Effectiveness of inhibitors in increasing chloride threshold value for steel corrosion
Jin-xia XU*, Lin-hua JIANG, Wei-lun WANG, Li TANG, Li CUI
1. College of Mechanics and Materials, Hohai University, Nanjing 210098, P. R. China 2. Shenzhen Durability Center for Civil Engineering, Shenzhen University, Shenzhen 518060, P. R. China
Effectiveness of inhibitors in increasing chloride threshold value for steel corrosion
Jin-xia XU*1, Lin-hua JIANG1, Wei-lun WANG2, Li TANG2, Li CUI1
1. College of Mechanics and Materials, Hohai University, Nanjing 210098, P. R. China 2. Shenzhen Durability Center for Civil Engineering, Shenzhen University, Shenzhen 518060, P. R. China
This investigation was aimed at evaluating the effectiveness of corrosion inhibitors in increasing the chloride threshold value for steel corrosion. Three types of corrosion inhibitors, calcium nitrite (Ca(NO2)2), zinc oxide (ZnO), and N,N'-dimethylaminoethanol (DMEA), which respectively represented the anodic inhibitor, cathodic inhibitor, and mixed inhibitor, were chosen. The experiment was carried out in a saturated calcium hydroxide (Ca(OH)2) solution to simulate the electrolytic environment of concrete. The inhibitors were initially mixed at different levels, and then chloride ions were gradually added into the solution in several steps. The open-circuit potential (Ecorr) and corrosion current density (Icorr) determined by electrochemical impedance spectra (EIS) were used to identify the initiation of active corrosion, thereby determining the chloride threshold value. It was found that although all the inhibitors were effective in decreasing the corrosion rate of steel reinforcement, they had a marginal effect on increasing the chloride threshold value.
steel corrosion; chloride threshold value; electrochemical impedance spectra (EIS); corrosion inhibitor
1 Introduction
Steel embedded in concrete is protected against corrosion by a thin iron oxide layer that is formed on the steel surface due to the high alkaline environment of concrete. Despite this, severe corrosion problems are often encountered in many structures. Most frequently, corrosion is induced by the ingress of chloride ions (Cl) (Wang and Ueda 2009; Xu et al. 2009b). As soon as the concentration of invaded chloride ions from seawater and de-icing salts exceeds a critical level on the steel surface, which is called the chloride threshold value, the passive layer is destroyed, and the corrosion is initiated (Ann and Song 2007; Xu et al. 2009a; Song et al. 2008).
The initiation phase of a reinforced concrete structure is normally considered the servicelife of the structure exposed to chlorides (Frederiksen 2009). Accordingly, the chloride threshold value is one of the crucial parameters for determining the service life. In particular, it has been indicated that the chloride threshold value intensively affects the initiation phase of steel corrosion, of which a small increase results in a great extension of the useful lifespan (Trejo and Pillai 2003). Therefore, it is considered that increasing the chloride threshold value is an effective way to prolong the service life of a reinforced concrete structure exposed to chlorides.
Due to low cost and easy application, addition of corrosion inhibitors to concrete has received more and more attention in recent years. In a previous review (Ann and Song 2007), the application of inhibitors was considered a promising method to increase the chloride threshold value so as to improve the durability of concrete structures exposed to chlorides. However, except for the nitrite-based inhibitor, the effectiveness of other inhibitors in raising the chloride threshold value for the corrosion of steel reinforcement in concrete has rarely been investigated (Ormellese et al. 2008; Mammoliti et al. 1999). Besides, the reported trends are not consistent. As a result, the actual effectiveness of corrosion inhibitors in increasing the threshold value is still not clearly known.
In this study, the effectiveness of corrosion inhibitors in increasing the chloride threshold value for the corrosion of steel reinforcement was investigated. Three types of corrosion inhibitors, calcium nitrite (Ca(NO2)2), zinc oxide (ZnO), and N,N'-dimethylaminoethanol (DMEA), were chosen. The experiment was carried out in a saturated calcium hydroxide (Ca(OH)2) solution to simulate the electrolytic environment of concrete in order to investigate the regularity about the effectiveness of corrosion inhibitors in increasing the chloride threshold value by removing the effect of the concrete cover on the experimental data. The inhibitors were initially mixed at different levels, and then chloride ions were gradually added into the solution in several steps. The open-circuit potential (Ecorr) and corrosion current density (Icorr) determined by electrochemical impedance spectra (EIS) were used to identify the initiation of active corrosion, in order to obtain the chloride threshold value.
2 Experiments
Specimens with a thickness of 5 mm and a diameter of 10 mm were cut from a steel bar with a cylindrical surface. The chemical compositions were 0.22% of carbon, 0.30% of silicon, 0.65% of manganese, 0.05% of sulfur, and 0.045% of phosphorus by weight, and the residual was ferrum. One end surface of each specimen was used for corrosion testing in the subsequent electrochemical investigation, and the remaining area was sealed with epoxy resin. Prior to the test, the exposed surface was polished using a series of silicon carbide emery papers of grades 400, 800, and 1 000, degreased in acetone, and then washed in distilled water.
A saturated Ca(OH)2solution with a pH value of 12.5 was prepared using distilled water and was used to simulate the alkaline pore solution of concrete. Ca(NO2)2, ZnO, and N,N'-DMEA were added at the concentrations of 1% and 2% by volume, which respectivelyrepresented the anodic inhibitor, cathodic inhibitor, and mixed inhibitor. In order to simulate the field conditions, the specimens were first immersed in the solution for several days to promote passivation. The durations were determined by the stabilization of the measured open-circuit potential. Subsequently, the chloride ions supplied by sodium chloride were added gradually in several steps at an interval of one day with a dosage of 0.01 mol/L. During this process,EcorrandIcorrdetermined by EIS were periodically monitored. Moreover, a control electrolyte, that is, the saturated Ca(OH)2solution in the absence of corrosion inhibitors, was also prepared for comparison of the test results. In order to obtain a reproducible result, all the experiments were repeated at least three times.
For electrochemical tests, the saturated calomel electrode and platinum electrode were connected to test instruments and worked as a reference and auxiliary electrodes, respectively. Also, the Partstat 2273 advanced potentiostat/galvanostat/FRA system was applied. EIS with the frequency ranging from 10 mHz to 100 kHz was generated. The applied perturbing signal was 5 mV. The obtained EIS for the specimens in the absence and presence of various inhibitors displayed similar shapes, as shown in Fig. 1, whereZ′ andZ′′ depict the imaginary part and real part of impedances, respectively. Generally, only a capacitive loop of semicircle can be found in Fig. 1.
The range of high frequencies in EIS shows the electrolyte resistance and the interface resistance between the steel bar and electrolytic solution, and the range of low frequencies represents the charge transfer resistance, which is equal to the polarization resistance (Rp). For obtaining a better fit, only EIS with the frequency ranging from 10 mHz and 100 kHz was analyzed with the equivalent circuit to obtain the values of polarization resistance by ZsimpWin software, as shown in Fig. 2, whereRsis the electrolyte resistance, andQdlis a constant phase element, associated with the double layer capacitance. Then, the polarization resistance was introduced in the Stern-Geary equation for the calculation ofcorrI. The Stern-Geary equation is
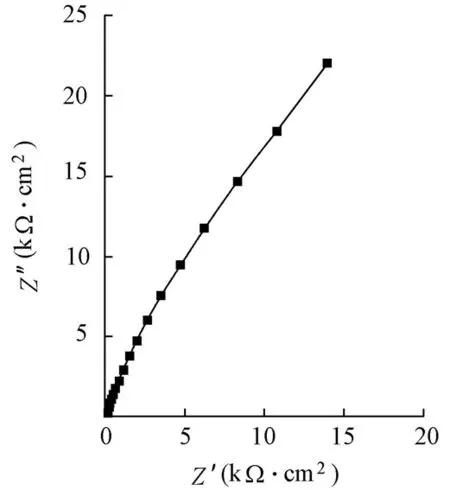
Fig. 1 Typical Nyquist plot of EIS response for specimens in absence and presence of various inhibitors

whereBis the Stern-Geary constant, assumed to be 26 mV (Andrade and Alonso 1996).
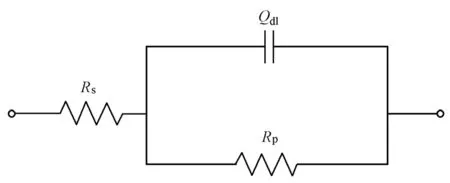
Fig. 2 Equivalent circuit for interpreting EIS results
All the potentials reported in this study were values relative to the saturated calomel electrode (SCE). All measurements were carried out at the temperature of 20±2.℃ Moreover, Ca(NO2)2was chemically pure, with a concentration of higher than 90% by weight, containing around 6% of Ca(NO3)2. The other chemical reagents used were analytically pure.
3 Results and discussion
3.1 Ca(NO2)2Inhibitor
As is well known, Ca(NO2)2is a typical anodic inhibitor. It is considered that nitrite ions compete with chloride ions for ferrous ions at the anode and react with ferrous ions to produce ferric oxide (Fe2O3), as indicated in the equations below (Soylev and Richardson 2008):
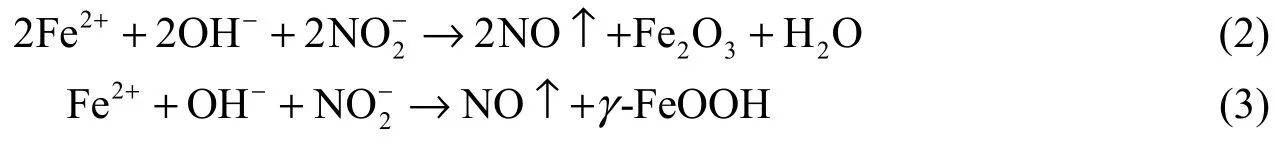
These reactions are much faster than the transport of ferrous ions via chloride-ion complex formation. Thus, nitrite ions help produce a stable passive layer even in the presence of chloride ions (withγ-FeOOH being the more stable oxide in the presence of chloride ions). The formed passive layer increases the corrosion potential at the steel surface, showing the characteristics of the anodic inhibitor.
The initial evolutions ofEcorrfor the specimens in the solutions with and without the Ca(NO2)2inhibitor in the absence of chloride ions are presented in Fig. 3. It can be seen thatEcorrfor the specimens in the solutions with the Ca(NO2)2inhibitor shifts more rapidly toward the positive potential compared with that in the control electrolyte. Also, with an increasing concentration of the Ca(NO2)2inhibitor, such a trend displays more distinctly. A possible reason for this change is the fact that there is a new mechanism of passivation in the solutions with the Ca(NO2)2inhibitor, which is different from that in the control electrolyte. The specimens are passivated more quickly by the mechanism. As a result, in the saturated Ca(OH)2solution in the absence of the Ca(NO2)2inhibitor, a passive layer is preliminarily produced on the steel surface after immersion for one or two hours. In contrast, the passivation duration of the specimens in the presence of the Ca(NO2)2inhibitor is less than 20 minutes.

Fig. 3 Initial evolution ofEcorrfor specimens in solutions with and without Ca(NO2)2inhibitor
Fig. 4 shows the variations ofEcorrfor the specimens in the solutions with the increase ofthe chloride level. It can be seen that, at the same chloride level,Ecorrfor the specimens in the presence of the Ca(NO2)2inhibitor is larger than that for the specimen in the absence of the Ca(NO2)2inhibitor, demonstrating the typical characteristics of the anodic inhibitor. Moreover, as a rule,Ecorrfor all the specimens shifts to a more negative value with the increase of the chloride level. For the specimen in the control electrolyte, there is a sudden sharp shift to a more negative value forEcorrwhen the chloride ion concentration is raised to 0.02 mol/L. BecauseEcorris closely related to the corrosion condition of the steel surface and maturation of the passive film, the sudden shift ofEcorrshould be attributed to the rupture of the passive film eroded by the aggressive chloride ions. Accordingly, the chloride threshold value for steel corrosion in the control electrolyte is assumed to be 0.02 mol/L. In contrast,Ecorrfor the specimens in the solutions with the Ca(NO2)2inhibitor displays a gentle decrease with the increase of the chloride level. The reason may be the effect of the Ca(NO2)2inhibitor on the Helmholtz electrical double layer on the interface between the specimen and solution. As a result, even though the active corrosion of the steel bar is initiated, the corrosion initiation of the specimens in the presence of the Ca(NO2)2inhibitor cannot be identified by the evolution ofEcorrwith the increase of the chloride level, and has to be distinguished byIcorrdetermined by EIS.
Fig. 5 shows the variations ofIcorrdetermined by EIS for the specimens in the solutions with and without the Ca(NO2)2inhibitor with the increase of the chloride level. It is indicated that at the same chloride level,Icorrdecreases with the increase of the Ca(NO2)2inhibitor, which indicates the positive effect of the Ca(NO2)2inhibitor. Furthermore,Icorrfor all the specimens increases with the increase of the chloride level. For the specimen in the control electrolyte, a sudden sharp increase occurs at a chloride ion concentration of 0.02 mol/L. At that moment, the corresponding value ofIcorris just over 0.1 μA/cm2. Moreover, the sudden sharp shift ofIcorroccurs at a chloride ion concentration of 0.07 mol/L for the specimen in a solution with a 1% Ca(NO2)2addition and 0.12 mol/L for the specimen in a solution with a 2% Ca(NO2)2addition. The corresponding values ofIcorrare 3.80 μA/cm2and 3.46 μA/cm2, respectively, being far more than 0.1 μA/cm2. Since the corrosion rate of steel is often regarded as being significant whenIcorrjust exceeds 0.1 μA/cm2, the sudden sharp shift ofIcorrfor the specimen in the control electrolyte should be attributed to the rupture of the passive film on the steel surface. Accordingly, the chloride threshold value determined byIcorrfor the specimen in the control electrolyte was 0.02 mol/L, which was identical to that determined byEcorr. However, the sudden sharp shift ofIcorrfor the specimens in the presence of the Ca(NO2)2inhibitor should not be attributed to the rupture of the passive film, sinceIcorrcorresponding to its sudden shift is far more than 0.1 μA/cm2. The reason may be the inhibition failure of Ca(NO2)2when the molar ratio between nitrite ions and chloride ions is greater than a critical value. The value obtained in this study was 0.21, which was close to the value of 0.25 provided by Valcarce and Vazquez (2008, 2009). The chloride ionconcentrations remained constant at 0.02 mol/L in the presence of the Ca(NO2)2inhibitor whenIcorrwas just over 0.1 μA/cm2. Therefore, it is assumed that the Ca(NO2)2inhibitor exhibits no effect on the chloride threshold value.
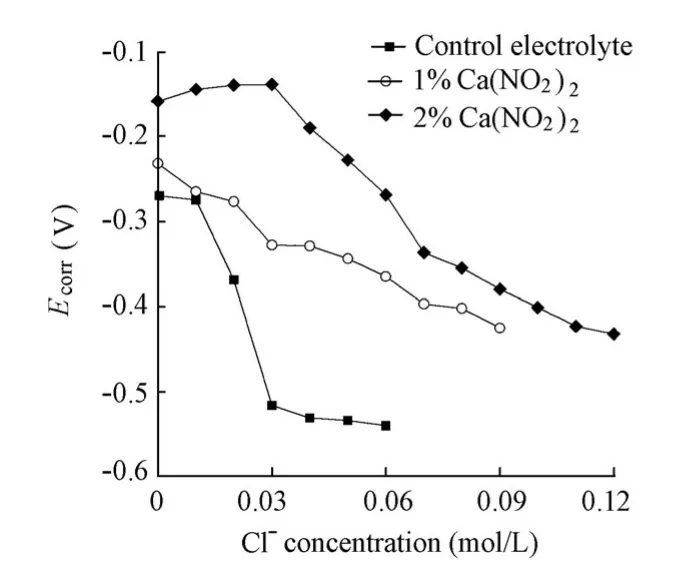
Fig. 4 Variations ofEcorrfor specimens in solutions with chloride level in presence and absence of Ca(NO2)2
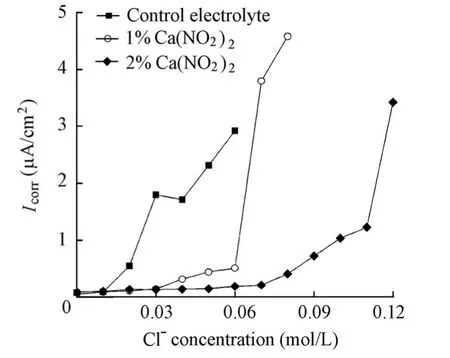
Fig. 5 Variations ofIcorrfor specimens in solutions with chloride level in presence and absence of Ca(NO2)2
3.2 ZnO Inhibitor
According to Oladis et al.’s viewpoints (De Rincón et al. 2002), ZnO acts as a cathodic inhibitor due to the fact that it can produce chemical precipitation at the cathode because of its high alkalinity in comparison with the anode. However, concrete has high alkalinity, so it is expected that ZnO produces chemical precipitation both in the cathodic and anodic areas, as well as within concrete itself. The mechanism of ZnO in corrosion inhibition is as follows (De Rincón et al. 2002):
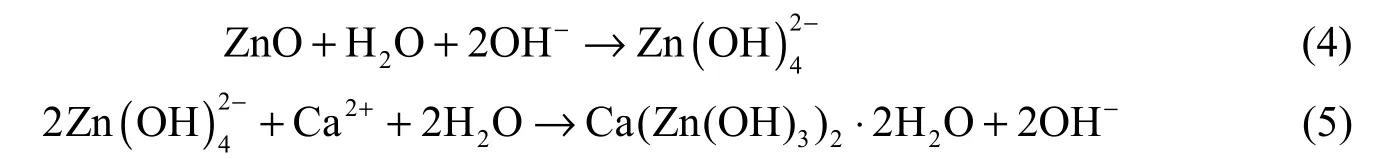
The final product (Ca(Zn(OH)3)2·2H2O) of these reactions can adhere to the steel surface and maintain the passivity of the steel in a concrete structure exposed to chlorides.
Fig. 6 shows the initial evolution ofEcorrfor the specimens in the solutions with and without the ZnO inhibitor in the absence of chloride ions. It can be found that the addition of the ZnO inhibitor does not change the time needed for the initialEcorrto reach a steady state. Therefore, although the addition of the ZnO inhibitor slightly increases the pH value of the solution (The pH value was 12.50 for the control electrolyte, but 12.57 for the solution with a 1% ZnO addition and 12.59 for the solution with a 2% ZnO addition, respectively), it does not alter the passivation rate of the steel in the solutions.
In spite of the fact that ZnO is classified as a cathodic inhibitor, at the same level of chlorides,Ecorrdoes not decrease, but increases with the addition of the ZnO inhibitor (Fig. 7). The reason may be the fact that ZnO acts not only at the cathodic site, but also at the anodic site on the steel surface in the alkaline solution. Moreover, as with the addition of the Ca(NO2)2inhibitor,Ecorrfor the specimens in the solutions with the ZnO inhibitor displays a gentle variation with the increase of the chloride level. As a result, the variations ofEcorrfor the specimens in the solutions with the increase of the chloride level cannot be used to identify the corrosion initiation and further to determine the chloride threshold value.
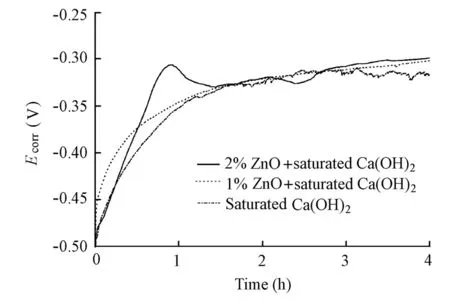
Fig. 6 Initial evolution ofEcorrfor specimens in solutions with and without ZnO inhibitor
The addition of the ZnO inhibitor significantly reduces the corrosion rate of steel, indicating its great inhibition effect on steel corrosion in aqueous solutions (Fig. 8). When 1% ZnO inhibitor was added, the inhibition efficiency was 69% at a chloride ion concentration of 0.06 mol/L. This efficiency was increased to 74% when 2% ZnO inhibitor was added. Moreover, unlike the case in which the Ca(NO2)2inhibitor was added,Icorrdetermined by EIS for the specimens in the solutions with the ZnO inhibitor did not display a sudden sharp shift, but a gentle increase with the increase of the chloride level. It could also be found that the chloride ion concentration corresponding toIcorrjust over 0.1 μA/cm2for the specimens in the solutions with 1% and 2% ZnO inhibitors remains at 0.02 mol/L, which is equal to the value for the specimen in the control electrolyte. Thus, it is believed that the ZnO inhibitor also has little influence on the chloride threshold value.
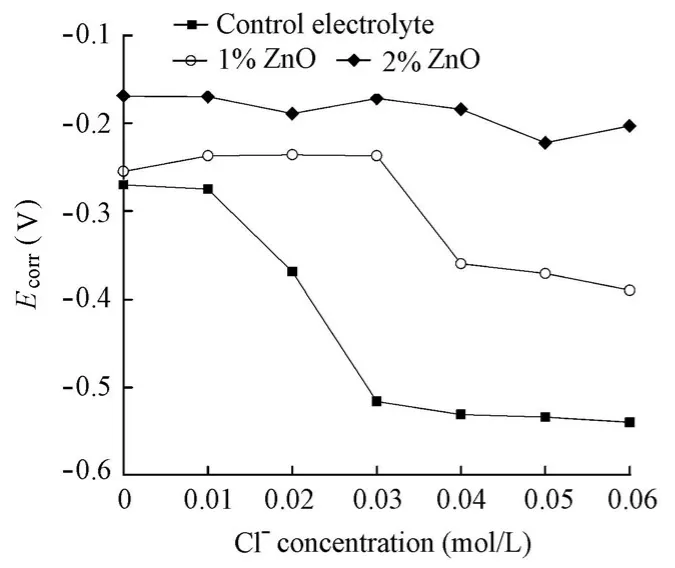
Fig. 7 Variations ofEcorrfor specimens in solutions with chloride level in presence and absence of ZnO
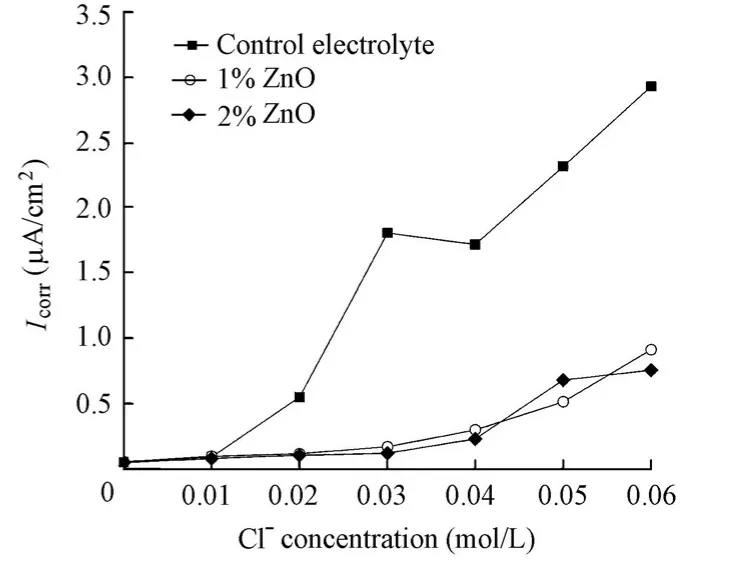
Fig. 8 Variations ofIcorrfor specimens in solutions with chloride level in presence and absence of ZnO
3.3 DMEA Inhibitor
DMEA is the main active component of an organic surface-applied corrosion inhibitor(SACI), and it has been extensively applied to real structures (Soylev and Richardson 2008). The corrosion inhibition of DMEA can be interpreted by a simple mechanism: DMEA is strongly bonded to the steel surface and displaces the ionic species from the solution/substrate interface (in particular, the chloride ion, which causes corrosion), producing a durable film to protect the steel from corrosion (Brundle et al. 1996; Welle et al. 1997). Owing to the uniform action of DMEA on both the anode and cathode on the steel surface, DMEA is classified as a mixed inhibitor.
Fig. 9 shows the initial evolution ofEcorrfor the specimens in the solutions with and without the DMEA inhibitor in the absence of chloride ions. It can be observed that the additions of the DMEA inhibitor largely prolonged the time forEcorrto reach a steady state. For the specimens in the solutions containing 1% and 2% of DMEA, the testing time of four hours was still not enough. In experiments, DMEA did not change the pH value of the solution. As a result, it is assumed that such a result was due to the fact that DMEA is chemisorbed intensively onto the steel surface so as to impede passivation of the steel.

Fig. 9 Initial evolution ofEcorrfor specimens in solutions with and without DMEA inhibitor
Prior to the chloride addition,Ecorrfor the specimens in the presence of the DMEA inhibitor was significantly lower than those in the absence of the DMEA inhibitor (Fig. 10). However, when chlorides were gradually added into the solution, the sequences ofEcorrfor the specimens in the absence and presence of the DMEA inhibitor were reversed. Moreover, as in the cases of the aforementioned Ca(NO2)2and ZnO inhibitors, the specimens in the solutions containing 1% and 2% of DMEA showed a gradual decrease ofEcorr. A sudden shift ofEcorrwas difficult to discover. Therefore, the variations ofEcorrfor the specimens in the solutions with the increase of the chloride level cannot be used to determine the chloride threshold value.
Fig. 11 presents the variations ofIcorrdetermined by EIS for the specimens with and without the DMEA inhibitor with the increase of the chloride level. The DMEA inhibitor has a strong inhibition effect. At a chloride ion concentration of 0.06 mol/L,Icorrfor the specimen in the solution with a 1% DMEA addition was 0.687 μA/cm2, and the value for the specimen in the solution with a 2% DMEA addition was 0.352 μA/cm2, both of which were much lower than the value of 2.92 μA/cm2for the specimen in the control electrolyte. The inhibition efficiency was 76% for the addition of 1% DMEA and 88% for the addition of 2% DMEA,respectively. When 0.1 μA/cm2was considered the criticalIcorrvalue for the identification of the corrosion initiation, all the chloride threshold values for the specimens in the absence and presence of the DMEA inhibitor were equal to 0.02 mol/L.

Fig. 10 Variations ofEcorrfor specimens in solutions with chloride level in presence and absence of DMEA
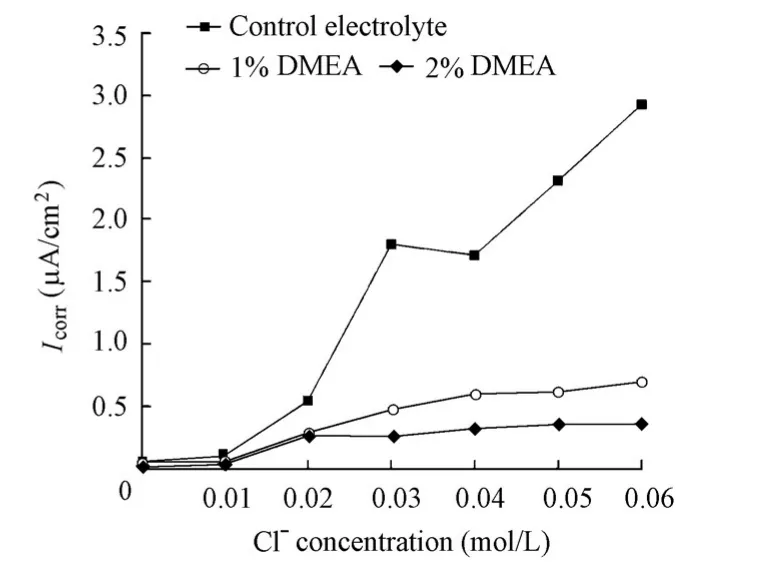
Fig. 11 Variations ofIcorrfor specimens in solutions with chloride level in presence and absence of DMEA
4 Conclusions
(1) The Ca(NO2)2inhibitor exhibits an inhibition effect only when the molar ratio between nitrite ions and chloride ions is less than 0.21. The ZnO and DMEA inhibitors can also effectively reduce the corrosion rate of steel in a saturated Ca(OH)2solution.
(2) All the inhibitors have a marginal effect on increasing the chloride threshold value for steel corrosion in a saturated Ca(OH)2solution. The reason may be due to the fact that the composition of the passive film on the steel surface does not change with the additions of the inhibitors.
(3) The addition of the Ca(NO2)2inhibitor into the saturated Ca(OH)2solution accelerates the formation of the passive film on the steel surface. The addition of the ZnO inhibitor does not alter the passivation rate of the steel. However, the addition of the DMEA inhibitor significantly retards the formation of the passive film.
(4) The open-circuit potential can be used to distinguish the critical point of corrosion initiation in a saturated Ca(OH)2solution. However, it is difficult to determine the corrosion initiation of steel reinforcement in the solutions containing various inhibitors by the same method according to the sudden sharp shift ofEcorr. The corrosion initiation of steel reinforcement can be correctly identified with a corrosion current density of greater than 0.1 μA/cm2.
(5) It has to be pointed out that although the results obtained in this study provide valuable information for investigation of the durability of a reinforced concrete structure when inhibitors are applied, the condition of the alkaline solution is different from that of real concrete. Some other factors, such as the changes of concrete resistivity and the micro-structure and the interaction of cement phases during cement hydration due to the application of variousinhibitors in concrete, can lead to a change of the chloride threshold level. Therefore, the results obtained in this study still need to be proven by further investigation in real reinforced concrete structures.
Andrade, C., and Alonso, C. 1996. Corrosion rate monitoring in the laboratory and on-site.Construction and Building Materials, 10(5), 315-328. [doi:10.1016/0950-0618(95)00044-5]
Ann, K. Y., and Song, H. W. 2007. Chloride threshold level for corrosion of steel in concrete.Corrosion Science, 49(11), 4113-4133. [doi:10.1016/j.corsci.2007.05.007]
Brundle, C. R., Grunze, M., Mader, U., and Blank, N. 1996. Detection and characterization of dimethylethanolamine-based corrosion inhibitors at steel surfaces, I: The use of XPS and ToF-SIMS.Surface and Interface Analysis, 24(9), 549-563. [doi:10.1002/(SICI)1096-9918(19960916)24:9<549::AID-SIA164>3.0.CO;2-Z]
De Rincón, O. T., Pérez, O., Paredes, E., Caldera, Y., Urdaneta, C., and Sandoval, I. 2002. Long-term performance of ZnO as a rebar corrosion inhibitor.Cement and Concrete Composites, 24(1), 79-87. [doi:10.1016/S0958-9465(01)00029-4]
Frederiksen, J. M. 2009. On the need for more precise threshold values for chloride initiated corrosion.Materials and Corrosion, 60(8), 597-601. [doi:10.1002/maco.200905273]
Mammoliti, L., Hansson, C. M., and Hope, B. B. 1999. Corrosion inhibitors in concrete, Part II: Effect on chloride threshold values for corrosion of steel in synthetic pore solutions.Cement and Concrete Research, 29(10), 1583-1589. [doi:10.1016/S0008-8846(99)00137-4]
Ormellese, M., Bolzoni, F., Lazzari, L., and Pedeferri, P. 2008. Effect of corrosion inhibitors on the initiation of chloride-induced corrosion on reinforced concrete structures.Materials and Corrosion, 59(2), 98-106. [doi:10.1002/maco.200804155]
Song, H. W., Saraswathy, V., Muralidharan, S., and Thangavel, K. 2008. Tolerance limit of chloride for steel in blended cement mortar using the cyclic polarisation technique.Journal of Applied Electrochemistry, 38(4), 445-450. [doi:10.1007/s10800-007-9457-3]
Soylev, T. A., and Richardson, M. G. 2008. Corrosion inhibitors for steel in concrete: State-of-the-art report.Construction and Building Materials, 22(4), 609-622. [doi:10.1016/j.conbuildmat. 2006.10.013]
Trejo, D., and Pillai, R. G. 2003. Accelerated chloride threshold testing, Part I: ASTM A615 and A706 reinforcement.ACI Materials Journal, 100(6), 519-527.
Valcarce, M. B., and Vazquez, M. 2008. Carbon steel passivity examined in alkaline solutions: The effect of chloride and nitrite ions.Electrochimica Acta, 53(15), 5007-5015. [doi:10.1016/ j.electacta.2008.01.091]
Valcarce, M. B., and Vazquez, M. 2009. Carbon steel passivity examined in solutions with a low degree of carbonation: The effect of chloride and nitrite ions.Materials Chemistry and Physics, 115(1), 313-321. [doi:10.1016/j.matchemphys.2008.12.007]
Wang, L. C., and Ueda, T. 2009. Meso-scale modeling of chloride diffusion in concrete with consideration of effects of time and temperature.Water Science and Engineering, 2(3), 58-70. [doi:10.3882/j.issn.1674-2370.2009.03.006]
Welle, A., Liao, J. D., Kaiser, K., Grunze, M., Mäder, U., and Blank, N. 1997. Interactions of N, N'-dimethylaminoethanol with steel surfaces in alkaline and chlorine containing solutions.Applied Surface Science, 119(3-4), 185-198. [doi:10.1016/S0169-4332(97)00216-X]
Xu, J. X., Jiang, L. H., and Wang, J. X. 2009a. Influence of detection methods on chloride threshold value for the corrosion of steel reinforcement.Construction and Building Materials, 23(5), 1902-1908. [doi:10.1016/j.conbuildmat.2008.09.011]
Xu, J. X., Jiang, L. H., and Wang, Q. 2009b. Finite element model of reinforcement corrosion in concrete.Water Science and Engineering, 2(2), 71-78. [doi:10.3882/j.issn.1674-2370. 2009.02.008]
(Edited by Ye SHI)
This work was supported by the National Natural Science Foundation of China (Grants No. 51278168 and 51278167), the China Postdoctoral Science Foundation Funded Project (Grant No. 20100481082), the China Postdoctoral Science Foundation Special Funded Project (Grant No. 201104544), the Jiangsu Planned Projects for Postdoctoral Research Funds (Grant No. 1002019B), the Qing Lan Project, and the Opening Project of Shenzhen Durability Center for Civil Engineering, Shenzhen University (Grant No. SZDCCE11-03).
*Corresponding author (e-mail:xujinxia@hhu.edu.cn)
Oct. 10, 2011; accepted Jun. 13, 2012
 Water Science and Engineering2013年3期
Water Science and Engineering2013年3期
- Water Science and Engineering的其它文章
- Present and future of hydrology
- Pollutant mixing and transport process via diverse transverse release positions in a multi-anabranch river with three braid bars
- Characteristics of phosphorus adsorption by sediment mineral matrices with different particle sizes
- Joint probability distribution of winds and waves from wave simulation of 20 years (1989-2008) in Bohai Bay
- Optimized operation of cascade reservoirs on Wujiang River during 2009-2010 drought in southwest China
- Towards full predictions of temperature dynamics in McNary Dam forebay using OpenFOAM
