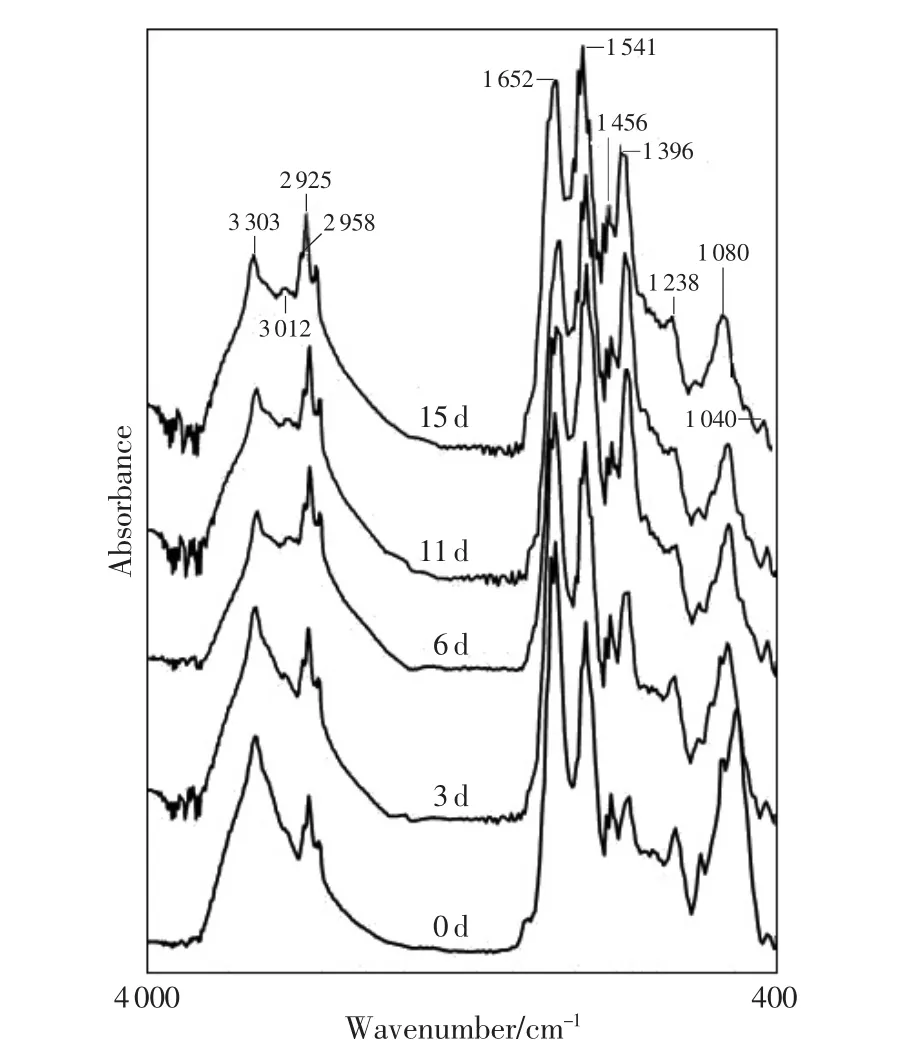
Fig.1 FTIR spectra absorption peaks changes trend in the rat liver postmortem
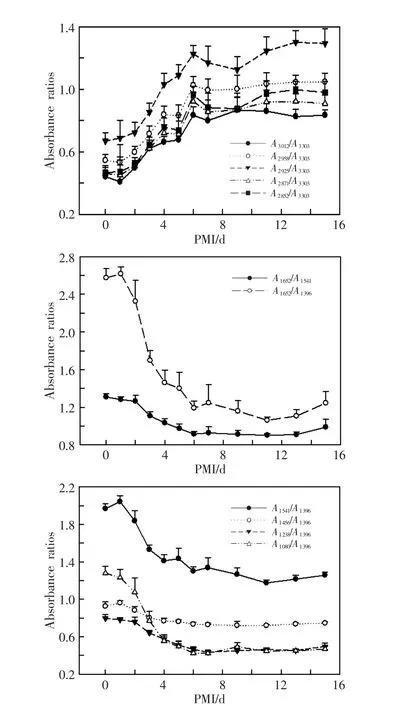
Fig.2 FTIR spectra absorbance ratios changes trend in the rat liver postmortem
In 7 organs,most band absorbance ratios showed the plateau stage after 6 or 7 days postmortem and didn’t significantly change from 6 d or 7 d to 15 d. But the band absorbance ratios at 3 600-2 800 cm-1region in the brain,heart and kidney cortex samples still showed increasing tendencies,which were significantly slower when compared with those within 6 days postmortem.
Six mathematical model functions showed the relation between absorbance ratios and PMI(Fig.3),and the cubic model functions were presented(Table 2). The cubic model function showed a better correlation than the other models(P<0.05).A1652/A1396and A1080/A1396showed the best correlation in the liver at 20℃ with the highest coefficient of determination(0.903,0.909).
The absorbance bands with obvious changes at 20℃ showed stabilized tendencies at 4℃ and significant changes at 30℃ within 15 days postmortem. For example,the spectra of the liver and kidney cortex were observed at three different temperatures on 6d postmortem(Fig.4 and 5).The spectra at 30℃within 3 days postmortem showed the similar patterns as those observed at 20℃ within 7 days postmortem, which may suggest that the changes of spectra are under the influence of temperature.
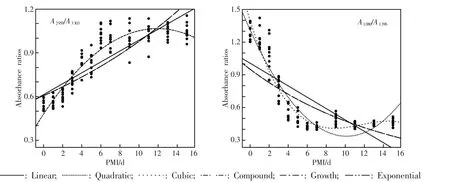
Fig.3 Model functions of absorbance ratios in the rat liver postmortem

Table 2 The cubic model functions used to describe the time courses of various absorbance ratios in the rat liver postmortem
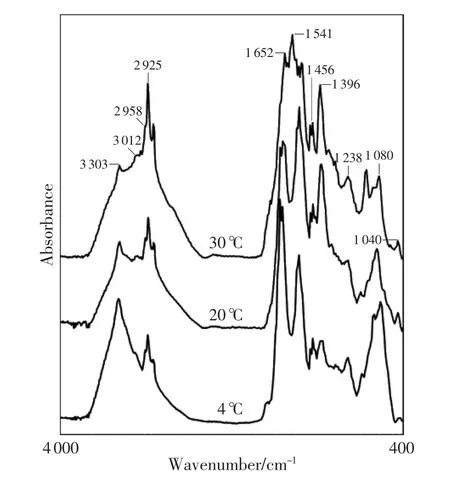
Fig.4 In different environmental temperature,6d after the rats’death,FTIR spectra of liver

Fig.5 In different environmental temperature,6d after the rats’death,FTIR spectra of kidney cortex
4 Discussion
4.1 Early studies of postmortem metabolic changes
In our previous studies[6,7,9-13]involving KBr disk and ATR FTIR spectroscopy methods have revealed time-dependent postmortem changes in liver,spleen, kidney,and others in rats and humans.The ATR, KBrspectralchangesand absorbanceratioswere found to be similar in both humans and rats.The previous study showed time-dependent FTIR spectral changes in the rats of craniocerebral injury death during 7 dayspostmortem,whose majorchanges were manifest,as indicated by an increase in A3012, A2958,A2925,A2871,A2852,A1541,A1396and a decrease in A1238,A1153,A1080,A1040,and maintained stability in A3303,A1652,A1338,A1313.Curve estimation analysis between band absorbanceratiosand PMIwasperformed to obtain the best mathematical model function.The cubic model function showed a better correlation than others.
The results reported in the preliminary studies showed that the changes in rat occipito-cervical injury model were significant.The former studies extended only to 144 hours or 168 hours of death. However,the longer term postmortem changes need to be quantitatively extrapolated,i.e.,over 7 days after death.In the current study,the postmortem FTIR spectral changes extended to 15 days in the brain, heart,lung,liver,spleen,kidney cortex and muscle samples.Our recent study reported the time-dependent FTIR spectral changes in rat occipito-cervical injury model.Massive hemorrhage is due to the loss of a large amount of blood,affecting the organ systems and the biochemical components in tissues.Yet it remains unclear whether FTIR spectra changes in rats of massive hemorrhagic deaths are associated with those in occipito-cervical trauma death.
4.2 The current study
4.2.1 The changes ofabsorption bands and absorbance ratios
There was an obvious increase in the A3012,A2958, A2925,A2871and A2852where the spectrum was populated by absorptions arising from the C-H stretching vibrations.Allfive absorbance ratiosshowed the changes of saturated fat content and unsaturated fat content[14-16].There was a dramatic increase in the area of the 5 bands after death.The changes in the C-H stretching region may be explained by the growth of bacteria,which lead to an increase of lipid synthesis to form the bacterial membrane.
The band at 1 396 cm-1was attributed to COO-vibrations of fatty acid.This absorption band area showed an increase after death.The absorbance at 1541cm-1assigned to amideⅡincreased after death, which suggested a contradiction with the postmortem protein degradation theory[17].In this case,the increase of amideⅡ postmortem was probably the accumulation of new metabolites due to complicated postmortem chemical reactions.Thus,it is worthwhile to further study the increase of this band absorbance using other methods.The absorbance of the bands at 3303,1456,1338,1313cm-1.These changes all showed the corresponding chemical substance degradation processes after death[18].The bands at 1 238 cm-1and 1 080 cm-1arose mainly from the antisymmetric and symmetric stretching modes,respectively,of phosphodiester groups in nucleic acids rather than in phospholipids[17].The absorbance of these two bands displayed dramatic reduction,which could be used to study the degrading of nucleic acid.The band at 1040cm-1was assigned to the C-O stretching vibration of polysaccharide[19].It appeared in the liver and its absorbance decreased as time progressed.Our inference is that the effects of phosphorylase enzyme and debranching enzyme may cause the phosphorylation and hydrolyzation of hepatic glycogen,the activity of which can be kept for a period of time to degrade the glucogen.
The changes of the following absorbance ratios of A3012/A3303,A2958/A3303,A2925/A3303,A2871/A3303,A2852/A3303increased progressively,while those of A1652/A1541, A1652/A1396,A1541/A1396,A1456/A1396,A1238/A1396,A1080/A1396declined with the time.
4.2.2 The impact of temperature
In the current study,the temperature was controlled at 4℃,20℃ and 30℃.In each case,the results were found to be similar,i.e.,the same trends of the band absorbance ratios to grow or decline. The plateau stage,however,appeared earlier at 30℃, which may be explained by the higher temperature speeding up the decomposition. The absorbance bands with obvious changes at 20℃ produced a stable trend at 4℃ within 15 days postmortem and a significant change at 30℃,which may suggest that postmortem metabolic changes are heavily dependent on environmental temperature.
4.2.3 The analysis of the plateau stage
From the changes of the band absorbance ratios in each organ developed the plateau stage on the 7th day,which could be hypothesized as follows:1)Due to the decreasement in the degradation rate after 7 days,the changes of the functional groups were not obvious,and those of the band absorbance ratios were no longer great,which could lead to a smooth trend in general;2)The tissues degraded completely after 7 days,the device couldn’t accurately measure the slight changes;3)The individual difference of the samples.
5 Conclusion
Based on the results,wecompared different FTIR spectral changes in the rats of massive hemorrhage death at(20±2)℃ and different time intervals. With the extension of PMI,we found that FTIR spectral changes of 7 organs were significant during 15 days,different organs spectral changes presenting similar trend,and the plateau stage appearing on the 7th day postmortem;that FTIR spectral changes reflected the changes of chemical functional groups, the nucleic acid contentdecreasing dramatically, while the amideⅠ content reduced gradually,the amideⅡ and lipid content increased relatively;that the cubic model function best showed a correlation; that postmortem metabolic changes may be heavily dependenton environmentaltemperature;and that FTIR spectra changes were similar in the rats of massive hemorrhagic deaths and those of occipitocervical trauma death.
There are several contributing factors that can influence tissue chemical reaction,such as temperature,humidity,cause of death.Therefore further investigations are needed in different tissues and organs under various conditions.
Acknowledgements
This study was funded by the Council of NationalNaturalScience Foundation ofChina (No. 81001350)and the Science and Technology Commission of Shanghai Municipality(No.09ZR1432900). References:
[1] Matuszewski S.Estimating the preappearance interval from temperature in Creophilus maxillosus L.(Coleoptera: Staphylinidae)[J].J Forensic Sci,2012,57(1):136-145.
[2] Bortolotti F,Pascali JP,Davis GG,et al.Study of vitreous potassium correlation with time since death in the postmortem range from 2 to 110 hours using capillary ion analysis[J].Med Sci Law,2011,51(S1):S20-S23.
[3] Zhou C,Byard RW.Factors and processes causing accelerated decomposition in human cadavers-An overview[J].J Forensic Leg Med,2011,18(1):6-9.
[4] Cohenford MA,Rigas B.Cytologically normal cells from neoplastic cervical samples display extensive structural abnormalities on IR spectroscopy:implications for tumor biology[J].Proc Natl Acad Sci U S A,1998,95(26): 15327-15332.
[5] Wong PT,Wong RK,Caputo TA,et al.Infrared spectroscopy of exfoliated human cervical cells:evidence of extensive structural changes during carcinogenesis[J]. Proc Natl Acad Sci U S A,1991,88(24):10988-10992.
[6] Huang P,Su CP,Li SS,et al.Estimation of postmortem intervalusing FTIR spectroscopy in rats’cardiac muscle[J].Fa Yi Xue Za Zhi,2010,26(1):1-5.
[7] Huang P,Tian WP,Yang GD,et al.Study on estimation of postmortem interval using FTIR in rat lung[J]. Guang Pu Xue Yu Guang Pu Fen Xi,2007,27(10): 1962-1965.
[8] Huang P,Tuo Y,Wang ZY.Review on estimation of postmortem interval using FTIR spectroscopy[J].Fa Yi Xue Za Zhi,2010,26(3):198-201.
[9] Huang P,Yang GD,Tuo Y,et al.The analysis of vital reaction following skin injury close to death in mouse by FTIR[J].Guang Pu Xue Yu Guang Pu Fen Xi,2007,27(6):1074-1076.
[10]Tuo Y,Huang P,Ke Y,et al.Attenuated total reflection Fourier transform infrared spectroscopic investigation of the postmortem metabolic process in rat and human kidney cortex[J].Appl Spectrosc,2010,64(3):268-274.
[11]Huang P,Ke Y,Lu Q,et al.Analysis of postmortem metabolic changes in rat kidney cortex using Fourier transform infrared spectroscopy[J].Spectroscopy,2008, 22(1):21-31.
[12]Huang P,Tian W,Tuo Y,et al.Estimation of postmortem interval in rat liver and spleen using Fourier transform infrared spectroscopy[J].Spectrosc Lett,2009, 42(2):108-116.
[13]Li SY,Shao Y,Li ZD,et al.Relationship between PMI and fourier transform infrared spectral changes in muscle of rats after death caused by mechanical asphyxial[J].Fa Yi Xue Za Zhi,2012,28(3):161-166.
[14]Diem M,Romeo M,Boydston-White S,et al.A decade of vibrational micro-spectroscopy of human cells and tissue(1994-2004)[J].Analyst,2004,129(10):880-885.
[15]Dogan A,Ergen K,Budak F,et al.Evaluation of disseminated candidiasis on an experimental animal model:a fourier transform infrared study[J].Appl Spectrosc, 2007,61(2):199-203.
[16]Fernandez DC,Bhargava R,Hewitt SM,et al.Infrared spectroscopic imaging for histopathologic recognition[J]. Nat Biotechnol,2005,23(4):469-474.
[17]Toyran N,Lasch P,Naumann D,et al.Early alterations in myocardia and vessels of the diabetic rat heart:an FTIR microspectroscopic study[J].Biochem J, 2006,397(3):427-436.
[18]Toyran N,Zorlu F,Severcan F.Effect of stereotactic radiosurgery on lipids and proteins of normal and hypoperfused rat brain homogenates:a Fourier transform infrared spectroscopy study[J].Int J Radiat Biol,2005, 81(12):911-918.
[19]Cakmak G,Togan I,Ugˇuz C,et al.FT-IR spectroscopic analysis ofrainbow troutliverexposed to nonylphenol[J].Appl Spectrosc,2003,57(7):835-841.
date:2012-04-23)
(Editor:LIANG Lu)
DF795.1
A
10.3969/j.issn.1004-5619.2012.04.001
Article IC:1004-5619(2012)04-0241-06
Author:LI Shi-ying(1987—),female,postgraduate in forensic pathology;E-mail:11211010057@fudan.edu.cn
HUANG Ping,male,associate research fellow,associate chief forensic expert,MD,Ph.D in forensic pathology;E-mail:huangpingifs@gmail.com
Corresponding author:CHEN Yi-jiu,male,research fellow, Ph.D tutor in forensic pathology;E-mail:yijiuchen@yahoo.com.cn

