Extraction of Metal Ions with 8-Hydroxyquinoline as Chelating Ligands in Supercritical Carbon Dioxide
Yang Haijian, Peng Jing, Yang Hongwei
(Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission & Ministry of Education,College of Chemistry and Materials Science, South-Central University for Nationalities, Wuhan 430074, China)
Nowadays the metal contamination is becoming a seriously worldwide environmental issue. The most widely used technique for separating the metal ions from samples such as water and soil is chelation combined with solvent extraction[1]. However, there are several defects in the extraction procedures include the non-negligible amounts of the residual solvent in products, the use and loss of excess amounts of toxic solvents, the poor selectivity, and the associated health, environment and safety problems. Recently the supercritical fluid extraction (SFE) has been come up with as an attractive technique in many separation process. Among the various kinds of fluids, the most commonly used fluid is supercritical CO2(sc-CO2) because of its non-toxic, chemical inertness, low-price, widely found in nature. The most important is its reasonably accesible critical constants, i.e.,Tc=31.1 ℃ andPc=7.38 MPa[2]. Usually it′s not common to extract metal ions by sc-CO2directly because the polar metal ions can be hardly dissolved in sc-CO2and lead to inefficiency. Fortunately, when metal ions are chelated with ligands, the resultant metal complexes can be easily dissolved in sc-CO2, accordingly removed from the matrices[3-5]. Thus far, many chelating ligands have been used to exact with β-dike-tones, amines, crown ethers, organic phosphate, and dithiocarbamates[6].
As a kind of amine, 8-hydroxyquinolin is a strong chelating ligand in coordination that can form stable complexes with almost all heavy metal ions. In previous work, we had measured the the solubility of 8-hydroxyl-quinoline in sc-CO2. According to research results, we can come to a conclusion that the 8-hydroxyl-quinoline is relatively CO2-philic.
In the present work, the effect of pressure 10 ~25 MPa, temperature 313 ~ 343 K, time 10 ~ 90 min, and ligand to metal molar ratio (50∶1) ~ (200∶1) on the extraction efficiency (E%) of metal ions were systematically investigated and the relevant extraction constants were also calculated.
1 Experiment
1.1 Reagents and apparatus
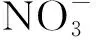
A CO2delivery pump (JASCO PU-CO2) was used to cool and deliver fluid CO2fluid and a back-pressure regulator (JASCO BP-1580-81) was used to keep the pressure of 0~35.0 MPa. The temperature was controlled by a temperature magnetic stirrer with an accuracy of ±0.01 K. Atomic absorption spectrophotometry (AAS) was recorded by AA-6300 from Shimadzu.
1.2 Preparation of extracted sample
A 1cm×1cm cellulose-based filter paper was used as an absorbent. A 10 μL of metal ion solution from a stock solution was absorbed onto the filter papers and the spiked papers were dried overnight in an electric vacuum oven at 373 K.
1.3 Metal ion extraction procedure
All the extraction of metal ions from spiked filter papers byinsituchelation-SFE were carried out with a lab-built SFE apparatus described by Wai and his co-workers[7]. The spiked filter paper and the extractants were placed in the extraction vessel (20 mL). After stirring for 20 min, pressurized CO2was introduced into the extraction vessel. The extraction system was vigorously stirred with a magnetic stirrer, and was kept at a constant temperature during the extraction procedure by a temperature controller jacket with a circulator. After the extraction, the system was depressurized and cooled to room temperature.
1.4 The analysis of the extracted samples
The spiked samples were digested with HNO3solution in water (5 mL, 1 mol/L) and then analyzed by AAS to measure the residual metal ion concentrations. The extraction behavior was evaluated by the extraction efficiency (E%), which represents the distinction in the concentration of metal ions before and after extraction. TheE% was calculated based on Eq.1:
E% = (1-Cf/Ci)×100 %.
(1)
whereCiandCfrepresented the concentration of the metal ion before and after the extraction, respectively[8].
2 Results and discussion
2.1 Influencing factors
Five parameters such as pressure, temperature, extraction time, and ligand to metal ratio would affect the extraction efficiency. Throughout the current work, all extraction procedures were performed in the presence of a fixed amount of ultrapure water (15 μL). Since the water could promote rapid desorption of metal complexes from the solid matrix to the sc-CO2, the extraction efficiency could increase[9].
2.2 Effect of pressure on extraction efficiency
The fluid pressure played an important role in supercritical fluid extraction because of the direct correlation between fluid density and pressure. With the increase of the pressure, the density of the CO2phase increases, which increases its solubilizing ability[10,11]. As shown in Fig.1, the extraction efficiency for all eight ions was improved by increasing the CO2pressure from 10 MPa to 25 MPa, at 323 K, 30 min of extraction time, andr(ligand ∶ metal ratio) =( 50∶1). The result turned out as predicted.
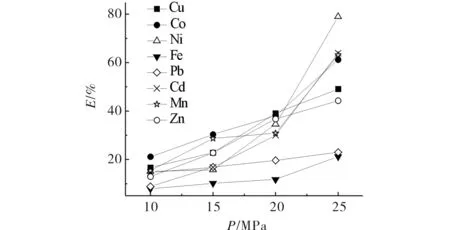
Fig.1 Effect of pressure on extraction efficiency of metal ions图1 压力对金属离子萃取的影响
2.3 Effect of temperature on extraction efficiency
The effect of temperature on the extraction of eight ions was complex. On one hand, with the increase of the temperature, the saturated vapor pressure increased and the thermal motion of the complexes at the active aites of the matrix intensified. On the other hand, the density of the fluid decreased with the increase of temperature[12]. Therefore, these three factors might have competed with each other and there would be a optimum extraction temperature representing the best compromise among them. As shown in Fig. 2, with the temperature of the system from 313 to 343 K(at 25 MPa, 30 min 15 μL ultrapure water andr(M∶chelating ligand = 1∶50), the extraction efficiencies ions increased, but they began to fall if the temperature continued to rise.
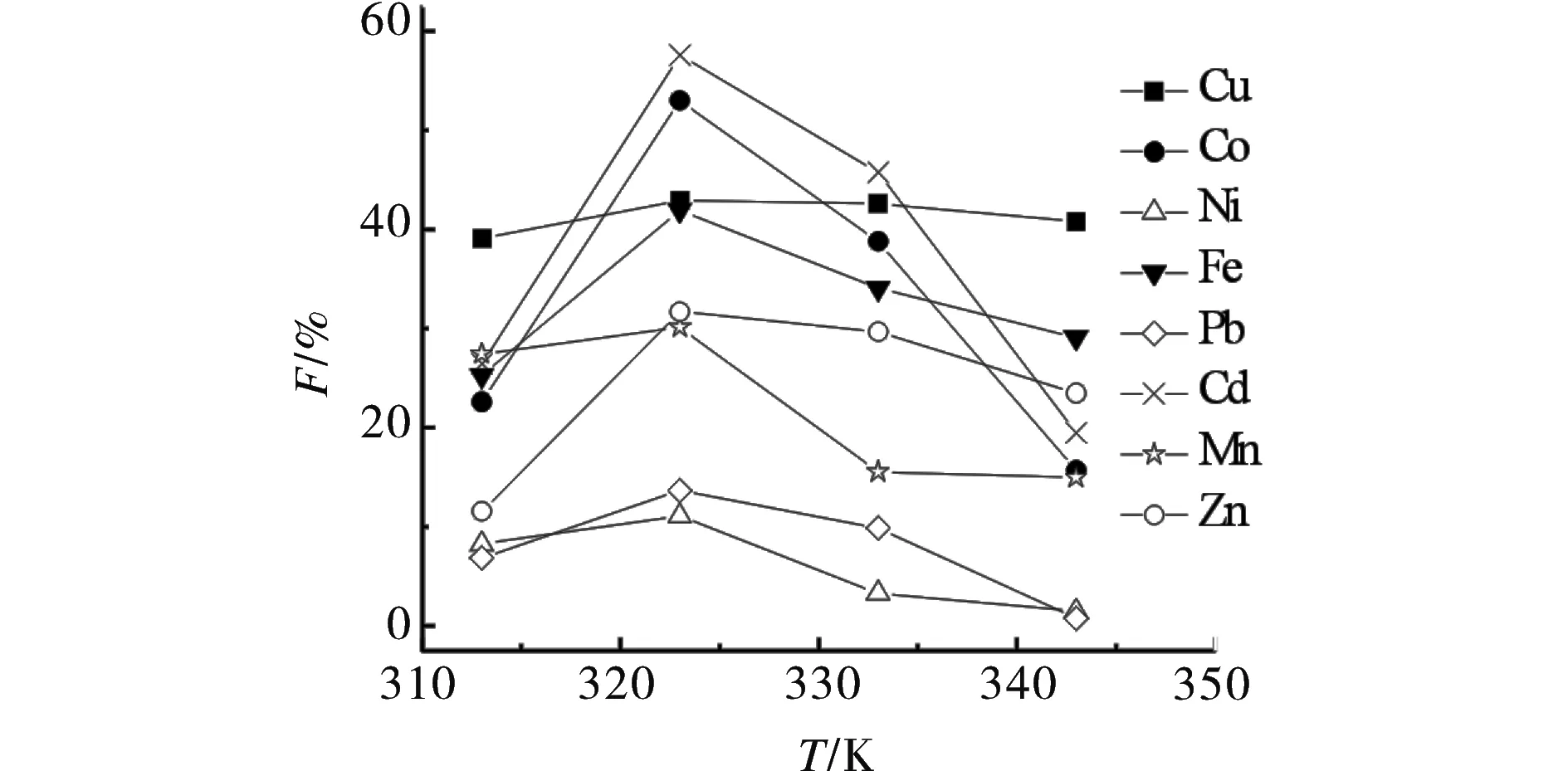
Fig.2 Effect of temperature on extraction efficiency of metal ions图2 温度对金属离子萃取的影响
2.4 Effect of time on extraction efficiency
As we can see in Fig.3, the extraction efficiencies of eight ions increased as the extraction time increased at 323 K especially for Ni2+. They all reached their maximum levels at 30 min and decreased immediately because the complexes or the ligands decomposed during the extraction procedure, which prolong the contact to acid environment of sc-CO2with ultrapure water. Obviously, longer extraction time didn′t result in distinct increase of efficiency after 30 min.

Fig.3 Effect of time on extraction efficiency of metal ions图3 时间对金属离子萃取的影响
2.5 Effect of ligand to metal ratio on extraction
efficiency
The effect of ligand to metal ratio on extraction efficiency was investigated at four different cases (50, 100, 150, 200) at 25 MPa, 323 K, and 30 min for all eight ions. As shown in Fig.4, the extraction efficiencies increased until the molar ratio of ligand to metal reached 100.
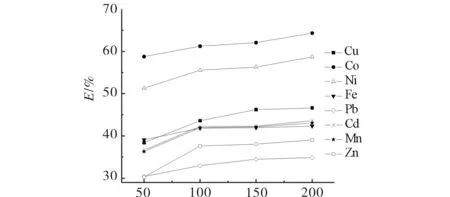
Fig.4 Effect of molar ratio of ligand to metal on extraction efficiency of metal ions图4 螯合剂与金属离子的配比对萃取效率的影响
2.6 Supercritical CO2 extraction of metal ions without PFOAT
Following the previous study, the optimum experimental condition for eight metal ions extraction (25 MPa, 323 K, 30 min, and ligand to metal ratio was 50) was obtained for the following study. As shown in Fig.5, the extraction efficiencies were around 60% except Ni2+(83.70%), which indicated that despite forming complexes with the heavy metal ions, the chelating ligands′ equilibrium constants were small, or the solubilities of the complexes in sc-CO2were low. Meanwhile, the efficiencies of SFE for eight heavy metal ions differed from element to element.
2.7 Supercritical CO2 extraction of various single metal ions with PFOAT
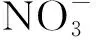
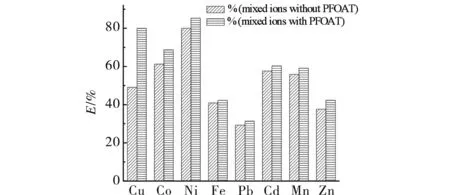
Fig.5 Effect of metal type on the extraction efficiency of metal ions with PFOAT as co-extractant图5 有共萃取剂时不同金属种类对萃取效率的影响
2.8 Supercritical CO2 extraction of mixed metal ions
The extraction efficiency of Cu2+could stay at a very high degree whether adding PFOAT or not. Moreover, the extraction efficiencies of Cu2+and Ni2+were considerable with PFOAT as co-extractant. In order to investigate the selectivity of ligand for Ni2+and Cu2+, the extraction of mixed metal ions was performed at 25 MPa, 323 K, 30 min,r(M∶chelating ligand) =1∶100 without PFOAT as additive. As shown in Fig.6, the mixed extraction efficiencies for all eight metal ions were not very high except for Ni2+(80.02%) and lower than those observed in the case of single extraction, without PFOAT. For increasing the extraction efficiencies, the extraction was performed in the presence of PFOAT. As shown in Fig.7, the extraction efficiency increased dramatically, especially for Cu2+(79.98%) and Ni2+(85.36%). So the selectivity of 8-hydroxyquinolin as chelating ligands for Ni2+and Cu2+was remarkable under the same conditions, with single or mixed ions, adding PFOAT or not.

Fig.6 The extraction efficiency of mixed metal ion without PFOAT as co-extractant 图6 无共萃取剂时混合离子的萃取效率
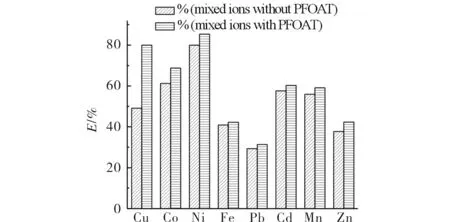
Fig.7 The extraction efficiency of mixed metal ion extraction with PFOAT as co-extractant 图7 有共萃取剂时混合离子的萃取效率
2.9 Analysis of the extraction mechanism
According to the literature[11,14], the kinetics of the cations solvent extraction is complex since it involves mass transfer coupled with chemical reaction in a heterogeneous system. Based on the measurements and literature data with these systems, the reaction of 8-hydroxyquinolin with metal ions in the solid phase could be represented as following Eq.2:
(2)
wherejandrrepresent the stoichiometric numbers, and Mn+, B and (Mn+)rBjrepresent the metal ions, chelating ligands, and metallic complex, respectively. This reaction is characterized by an overall extraction constant,Kex, which is defined by following Eq.3:
Kex=[(Mn+)rBj]/ [Mn+]r[B]j.
(3)
Where [Mn+], [B], and [(Mn+)rBj] represent the equilibrium concentrations of the metal ions, cheelating ligands, and chelates,respectively.
The measured extraction constants for the extraction of single metal ions without PFOAT(25 MPa, 323 K, 30 min, 15 μL ultrapure water andr(M∶chelating ligand)= 1∶100 were showed in Tab.1.
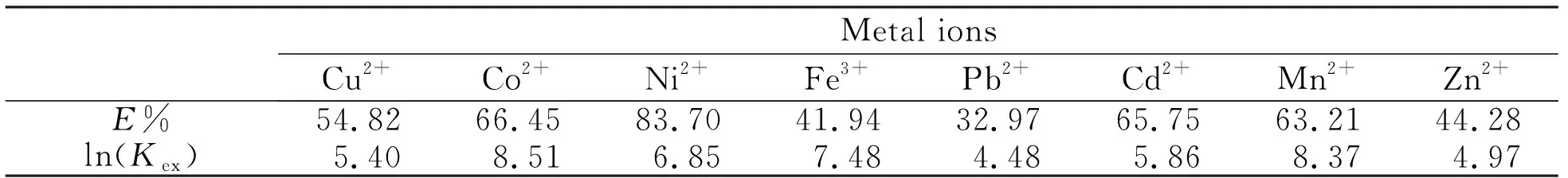
Tab.1 The values of Kex and extraction efficiency for single metal ion extraction without PFOAT as additive表1 无共萃取剂时对单一金属离子的萃取效率E%和萃取常数Kex
When PFOAT was used as a co-ligand to extract metal ions from the paper matrix, with water as an additive, part of the PFOAT dissolved in the supercritical CO2phase became distributed on the surface of the solid phase. PFOAT can dissociate to give a CO2-philic PFOA-anion and this associates with the (Mn+)rBjcomplex to form an ion pair (Mn+)rBj(PFOA-)n. The reaction between the species can be represented as below Eq.4:
rMn++jB +nPFOAT
(Mn+)rBj(PFOA-)n+nT+.
(4)
In the same way, the overall extraction constant of this extraction process can be expressed by Eq.5, and the measured extraction constants for the extraction of single metal ions in the presence of PFOAT are represented in Tab. 2.
Kex= [(Mn+)rBj(PFOA-)n][T+]n/ [Mn+]r[B]j[PFOAT]n.
(5)

Tab.2 The values of Kex and extraction efficiency for single metal ion extraction in supercritical CO2 with PFOAT as additive表2 有共萃取剂时对单一金属离子的萃取效率E%和萃取常数Kex
According to Tab.1 and Tab.2, the extraction constantKexof the chelating ligand increased as the single ions extraction efficiency increased. Adding PFOAT or not, for the same chelating ligand, 8-hydroxyquinolin, the metal ions of Cu2+, Ni2+, Pb2+, Cd2+, Zn2+and the chelating ligand formed complexes in a 1∶2 ratio[15], the metal ions of Co2+, Fe3+and Mn2+formed complexes with the chelating ligand at the ratio of 1∶3[15,16], and the valueKexof the chelating ligand increased as the extraction efficiency increased. The value ofKexwas not only related to the concentration of metal ions, but also to the value ofr,jandn, which can be suggested from Eq.3 and Eq.5.
3 Conclusion
All in all, the chelating ligand 8-hydroxyquinolin has been proved to be a comparatively suitable chelating ligand for ion extraction, and the chelating ligand showed good selectivity for ion Ni2+(83.70% without PFOAT, 88.96% with PFOAT). Detailed calculations showed that the extraction constants andKexwere seen to increase with the increasing extraction efficiency for the same metal ion in the same extraction system. The study has represented a new chelating ligand which was appropriate for metal ion (especially for Ni2+) extraction in supercritical CO2, offering a new promising candidate for the application of chelating ligands.
[1] Yang H,Kim H,Guo C. Metal ion extraction with bipyridine derivatives as chelating ligands in supercritical carbon dioxide [J]. Clean,2010 ,38(2): 159-166.
[2] Yamini Y,Saleh A,Khajeh M. Orthogonal array design for the optimization of supercritical carbon dioxide extraction of Platinum(IV) and Rhenium(VII) from a solid matrix using Cyanex 301 [J]. Sep Purif Technol,2008,61(1): 109-114.
[3] Wang W,Yang H,Hu J. Extraction of metal ions with non-fluorous bipyridine derivatives as chelating ligands in supercritical carbon dioxide [J]. J Supercrit Fluids,2009,51(2): 181-187.
[4] Wang W,Yang H,Hu J. Solubilities of diglycolic acid esters at temperatures ranging from (343 to 363) K in supercritical carbon dioxide [J]. J Chem Eng Data,2010,55(2): 694-697.
[5] Xie Y,Yang H,Wang W. Solubilities of diglycolic acid esters in supercritical carbon dioxide [J]. J Chem Eng Data,2009,54(1): 102-107.
[6] Erkey C. Supercritical carbon dioxide extraction of metals from aqueous solutions: a review [J]. J Supercrit Fluids,2000,17(3): 259-287.
[7] Wai C. Evaluation of dithiocarbamates and β-diketones as chelating agents in supercritical fluid extraction of Cd,Pb,and Hg from solid samples [J]. Talanta,1996,43(12): 2083-2091.
[8] Iwao S. Recovery of palladium from spent catalyst with supercritical CO2and a chelating agent [J]. J Supercrit Fluids,2007,42(2): 200-204.
[9] Kersch C,Van Roosmalen M,Woerlee G,et al. Extraction of heavy metals from fly ash and sand with ligands and supercritical carbon dioxide [J]. Ind Eng Chem Res,2000,39(12): 4670-4672.
[10] Liu J,Han B,Li G,et al. Investigation of nonionic surfactant Dynol-604 based reverse microemulsions formed in supercritical carbon dioxide [J]. Langmuir,2001,17(26): 8040-8043.
[11] Chang F,Kim H,Joo B,et al. Novel CO2-soluble pyridine derivatives and the extraction of heavy metals into Sc-CO2[J]. J Supercrit Fluids,2008,45(1): 43-50.
[12] EI-Fatah S,Goto M,Kodama A,et al. Supercritical fluid extraction of hazardous metals from CCA wood [J]. J Supercrit Fluids,2004,28(1): 21-27.
[13] Liu J,Yang H,Wang W,Li Z. Solubilities of amide compounds in supercritical carbon dioxide [J]. J Chem Eng Data,2008,53(9),2189-2192.
[14] Mochizuki S,Wada N,Smith R,et al. Perfluorocaboxylic acid counter ion enhanced extraction of aqueous alkali metal ions with supercritical carbon dioxide [J]. Analyst,1999,124: 1507-1511.
[15] Lorena M,Franklyn B,Coey J. Optical,magnetic,elec-trochemical,and electrical properties of 8-hydroxyquinoline-based complexes with Al3+,Cr3+,Mn2+,Co2+,Ni2+,Cu2+,and Zn2+[J]. J Phys Chem C,2011,115(18): 9182-9192.
[16] Muegge B,Brooks S,Richter M. Electrochemilumine-scence of tris(8-hydroxyquinoline-5-sulfonic acid)aluminum(III) in aqueous solution [J]. Anal Chem,2003,75(5): 1102-1105.

