配位聚合物及其在分析传感方面的应用
唐志勇,何 芳,部丽娟,谷铁安,杨大威,傅迎春,谢青季,姚守拙
(湖南师范大学化学生物学及中药分析教育部重点实验室,化学化工学院,湖南长沙410081)
配位聚合物及其在分析传感方面的应用
唐志勇,何 芳,部丽娟,谷铁安,杨大威,傅迎春,谢青季*,姚守拙
(湖南师范大学化学生物学及中药分析教育部重点实验室,化学化工学院,湖南长沙410081)
该文简要综述了配位聚合物(即金属有机框架)及其用于分析传感的近期进展,包括电分析、光分析和色谱分析等方面的应用,引用文献83篇。
配位聚合物;分析传感应用;综述
0 引言
配位聚合物(Coordination Polymer)指具有结构重复单元、含有金属离子配位键的聚合物,主要指金属中心离子与有机配体通过自组装而形成的具有周期性网络结构的金属有机骨架材料[1],有时也叫金属有机框架 (metal organic frameworks,MOFs)、多孔配位网络结构(porous coordination networks,PCNs)、杂化有机-无机材料(hybrid organic-inorganic materials,HOIMs)、或者金属有机聚合物 (metal-organic polymers,MOPs)。与通过共价键形成的常规聚合物不同,配位聚合物的形成主要是通过金属离子与有机配体的配位作用,也涉及到氢键、静电作用、范德华力、π-π堆积作用等分子间弱相互作用力。配位聚合物一词最早由Bailer在上世纪60年代提出[2~3],近年来发展迅速[4]。在ISI Web of Science中,分别以coordination polymers和metal organic frameworks为主题,搜出近十年来出版的论文数,发现该领域的论文数量呈逐年增加趋势(图1)。
因其具有独特的结构和性质,配位聚合物可用于载药和给药[5]、多相催化[6~7]、化学/生物传感[8]及气体储存与分离[9~15]等诸多领域,受到了极大的关注。目前已有一些有关配位聚合物的综述报道[16~26]。但是,配位聚合物的分析传感应用似乎是一个很新的研究领域,鲜见相关综述。故此,该文主要综述配位聚合物及其在分析传感方面的应用。
1 配位聚合物的合成、结构与性能
从配位聚合物主要组分上来看,构筑配位聚合物的配体已经由最初的含氮杂环配体拓展到目前研究比较多的含氧、含磷的多功能配体甚至混合配体[27~43],构筑配位聚合物的金属中心离子也已经从常见的过渡金属离子拓展到主族和镧系元素[44~47]。同时,配位聚合物也从单一的同金属发展到含有两种乃至两种以上金属离子的杂金属配位聚合物[48~53]。
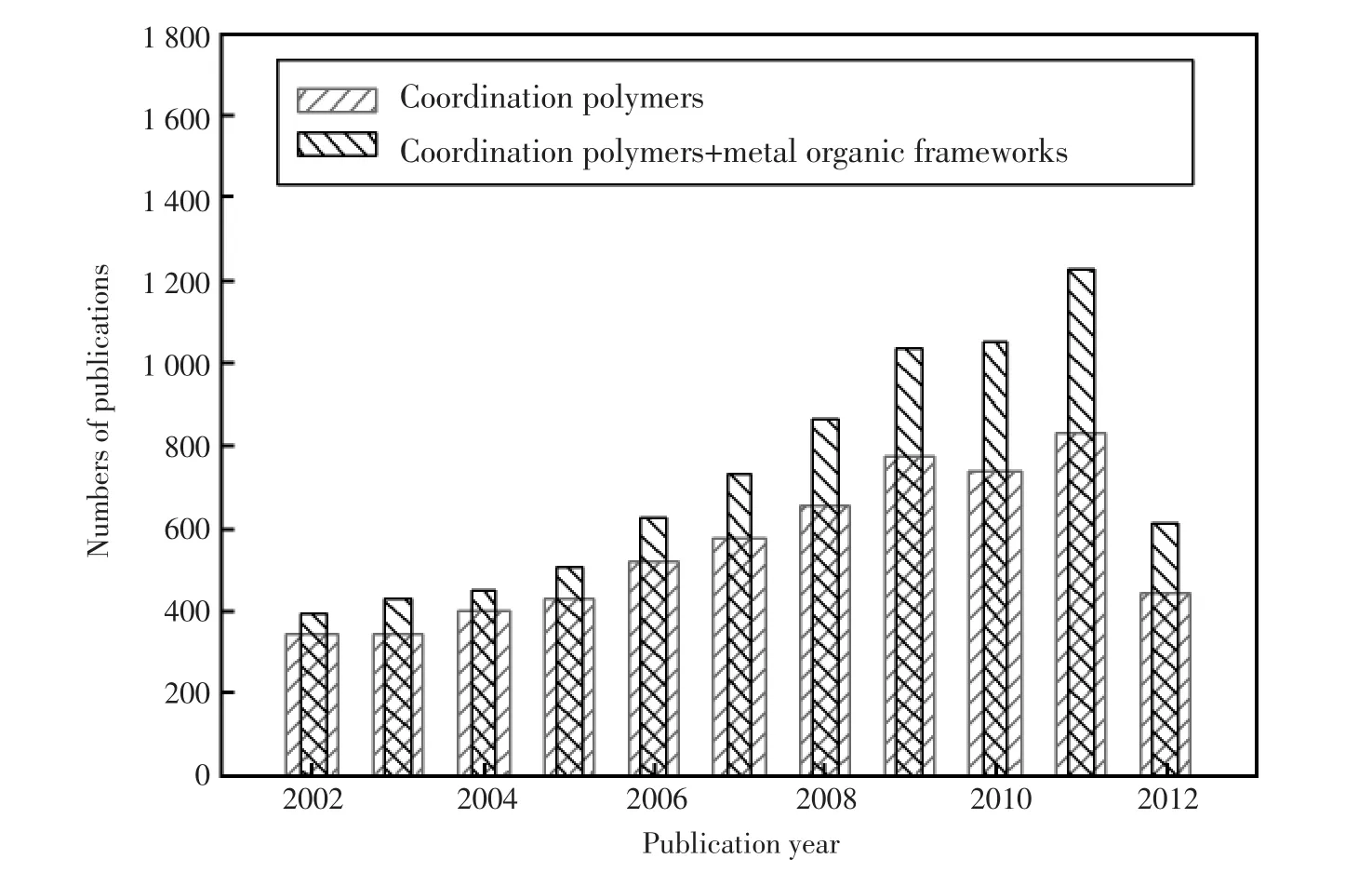
图1 在ISI Web of Science中分别以“配位聚合物”和“配位聚合物和金属有机框架”为主题搜索到的近十年发表的论文数量Fig.1 Number of publications of subjects of“coordination polymers”and“coordination polymers and metal organic frameworks”in recent 10 years(Source:ISI Web of Science,retrieved November 26,2012)
从配位聚合物的制备和构筑方法来看,目前常用的合成方法有溶液法,扩散法和水、溶剂热法,简述如下。
溶液法[54~55]:反应物溶解在溶剂中,在室温或加热回流条件下制得配位聚合物。该法操作简便,反应较为快速。但要求金属离子和配体可共溶于某一溶剂或混合溶剂,生成的副产物和同分异构体也较多,难以控制。
扩散法[56~57]:常用于培养配位聚合物单晶。主要有两种,一是两种反应液分别置于反应器的两端,静置,两种反应液通过扩散相互反应生成配位聚合物。二是在两种反应液中加入缓冲溶液进行扩散反应。
水、溶剂热法[58~60]:在水或有机溶剂存在的条件下,利用高温高压反应合成配位聚合物及培养高质量的晶体。在这种较为极端的反应条件下,水或溶剂的粘度降低,扩散过程加快,从而可获得一些常规方法难以得到的配位聚合物。
1998年,Robson等将配位聚合物框架结构分为三大类:一维链状聚合物、二维层状聚合物和三维网状聚合物[61]。常见的配位聚合物结构有:一维结构有直线形、梯形、铁轨形、书架形和双绳链等;二维结构有长方形和方格形、砖墙形和蜂窝形结构等;三维有金刚石结构、八面体和类八面体结构,以及其它的三维结构[62]。
配位聚合物具有独特的孔洞、形状和表面性质,如具有高孔隙率、大容量的孔体积、大的表面积、多种规格的孔径和结构丰富的拓扑结构,在分子识别、气体吸附和催化方面表现出诱人的发展前景。另外,以发光的有机物作为配体,或以铁、钴、镍、猛等为金属中心离子的配位聚合物,表现出独特的光学、磁性性能[63~64]。同时,选择合适的金属离子和配体,能改善配位聚合物的生物兼容性,有利于研发新型药物载体[23,65~66]。
2 配位聚合物用于电分析
配位聚合物在电化学分析中的主要应用可分为以下两方面:基于目标分子直接电化学的分析检测和用作电分析中的载体材料。
2.1 基于目标分子直接电化学的分析检测
配位聚合物及其复合材料对某些电活性分子具有电催化活性,藉此可构筑信号增强型电化学传感器。
Hosseini等[67]基于3-(三甲氧基甲硅烷基)-1-丙硫醇在SiO2纳米颗粒表面的水解,制得巯基化的SiO2纳米颗粒SH-SiO2,再通过均苯三甲酸(BTC)、SH-SiO2以及Cu(NO3)2的混合反应,制得铜的金属有机框架支撑的SH-SiO2纳米颗粒SH-SiO2@Cu-MOF,然后再吸附固定纳米金制得Au-SH-SiO2@Cu-MOF,如图2。在pH=5.0的0.1 mol/L磷酸缓冲溶液中,相对于裸玻碳电极及修饰Cu-MOF或者SH-SiO2@Cu-MOF的玻碳电极,Au-SH-SiO2@Cu-MOF的玻碳电极对L-半胱氨酸表现出更强的电催化氧化活性。该法检测L-半胱氨酸的线性范围为0.02~300 μmol/L,检测下限为0.008 μmol/L。该工作可能是MOF金属纳米颗粒电催化用于检测生物小分子的第一例。同时,这种传感器对肼也具有很强的电催化活性[68],线性范围为0.04~500 μmol/L,灵敏度为0.1 μA/(μmol/L),检测下限为0.01 μmol/L。
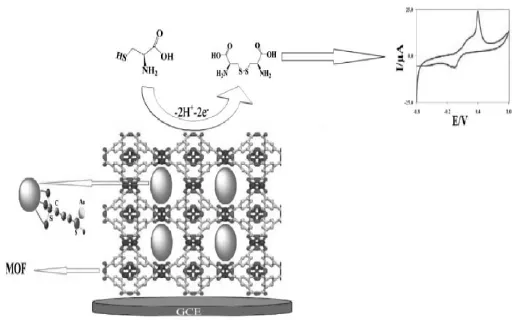
图2 石墨烯-金属配位聚合物复合物纳米片作为电化学生物传感的增强材料[67]Fig.2 Graphene-metal coordination polymer composite nanosheet as enhanced material for electrochemical biosensing
Yang等[69]以结构聚合物光纤(MPOFs)的气孔为模板,用葡萄糖化学还原银氨溶液,制得管状Ag-MPOF复合材料。相比于常用的银电极,这种修饰了管状银纳米晶结构聚合物的光纤电极表现出更高的电化学活性。在NO3-或NO2-的溶液中,在-0.4~1.3 V区间的循环伏安图只表现出一对氧化还原峰,因而可以用于NO3-或NO2-传感。Kumar等[70]使用电化学方法合成Cu3(BTC)2,用两块铜电极在含BTC、支持电解质和甲醇的混合溶液中恒电位电解,得到天蓝色的Cu3(BTC)2。在-3.0~0V电势范围的循环伏安扫描过程中,修饰了Cu3(BTC)2的玻碳电极对CO2表现出电催化还原活性。Xu等[71]用溶剂热法,通过5-(1H-间二氮茂-4-基甲基)氨基间苯二甲酸(H3L)、Cu(NO3)2或 AgNO3、 NaOH和H2O分别合成了{[Cu(HL)(H2O)]·H2O}n和[Ag(H2L)]n。修饰了这类配位聚合物的玻碳电极,对H2O2表现出优良的电催化性能,可实现其高性能电化学传感。
2.2 用作电分析的载体材料
配位聚合物具有多孔、内表面积大等特点,因而可用于固定酶和无机纳米材料,实现一些小分子的电分析。
Fu等[72]发现在中性磷酸缓冲溶液中,NaAuCl4或Na2PtCl6可与2,5-二巯基-1,3,4-噻二唑(DMcT)形成金属-有机配位聚合物(MOCPs)。采用紫外、红外、拉曼、电化学和石英晶体微天平(QCM)等方法详细研究了其配位聚合机制。以所制得的MOCPs固定葡萄糖氧化酶或酪氨酸酶用于葡萄糖或者酚类化合物的安培生物传感 (图3),分析性能优异。例如,采用NaAuCl4与DMcT形成的配位聚合物固定酪氨酸酶,对氢醌安培传感的线性范围为0.025 nmol/L~1.09 μmol/L,检测下限为15 nmol/L。因为MOCPs具有多孔结构和强吸附能力,用于酶的固定具有酶固定量大、酶活性好、酶膜传质效率高等优势,可望在生物催化、生物分析和环境监测等领域获得应用。

图3 基于金属有机配位聚合物和酶的生物复合材料的生物传感[72]Fig.3 Illustration of the preparation of the MOCPs-enzyme biocomposites-based biosensor and the biosensing mechanism
Guo等[73]用聚乙烯吡咯烷酮(PVP)、氧化石墨烯(GO)和肼的氨溶液,通过剧烈搅拌制得PVP功能化的石墨烯纳米片(PVP-GNs),然后将制得的功能化石墨烯分别与间苯二胺和H2PtCl6、间苯二胺、H2PtCl6和葡萄糖氧化酶(GOD)搅拌反应得到金属配位聚合物-石墨烯纳米片 (MCPGHs)和修饰了葡萄糖氧化酶的金属配位聚合物-石墨烯纳米片(MCPGHs/GOD)。这种纳米复合材料不但具有石墨烯的独特性能(卓越的导电性和高的比表面积),而且具有了配位聚合物(MCPs)的独特性质(可调的孔径和大的内表面积),能够高效固定葡萄糖氧化酶。与石墨烯相比,MCPGNs对H2O2的还原有更好的电催化活性。所制葡萄糖生物传感器响应葡萄糖的线性范围为50 nmol/L~ 1 mmol/L,检测下限为5 nmol/L。
3 配位聚合物用于光分析
特殊设计的配位聚合物可作为光分析的敏感材料,实现高性能的化学生物传感分析。
Lan等[74]用Zn(NO3)2·6H2O、联苯-4,4’-草酸(H2bpdc)和1,2-双吡啶基乙烯(bpee)在二甲基甲酰胺中加热制得无色块状的晶体 [Zn2(bpdc)2(bpee)]·2DMF(1),依次浸入甲醇和二氯甲烷进行溶剂交换,真空去除溶剂得到[Zn2(bpdc)2(bpee)],具有荧光活性。气相中,用这种微孔晶体金属-有机材料通过氧化还原淬灭机理可痕量检测爆炸物,如0.18 ppm的2,4-二硝基甲苯(DNT)和2.7 ppm的2,3-二甲基-2,3-二硝基丁烷(DMNB)。在10 s内,DNT的荧光淬灭效率为85%,DMNB的为84%,如图4。在150℃加热条件下,可恢复到初始的荧光强度。
An等[75]合成了一种新型多孔金属-腺嘌呤材料[Zn8(腺嘌呤)4(联苯双酯)6O·2Me2NH2,8DMF, 11H2O](bio-MOF-1),通过将其多次在Ln(NO3)3(Ln=Tb,Sm,Eu或Yb)浸泡,阳离子交换得到Ln3+@bio-MOF-1。Yb3+@bio-MOF-1固体可通过荧光淬灭来检测氧气,同时,在氮气中,能够恢复荧光强度,分析性能优异。Dang等[76]用水热法,以四羧基配体tetrakis[4-(carboxyphenyl) oxamethyl]methane acid(H4L)和Eu(NO3)3为原料在二甲基甲酰胺(DMF)中合成了Eu-MOF。这种金属有机框架可作为Fe3+的高敏高选择性冷光传感器,基于阳离子交换引起Eu的冷光淬灭。Xie等[77]利用具有高磷光强度的铱苯基吡啶的衍生物合成的多孔磷光配位聚合物,作为化学传感器传感氧气。氧气能够在这种配位聚的孔道快速扩散,且能可逆地淬灭铱的配位聚合物的磷光。Doty等[78]通过Zn(NO3)2和4,4’-反-二苯乙烯二羧酸(H2SDC)分别以N,N-二甲基甲酰胺(DMF)或N, N-二乙基甲酰胺(DEF)为溶剂合成黄色针形晶体Zn3(SDC)3(DMF)2(MOF-S2)和无色棱柱形晶体Zn4O(SDC)3(MOF-S1)。通过X射线单晶衍射等一系列表征,发现MOF-S1为多孔、3D互相贯通拓扑结构,MOF-S2为2D片状纳米多孔结构。用3 MeV质子引起的辐射发光,以及α粒子引起的闪烁来证实了电离辐射引起的发射与二苯乙烯的顺~反异构途径,引起的量子产率增加,具有相似性质。Feng等[79]以4,4’-反-二苯乙烯二羧酸 (H2SDC)为有机配体合成了两种发光体的MOF[78],研究了光致发光性能。Guo等[80]用 Yb (NO3)3和联苯-3,4’,5-三羧酸盐(H3BPT)在DMF、乙醇和水混合溶剂中,得到近红外冷光Yb(BPT) (H2O)·(DMF)1.5(H2O)1.25的MOF1材料。在温度25~ 200℃下,MOF1失去溶剂分子变成 MOF1a。MOF1a对C2H2,CO2和CH4的吸附量不同,可以用来分离C2H2/CH4或CO2/CH4。同时,其荧光光谱依赖溶剂分子,DMF和丙酮分别可以显著增强或淬灭荧光,可用做小分子传感器。
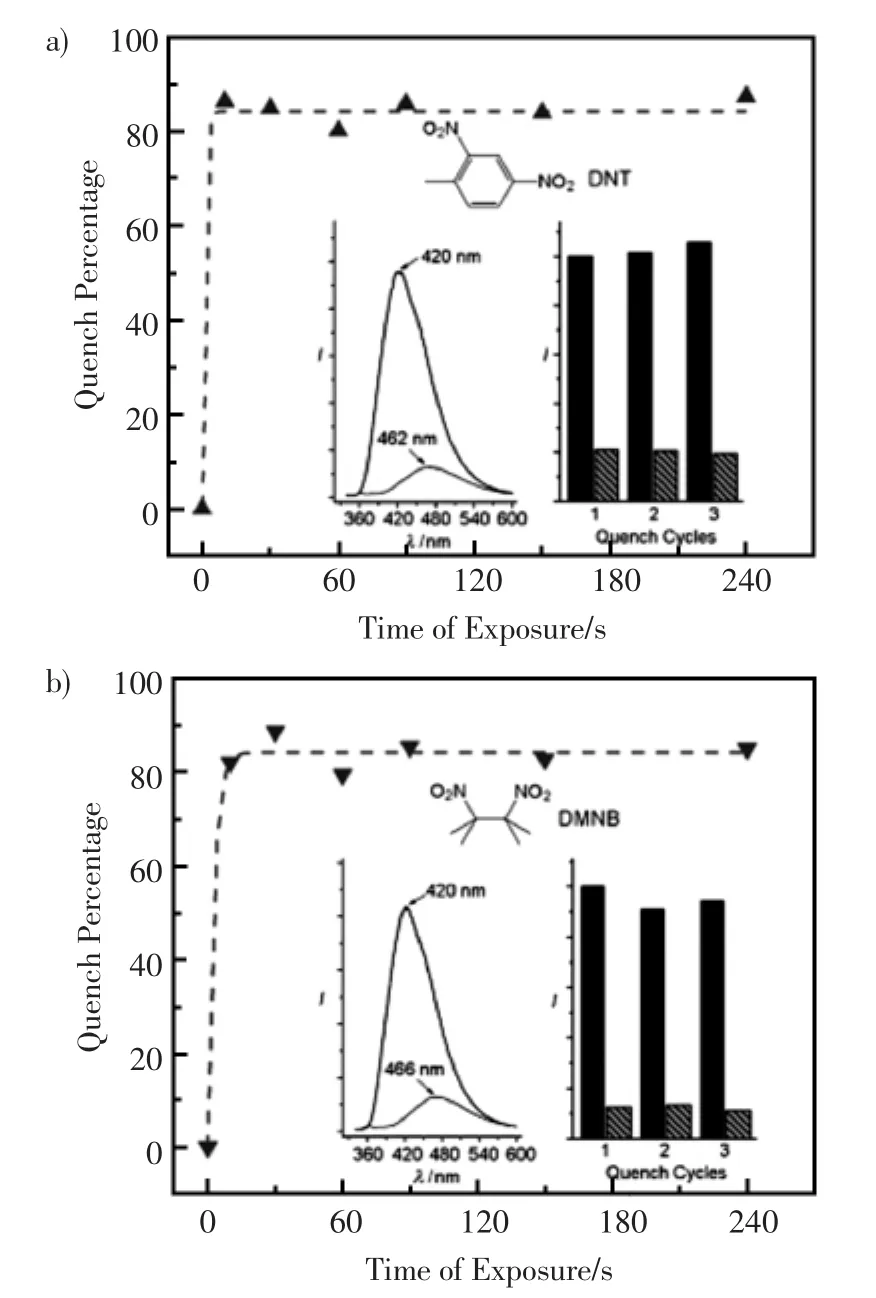
图4 时间-荧光淬灭图a)2,4-二硝基甲苯(DNT)和b)2,3-二甲基-2,3-二硝基丁烷(DMNB,激发波长为320 nm)。插图:暴露在分析物蒸气前和10 s后的荧光光谱(左),以及三个连续淬灭/再生周期(右)[74]Fig.4 Time-dependent fluorescence quenching by a)2,4-dinitrotoluene(DNT)and b)2,3-dimethyl-2,3-dinitrobutane(DMNB;excitation wavelength=320 nm). Insets:the corresponding fluorescence spectra before and after exposure to the analyte vapors for 10 s(left)and three consecutive quench/regeneration cycles(right)
4 配位聚合物用于色谱分析
相比于常规的固定相材料,配位聚合物具有可控和可剪裁的孔隙结构,表现出更优异的分离性能。尤其在分离小分子方面,具有典型微孔结构的配位聚合物引人注目。
Chen等[81]用1,4-苯二甲酸、4,4’-联吡啶和Zn(NO3)2在DMF和乙醇混合溶剂中,通过溶剂热法制得MOF-508,可用来分离天然气。用MOF-508填充的色谱柱,能分离正戊烷、正己烷、2-甲基丁烷、2-戊烷和2,2-二甲基丁烷。Jiang等[82]通过控制溶剂量或者反应时间和温度,用Cd(NO3)2、4,4’-二吡啶(bpy)和2-氨基-1,4-苯二羧酸(L)在二甲基甲酰胺(DMF)中,合成了三种不同通道大小(纳米,微米和介孔)的金属-有机框架同分异构体Cd(L)(bpy),Cd(L)(bpy)·4H2O·2.5DMF和Cd(L)(bpy)·4.5H2O·3DMF。用单晶X射线衍射、热重分析和元素分析进行了表征。同时,通过对溶剂或温度的控制,实现了微孔到介孔MOFs的可逆转化。微孔的MOF可用作冷光探针用于检测小分子,介孔MOF可用做高效液相色谱的固定相,用于分离染料分子。此外,Yan等[83]填充有机金属框架材料MIL-101到石英柱,通过高分辨气相色谱分离了二甲苯同分异构体和苯乙烷。
5 结论和展望
配位聚合物作为通过有机配体和金属离子间的配位键形成的聚合物,具有高度规整的长程网络结构,属于无机、有机、固态、材料化学的前沿研究领域。对配体和金属中心离子的多种选择和调控极大地丰富了配位聚合物材料的多样性和功能性,为其理论研究和实际应用提供了广阔的发展空间。近年来,配位聚合物作为分析传感新材料的应用已初见端倪,并呈现出良好的发展前景。设计和合成具有良好分析应用性能的配位聚合物,提高其分子识别特性和信号转换能力,将成为配位聚合物分析应用的研究前沿和热点。
[1]Champness N R,Schröder M.Extended networks formed by coordination polymers in the solid state[J].Current Opinion in Solid State and Materials Science,1998,3(4): 419~424.
[2]Kitagawa S,Kitaura R,Noro S.Functional porous coordination polymers[J].Angewandte Chemie International Edition,2004,43(18):2 334~2 375.
[3]Janiak C.Engineering coordination polymers towards applications[J].Dalton Transactions,2003,(14):2 781~ 2 804.
[4]Li H,Eddaoudi M,O'Keeffe M,et al.Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J].Nature,1999,402(18):276~279.
[5]Taylor-Pashow K M L,Della Rocca J,Huxford R C,et al.Hybrid nanomaterials for biomedical applications[J]. Chemical Communications,2010,46(32):5 832~5 849.
[6]Zhang S,Liu Q,Shen M,et al.A facile route for preparing a mesoporous palladium coordination polymer as a recyclable heterogeneous catalyst[J].Dalton Transactions, 2012,41(15):4 692~4 698.
[7]Babu K F,Kulandainathan M A,Katsounaros I,et al. Electrocatalytic activity of BasoliteTM F300 metal-organic-framework structures[J].Electrochemistry Communications,2010,12(5):632~635.
[8]Allendorf M,Bauer C,Bhakta R,et al.Luminescent metal-organic frameworks[J].Chemical Society Reviews, 2009,38(5):1 330~1 352.
[9]Keskin S,Liu J,Rankin R B,et al.Progress,opportunities,and challenges for applying atomically detailed modeling to molecular adsorption and transport in metal-organic framework materials[J].Industrial&Engineering Chemistry Research,2008,48(5):2 355~2 371.
[10]Düren T,Bae Y S,Snurr R Q.Using molecular simulation to characterise metal-organic frameworks for adsorption applications[J].Chemical Society Reviews,2009,38(5): 1 237~1 247.
[11]Mendoza-Cortés J L,Goddard III W A.Recent advances on simulation and theory of hydrogen storage in metalorganic frameworks and covalent organic frameworks[J]. Chemical Society Reviews,2009,38(5):1 460~1 476.
[12]Babarao R,Jiang J.Molecular screening of metal-organic frameworks for CO2storage[J].Langmuir,2008,24(12): 6 270~6 278.
[13]Li J R,Kuppler R J,Zhou H C.Selective gas adsorption and separation in metal-organic frameworks[J].Chemical Society Reviews,2009,38(5):1 477~1 504.
[14]Keskin S,Sholl D S.Screening metal-organic framework materials for membrane-based methane/carbon dioxide separations[J].The Journal of Physical Chemistry C, 2007,111(38):14 055~14 059.
[15]Keskin S,Sholl D S.Assessment of a metal?organic framework membrane for gas separations using atomically detailed calculations:CO2,CH4,N2,H2mixtures in MOF-5[J].Industrial&Engineering Chemistry Research, 2008,48(2):914~922.
[16]Ruckenstein E,Hong L.Binding catalytic sites to the surface of porous polymers and some catalytic applications[J].Chemistry of Materials,1992,4(1):122~127.
[17]James S L.Metal-organic frameworks[J].Chemical Society Reviews,2003,32(5):276~288.
[18]Tranchemontagne D J,Mendoza-Cortés J L,O’Keeffe M, et al.Secondary building units,nets and bonding in the chemistry of metal-organic frameworks[J].Chemical Society Reviews,2009,38(5):1 257~1 283.
[19]Long J R,Yaghi O M.The pervasive chemistry of metalorganic frameworks[J].Chemical Society Reviews,2009, 38(5):1 213~1 214.
[20]Wang Z,Cohen S M.Postsynthetic modification of metal -organic frameworks[J].Chemical Society Reviews, 2009,38(5):1 315~1 329.
[21]Cohen S M.Modifying MOFs:New chemistry,new materials[J].Chemical Science,2010,1(1):32~36.
[22]Huxford R C,Della Rocca J,Lin W.Metal-organic frameworks as potential drug carriers[J].Current Opinion in Chemical Biology,2010,14(2):262~268.
[23]Keskin S,Kızılel S.Biomedical applications of metal organic frameworks[J].Industrial and Engineering Chemistry Research,2011,50(4):1799.
[24]Zhang J P,Huang X C,Chen X M.Supramolecular isomerism in coordination polymers[J].Chemical Society Reviews,2009,38(8):2 385~2 396.
[25]Dhakshinamoorthy A,Garcia H.Catalysis by metal nanoparticles embedded on metal-organic frameworks [J].Chemical Society Reviews,2012.
[26]Czaja A U,Trukhan N,Müller U.Industrial applications of metal-organic frameworks[J].Chemical Society Reviews,2009,38(5):1 284~1 293.
[27]Fleischer E B,Shachter A M.Coordination oligomers and a coordination polymer of zinc tetraarylporphyrins[J]. Inorganic Chemistry,1991,30(19):3 763~3 769.
[28]Neels A,Neels B M,Stoeckli-Evans H,et al.Synthesisand X-ray powder structures of nickel(Ⅱ)and copper (Ⅱ)coordination polymers with 2,5-bis(2-pyridyl) pyrazine[J].Inorganic Chemistry,1997,36(16):3 402~3 409.
[29]Wang Q M,Guo G C,Mak T C W.A coordination polymer based on twofold interpenetrating three-dimensional four-connected nets of 42638 topology,[CuSCN(bpa)] [bpa=1,2-bis(4-pyridyl)ethane]?[J].Chemical Communications,1999,(18):1 849~1 850.
[30]Withersby M A,Blake A J,Champness N R,et al.Assembly of a three-dimensional polyknotted coordination polymer[J].Journal of the American Chemical Society, 2000,122(17):4 044~4 046.
[31]Chen Z F,Xiong R G,Zhang J,et al.The first chiral 2-D molecular triangular grid[J].Journal of the Chemical Society,Dalton Transactions,2000,22:4 010~4 012.
[32]Barnett S A,Blake A J,Champness N R,et al.Synthesis and structural characterisation of cadmium(Ⅱ)and zinc (Ⅱ)coordination polymers with an angular dipyridyl bridging ligand:parallel interpenetration of two-dimensional sheets with 4.82topology[J].Journal of the Chemical Society,Dalton Transactions:Inorganic Chemistry, 2001,5:567~573.
[33]Du M,Chen S T,Bu X H.{[Cd(bpo)(SCN)2]·CH3CN}n:A novel three-dimensional(3D)noninterpenetrated channel-like open framework with porous properties[J]. Crystal Growth&Design,2002,2(6):625~629.
[34]Paz F A A,Klinowski J.Supramolecular architecture of a novel salt of trimesic acid and 1,2-bis(4-pyridyl)ethane [J].CrystEngComm,2003,5(41):238~244.
[35]Al-Raqa S Y,Cook M J,Hughes D L.1,4-dibutoxy-2,3-di(4-pyridyl)-8,11,15,18,22,25-hexakis(hexyl)phthalocyaninato zinc,a self-assembled coordination polymer in the solid state[J].Chemical Communications,2003,1: 62~63.
[36]Siemeling U,Scheppelmann I,Neumann B,et al.Spontaneous chiral resolution of a coordination polymer with distorted helical structure consisting of achiral building blocks[J].Chemical Communications,2003,17:2 236~2 237.
[37]Qu Z R,Chen Z F,Zhang J,et al.The first highly stable homochiral olefin-copper(Ⅰ)2D coordination polymer grid based on quinine asa building block[J]. Organometallics,2003,22(14):2 814~2 816.
[38]Lee E W,Kim Y J,Jung D Y.A coordination polymer of cobalt(Ⅱ)-glutarate:Two-dimensional interlocking structure by dicarboxylate ligands with two different conformations[J].Inorganic Chemistry,2002,41(3):501~506.
[39]Kläui W,Mocigemba N,Weber-SchusterA,et al. [(C5H5)Co{P(O)(OH)2}3H]:A novelorganometallic trisphosphonic acid that dissolves glass to form a six-coordinate silicon complex[J].Chemistry-A European Journal,2002,8(10):2 335~2 340.
[40]Natarajan R,Savitha G,Dominiak P,et al.Corundum,diamond,and PtS metal-organic frameworks with a difference:Self-assembly of a unique pair of 3-connecting D2d-symmetric 3,3′,5,5′-tetrakis(4-pyridyl)bimesityl [J].Angewandte Chemie International Edition,2005,44 (14):2 115~2 119.
[41]Xue X,Wang X S,Xiong R G,et al.A cluster rearrangement of an open cubane(Cu4Br4)to a prismane(Cu6Br6)in a copper(Ⅰ)-olefin network[J].Angewandte Chemie, 2002,114(16):3 068~3 070.
[42]Yilmaz V T,Guney S,Andac O,et al.Different coordination modes of saccharin in the metal complexes with 2-pyridylmethanol:Synthesis,spectroscopic,thermal and structural characterization[J].Polyhedron,2002,21 (23):2 393~2 402.
[43]Kobayashi K,Sato A,Sakamoto S,et al.Solvent-induced polymorphism of three-dimensional hydrogen-bonded networks of hexakis(4-carbamoylphenyl)benzene[J]. Journal of the American Chemical Society,2003,125 (10):3 035~3 045.
[44]Murugavel R,Baheti K,Anantharaman G.Reactions of 2-mercaptobenzoic acid with divalent alkaline earth metal ions:Synthesis,spectral studies,and single-crystal X-ray structures of calcium,strontium,and barium complexes of 2,2'-dithiobis(benzoic acid)[J].Inorganic Chemistry,2001,40(27):6 870~6 878.
[45]Ren Y P,Long L S,Mao B W,et al.Nanoporous lanthanide-copper(Ⅱ)coordination polymers:Synthesesand crystal structures of[{M2(Cu3(iminodiacetate)6)}·8H2O]n(M=La,Nd,Eu)[J].Angewandte Chemie,2003,115(5): 550~553.
[46]Paz F A A,Klinowski J.Hydrothermal synthesis of a novel thermally stable three-dimensional ytterbium-organic framework[J].Chemical Communications,2003,13: 1 484~1 485.
[47]Liu C B,Yu M X,Zheng X J,et al.Structural change of supramolecular coordination polymers of itaconic acid and 1,10-phenanthroline along lanthanide series[J]. Inorganica Chimica Acta,2005,358(9):2 687~2 696.
[48]Kou H Z,Zhou B C,Gao S,et al.A 2D cyano-and ox-amidato-bridged heterotrimetallic CrⅢ-CuⅡ-GdⅢcomplex[J].Angewandte Chemie International Edition, 2003,42(28):3 288~3 291.
[49]Cai Y P,Su C Y,Li G B,et al.Syntheses and characterizations of two lanthanide(Ⅲ)-copper(Ⅱ)coordination polymers constructed by pyridine-2,6-dicarboxylic acid[J].Inorganica Chimica Acta,2005,358(4):1 298~1 304.
[50]Calvez G,Bernot K,Guillou O,et al.Sterically-induced synthesis of 3d-4f one-dimensional compounds:A new route towards 3d-4f single chain magnets[J].Inorganica Chimica Acta,2008,361(14):3 997~4 003.
[51]Ma J,Huang X,Song Y,et al.From metalloligand to interpenetrating channels:Synthesis,characterization,and properties of a 2p-3d-4f heterometallic coordination polymer{[Na5Cu8Sm4(NTA)8(ClO4)8(H2O)22]·ClO4·8H2O}n[J].Inorganic Chemistry,2009,48(14):6 326~6 328.
[52]Cai Y P,Zhou X X,Zhou Z Y,et al.Single-crystal-tosingle-crystal transformation in a one-dimensional Ag-Eu helical system[J].Inorganic Chemistry,2009,48(14): 6 341~6 343.
[53]Gheorghe R,Andruh M,Costes J P,et al.A rational synthetic route leading to 3d-3d′-4f heterospin systems: Self-assembly processes involving heterobinuclear 3d-4f complexes and hexacyanometallates[J].Chemical Communications,2003,22:2 778~2 779.
[54]Shekhah O,Liu J,Fischer R,et al.MOF thin films:Existing and future applications[J].Chemical Society Reviews,2011,40(2):1 081~1 106.
[55]Aoyagi M,Biradha K,Fujita M.Quantitative formation of coordination nanotubes templated by rodlike guests[J]. Journal of the American Chemical Society,1999,121 (32):7 457~7 458.
[56]Hong X L,Li Y Z,Hu H,et al.Synthesis,structure,luminescence,and water induced reversible crystal-toamorphous transformation properties of lanthanide(Ⅲ) benzene-1,4-dioxylacetates with a three-dimensional framework[J].Crystal Growth & Design,2006,6(6): 1 221~1 226.
[57]Zheng S L,Tong M L,Song-De Tan,et al.Syntheses, structures,and properties of three novel coordination polymers of silver(Ⅰ)aromatic carboxylates with hexamethylenetetramine exhibiting unique metal-π interaction [J].Organometallics,2001,20(25):5 319~5 325.
[58]Lu J Y,Babb A M.A simultaneous reduction,substitution,and self-assembly reaction under hydrothermal conditions afforded the first diiodopyridine copper(Ⅰ) coordination polymer[J].Inorganic Chemistry,2002,41 (6):1 339~1 341.
[59]Hu X X,Xu J Q,Cheng P,et al.A new route for preparing coordination polymers from hydrothermal reactions involving in situ ligand synthesis[J].Inorganic Chemistry,2004,43(7):2 261~2 266.
[60]刘冰.界面组装配位聚合物纳米结构材料[D].上海:复旦大学博士论文,2008.
[61]王宇婷.柔性含芳环梭酸构筑的配位聚合物的研究[D].郑州:郑州大学博士论文,2007.
[62]马景新.多羧酸配体金属配位聚合物的设计、合成、结构及性质的研究[D].兰州:兰州大学博士论文,2009.
[63]Falcaro P,Lapierre F,Marmiroli B,et al.Positioning an individual metal organic framework particle using a magnetic field[J].Journal of Materials Chemistry C,2013.
[64]Zeng M H,Feng X L,Zhang W X,et al.A robust microporous 3D cobalt(Ⅱ)coordination polymer with new magnetically frustrated 2D lattices:Single-crystal transformation and guest modulation of cooperative magnetic properties[J].Dalton Transactions,2006,44:5 294~5 303.
[65]Horcajada P,Serre C,McKinlay A C,et al.Biomedical applications of metal-organic frameworks[J].Metalorganic frameworks:Applications from catalysis to gas storage,2011,213~250.
[66]McKinlay A C,Morris R E,Horcajada P,et al.BioMOFs: Metal-organic frameworks for biological and medical applications[J].Angewandte Chemie International Edition,2010,49(36):6 260~6 266.
[67]Hosseini H,Ahmar H,Dehghani A,et al.A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of L-cysteine[J].Biosensors and Bioelectronics,2012.
[68]Hosseini H,Ahmar H,Dehghani A,et al.Au-SH-SiO2nanoparticles supported on metal-organic framework (Au-SH-SiO2@Cu-MOF)as a sensor for electrocatalytic oxidation and determination of hydrazine[J].Electrochimica Acta,2012.
[69]Yang X,Wang L.Silver nanocrystals modified microstructured polymer optical fibres for chemical and optical sensing[J].Optics Communications,2007,280(2): 368~373.
[70]Senthil Kumar R,Senthil Kumar S,Anbu Kulandainathan M.Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst[J].Electrochemistry Communications, 2012.
[71]Xu J,Su Z,Chen M S,et al.New metal complexes with 5-(1H-imidazol-4-ylmethyl)aminoisophthalic acid:Syntheses,structures,electrochemistry and electrocatalysis [J].Inorganica Chimica Acta,2009,362(11):4 002~ 4 008.
[72]Fu Y,Li P,Bu L,et al.Exploiting Metal-organic coordination polymers as highly efficient immobilization matrixes of enzymes for sensitive electrochemical biosensing [J].Analytical Chemistry,2011,83(17):6 511~6 517.
[73]Guo Y,Han Y,Shuang S,et al.Rational synthesis of graphene -metal coordination polymer composite nanosheet as enhanced materials for electrochemical biosensing[J].J.Mater.Chem.,2012.
[74]Lan A,Li K,Wu H,et al.A luminescent microporous metal-organic framework for the fast and reversible detection of high explosives[J].Angewandte Chemie International Edition,2009,48(13):2 334~2 338.
[75]An J,Shade C M,Chengelis-Czegan D A,et al.Zincadeninate metal-organic framework for aqueous encapsulation and sensitization of near-infrared and visible emitting lanthanide cations[J].Journal of the American Chemical Society,2011,133(5):1 220~1 223.
[76]Sun Z,Dang S.A layer-structured Eu-MOF as highly selective fluorescent probe for Fe3+detection through cation-exchange approach[J].Journal of Materials Chemistry,2012.
[77]Xie Z,Ma L,deKrafft K E,et al.Porous phosphorescent coordination polymers for oxygen sensing[J].Journal of the American Chemical Society,2009,132(3):922~923.
[78]Doty F,Bauer C,Skulan A,et al.Scintillating metal-organic frameworks:A new class of radiation detection materials[J].Advanced Materials,2009,21(1):95~101.
[79]Feng P L,Branson J V,Hattar K,et al.Designing metalorganic frameworks for radiation detection[J].Nuclear Instruments and Methods in Physics Research Section A: Accelerators,Spectrometers,Detectors and Associated Equipment,2011,652(1):295~298.
[80]Guo Z,Xu H,Su S,et al.A robust near infrared luminescent ytterbium metal-organic framework for sensing of small molecules[J].Chemical Communications,2011, 47(19):5 551~5 553.
[81]Chen B,Liang C,Yang J,et al.A microporous metal-organic framework for gas-chromatographic separation of alkanes[J].Angewandte Chemie,2006,118(9):1 418 ~1 421.
[82]Jiang H L,Tatsu Y,Lu Z H,et al.Non-,micro-,and mesoporous metal-organic framework isomers:Reversible transformation,fluorescence sensing,and large molecule separation[J].Journal of the American Chemical Society,2010,132(16):5 586~5 587.
[83]Gu Z Y,Yan X P.Metal-organic framework MIL-101 for high-resolution gas-chromatographic separation of xylene isomersand ethylbenzene[J].Angewandte Chemie International Edition,2010,49(8):1 477~1 480.
Coordination polymers for analysis and sensing applications
Tang Zhi-yong,He Fang,Bu Li-juan,Gu Tie-an,Yang Da-wei,Fu Ying-chun,Xie Qing-ji*,Yao Shou-zhuo
(Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research(Ministry of Education),College of Chemistry and Chemical Engineering,Hunan Normal University,Changsha 410081,China)
In this article,we briefly review the recent research progress of coordination polymers(i.e.metal organic frameworks),involving their applications in electroanalysis,optical analysis,and chromatographic analysis,with 83 references cited.
coordination polymers;analysis and sensing applications;review
国家自然科学基金(21075036,21175042,21105026)
*通讯联系人,E-mail:xieqj123@yahoo.com.cn

