Effect of Alkylimidazolium Ionic Liquids on the Corrosion Inhibition of Copper in Sulfuric Acid Solution
ZHANG Qi-Bo HUAYi-Xin
(Key Laboratory of Ionic Liquids Metallurgy,Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,P.R.China)
Effect of Alkylimidazolium Ionic Liquids on the Corrosion Inhibition of Copper in Sulfuric Acid Solution
ZHANG Qi-Bo*HUAYi-Xin
(Key Laboratory of Ionic Liquids Metallurgy,Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,P.R.China)
Abstract: The effects of three newly synthesized alkylimidazolium based ionic liquids:1-butyl-3-methylimidazolium hydrogen sulfate([BMIM]HSO4),1-hexyl-3-methylimidazolium hydrogen sulfate([HMIM]HSO4),and 1-octyl-3-methylimidazolium hydrogen sulfate([OMIM]HSO4),on the corrosion inhibition of copper in 0.5 mol·L-1H2SO4solution were investigated using potentiodynamic polarization and electrochemical impedance spectroscopy.All the measurements show that these alkylimidazolium ionic liquids are excellent inhibitors for copper in sulfuric acid media and the effectiveness of these inhibitors decreases as follows: [OMIM]HSO4>[HMIM]HSO4>[BMIM]HSO4at the same concentration.Potentiodynamic polarization studies indicate that the three inhibitors are mixed type inhibitors and that both the cathodic and anodic processes of copper corrosion are suppressed.The electrochemical impedance results were evaluated using an equivalent circuit in which two constant phase elements(CPE)were offered for these systems with two time constants.Changes in impedance parameters(charge transfer resistance and double layer capacitance)with the addition of the inhibitors also suggest that these imidazolium based molecules act by adsorbing at the copper/solution interface.The adsorption of these imidazolium based compounds on the copper surface in an acidic solution is found to fit the Langmuir adsorption isotherm.Thermodynamic calculations reveal that the adsorption of inhibitors on the metal surface occurs by a physisorption-based mechanism involving a spontaneous process.
Key Words:Corrosion inhibitor;Alkylimidazolium ionic liquid;Copper;Potentiodynamic polarization;Electrochemical impedance spectroscopy
1 Introduction
Copper and its alloys have been found widespread applications in many industrial processes such as industrial equipment,building construction,electricity,electronics,coinages and ornamental parts due to their electrical,thermal,mechanical and corrosion resistance properties.1However,the presence of aggressive ions like chlorides,sulphates or nitrates creates extensive localized attack.2-4One effective approach to protect metals against the general aggression of acid solutions is the use of organic inhibitors,which can effectively control the metal dissolution and eliminate the undesirable acid consumption.Many organic compounds including triazole,imidazole,thiazole,tetrazole,5-9indole and its derivatives10have been developed as corrosion inhibitors to inhibit copper corrosion in aggressive environments.It is noticed that,most of the effective organic inhibitors used contain heteroatoms such as O,S,N and multiple bonds in their molecules through which they can adsorb on the metal surface.11-15Generally,the adsorption depends mainly on certain physico-chemical properties of the inhibitor group,such as functional groups,π-orbital character,electron density at the donor atom and the electronic structure of the molecule.16,17However,most of commercially available corrosion inhibitors are toxic compounds that should be replaced with new environmental friendly inhibitors.18In the past two decades,research in the field of“green”corrosion inhibitors has been aimed at using cheap,effective molecules with low or“zero”environmental impact.19The inhibitive action of some non-toxic inhibitors,such as imidazole derivatives,18purine and adenine,19and phthalazin derivatives,20on copper corrosion in sulphuric acid was investigated.
Ionic liquids have attracted considerable attention in recent years.They have been identified as“green solvents”because of their attractive properties such as chemical and thermal stability,nonflammability,very low or negligible vapor pressure,high ionic conductivity,a wide electrochemical potential window and avirulence,21,22which makes them potentially attractive alternatives for volatile organic solvents.Recently,ionic liquids with imidazolium23and pyridinium cations24have showed excellent corrosion inhibition performance on mild steel in acidic environment.We have recently reported imidazolium ionic liquids for the corrosion inhibition of mild steel25and aluminum.26It was found that the action of such inhibitors depended on the specific interaction between the functional groups and the metal surface,due to the presence of the-C=N-group and electronegative nitrogen in the molecule.
The objective of the present study is to investigate the inhibitive action of three synthesized non-toxic imidazolium ionic liquids,namely 1-butyl-3-methylimidazolium hydrogen sulfate([BMIM]HSO4),1-hexyl-3-methylimidazolium hydrogen sulfate([HMIM]HSO4),and 1-octyl-3-methylimidazolium hydrogen sulfate([OMIM]HSO4)(Table 1)on copper in sulphuric acid,as well as to study the inhibition mechanism of the synthesized compounds.There is almost the same chemical structure,and the main difference is the carbon chain length of the alkyl connecting with N(3)of imidazolium ring of these compounds.The investigation was performed using potentiodynamic polarization,electrochemical impedance spectroscopy techniques,and thermodynamic calculations.
2 Experimental
The corrosion inhibitors,[BMIM]HSO4,[HMIM]HSO4,and[OMIM]HSO4,were synthesized in the laboratory as mentioned elsewhere.27,28The general structures of these inhibitors are shown in Table 1.
Potentiodynamic polarization and electrochemical impedance spectroscopy(EIS)measurements were carried out using an electrochemical work station(GAMRY USA,PCl4/300).All electrochemical experiments were performed in a conventional three-electrode electrochemical cell under atmospheric condition with a platinum disk(Ф1 mm,10 mm)and a saturated calomel electrode(SCE)as the counter and reference electrodes,respectively.The corrosive solution(0.5 mol·L-1H2SO4)was prepared by dilution of analytical grade H2SO4using double distilled water.The working electrode copper disk(Ф4 mm,99.995%)inserted in a Teflon tube with exposed surface of 0.1256 cm2,was placed into the degassed corrosive solution,and then the open circuit potential was measured after 30 min.Before each experiment the electrode was ground with a sequence of emery papers of different grades(600#,800#,and 1200#)and polished using 0.5 μm high-purity alumina,washed with double distilled water and dried with acetone.Potentiodynamic polarization studies were performed after 60 min immersion with a scan rate of 0.5 mV·s-1in the potential range from ca 150 mV below the open circuit potential to ca 150 mV above the open circuit potential(for Tafel extrapolation method).EIS measurements were performed at the open circuit potential over a frequency range of 100 kHz-10 mHz with a signal amplitude perturbation of 5 mV using ac signals.All potentials were recorded with respect to the SCE.
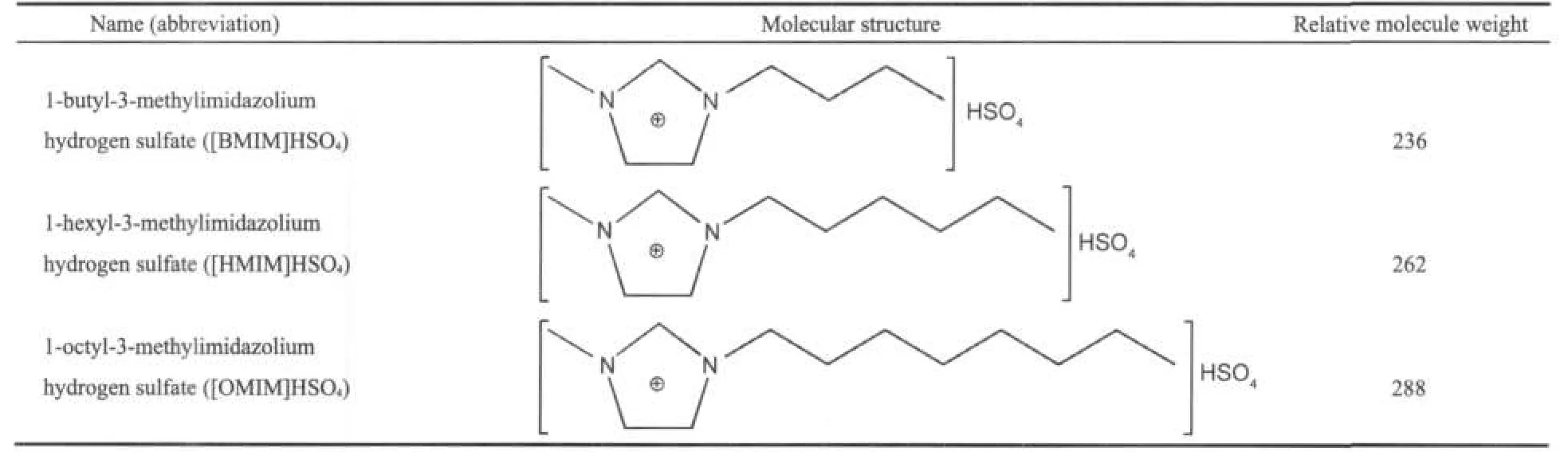
Table 1 Name and molecular structures of alkylimidazolium ionic liquids
3 Results and discussion
3.1 Potentiodynamic polarization measurements
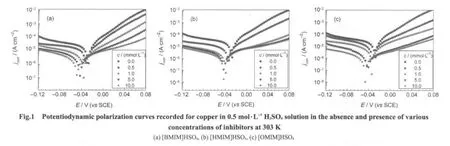
The potentiodynamic polarization curves for copper in 0.5 mol·L-1H2SO4solution in the absence and presence of various concentrations of inhibitors at 303 K are shown in Fig.1.It is observed that both the cathodic and anodic reactions are suppressed in the presence of ionic liquids studied in comparison to those recorded in the inhibitor-free solution.This behavior indicates that all the inhibitors have an inhibition effect on both cathodic and anodic reactions of the corrosion process.Generally,the presence of these compounds decreases the corrosion current density and causes more negative shift in corrosion potential(Ecorr)and this effect is more evident at higher concentrations.This can be explained by a small domination of the cathodic reaction inhibition.Thus these compounds could be classified as mixed type inhibitors with a predominantly cathodic action.
Corrosion kinetics parameters,i.e.,corrosion potential(Ecorr),cathodic Tafel slope(βc),anodic Tafel slope(βa),and corrosion current density(jcorr),can be obtained from the Tafel extrapolation of the polarizationcurves and the corresponding data are given in Table 2.The inhibition efficiency(η)was calculated by using the following equation:29

wherejcorrandjcorr(inh)are the corrosion current densities without and with addition of the inhibitors,respectively.As can be seen from Table 2,Tafel slope valuesβcandβado not change significantly in the inhibited solution as compared to the uninhibited solution;this observation indicates that the inhibiting action simply blocks the metal surface without affecting the mechanism of corrosion reactions.Theηincreases with the addition of inhibitors,which could be explained on the basis of the adsorption of inhibitor on the copper/solution interface where theadsorbed molecules partly hinder the active sites of the corrodent and then decrease the dissolution rate of the metallic material occurred.Additionally,the increase in η observed at higher inhibitor concentrations suggests that the adsorption process enhances with increasing inhibitor concentration,which leads to more adsorption of inhibitor molecules on the metal surface and results in larger surface coverage.The η obtained in the presence of inhibitors studied at a given concentration follows the order:[OMIM]HSO4>[HMIM]HSO4>[BMIM]HSO4.This behavior could be due to the surface adsorbability of the inhibitors enhancing with increasing molecular size and hence molecular mass.30,31

Table 2 Electrochemical polarization parameters for copper in 0.5 mol·L-1H2SO4solution in the absence and presence of various concentrations of inhibitors at 303 K

3.2 Electrochemical impedance spectroscopy measurements
The typical set of Nyquist plots for copper in 0.5 mol·L-1H2SO4solution in the absence and presence of various concentrations of inhibitors are shown in Fig.2.The impedance spectra obtained yield depressed semicircles and the diameters of the semicircles increase with increasing inhibitor concentrations.In addition,the presence of these inhibitors increases the impedance but does not change the profile of the Nyquist plots.These results support the results obtained from polarization measurements that these inhibitors do not alter the electrochemical reactions responsible for corrosion.They inhibit corrosion primarily through their adsorption on the copper surface.
It is significantly to note that each curve in Fig.2 appears only one depressed semicircle;however,this semicircle with a center that lies below the real axis should be interpreted in terms of a process with two time constants as observed from the Bode plots(Fig.3).The first time constant can be assigned to the charge transfer step of the corrosion process while the second time constant accounts for the adsorption of the adsorbed species on the metal surface.The only one depressed semicircle with its center below the real axis observed is actually composed of two capacitive semicircles that merge together.The depressed form of the impedance loop reflects the surface inhomogeneity of structural or interfacial origin such as those found in adsorption processes.32
According the above-mentioned discussion,the impedance data of copper in 0.5 mol·L-1H2SO4are analyzed using the equivalent circuit in Fig.4 as previously reported.33The equivalent circuit consists of solution resistance,Rs,in series with a constant phase element,CPEd,in parallel to the charge resistance,Rc,which is in series to the parallel combination of another constant phase element,CPEa,and adsorption resistance,Ra.The impedance of CPE is given by:34


where ω is the angular frequency,Y0is frequency-independent parameter,n has the meaning of a phase shift which can be explained as a degree of surface inhomogeneity.35Depending on the value of n,Y0may be a resistance,R(n=0);a Warburg impedance,W(n=0.5);a capacitance,C(n=1)or an inductance,L(n=-1)36.Considering that the impedance of a double layer does not behave as an ideal capacitor in the presence of dispersing effect,CPE is used in place of capacitor in Fig.4 to fit more accurately the impedance behavior of the electric double-layer.37Hosseini et al.38had discussed previously that the idealized capacitance values can be calculated from CPE parameter values Y0and n using the expression:


whereω=(1/RctY0)1/n.Values of these components are derived from impedance measurements and theηof all examined inhibitors is presented in Table 3.In the case of the electrochemical impedance spectroscopy,theηis calculated using charge transfer resistance as follow:39

whereRctandRct(inh)are the charge transfer resistances without and with inhibitor,respectively,andRctis the sum ofRcandRaaccording to literature.38,40
From Table 3,it could be found that theηand theRctvalues increase with an increase in the concentration of inhibitor while the values ofCdl(includingCdl,dandCdl,a,respectively)decrease,and this trend is more pronounced at higher inhibitor concentrations.The decrease inCdlvalues which can result from a decrease in local dielectric constant and/or an increase in the thickness of double layer is attributed to the gradual replacement of water molecules and other ions originally adsorbed on the surface by adsorption of inhibitor molecules on the metal surface.41The value of the phase shift(n),which is an indication for a depression of the semicircle,can be used as a measure of the surface inhomogeneity.It gives certain information on the inhibitor′s adsorption as well.No significant change in the values ofn(bothncandna)is observed after the addition of various concentrations of inhibitors.This result indicates that the charge transfer process controls the dissolution mechanism of copper in 0.5 mol·L-1H2SO4solution in the absence and presence of various concentrations of inhibitors.42The adsorption of inhibitor molecules at metal/solution interface results in an increase of resistance,which reduces corrosion rate of copper.These results obtained from the EIS method support those results obtained from potentiodynamic polarization measurement.Theηobtained in the presence of inhibitors at a given concentration follows the order:[OMIM]HSO4>[HMIM]HSO4>[BMIM]HSO4.
3.3 Effect of temperature
The effect of temperature on theηfor copper in 0.5 mol·L-1H2SO4solution in the absence and presence of 5 mmol·L-1inhibitors at temperatures ranging from 303 to 333 K was investigated by potentiodynamic polarization measurements.Electrochemical parameters and the correspondingηvalues are given in Table 4.
In both cases of uninhibited and inhibited solutions,thejcorrincreases with increasing temperature,while theηis found to decrease with increasing the solution temperature from 303 to 333 K.This behavior can be interpreted on the basis that the increase in temperature results in the desorption of the inhibitors from the copper surface.
3.4 Thermodynamics parameters
A plot of the natural logarithm of the corrosion rate(jcorr)of copper obtained from polarization measurementsvs1/Tgave straight lines as shown in Fig.5.The apparent activation energy(Ea)was calculated by using following relationship and the values are given in Table 5.

whereRis the general gas constant,Ais the Arrhenius pre-exponential factor andTis the absolute temperature.An alternative formula of the Arrhenius equation is the transition state equation:43

wherehis the Planck′s constant,Nis the Avogadro′s number,ΔSis the entropy of activation,and ΔHis the enthalpy of activation.A plot of ln(jcorr/T)vs1/Tshould give a straight line(Fig.6)with a slope of(-ΔH/R)and an intercept of[(ln(R/Nh))+(ΔS/R)],from which the values of ΔSand ΔHare calculated and presented in Table 5.It is observed that the thermodynamic parameters(Eaand ΔH)for the corrosion of copper in 0.5 mol·L-1H2SO4solution in the presence of the inhibitors arehigher than those in the inhibitor-free acid solution,indicating more energy barrier for the anodic and cathodic reactions is attained,and as a result the corrosion process on the copper surface is mitigated.The entropy of activation ΔSin the presence of the inhibitor is large.This suggests that an increase in disordering takes place on going from reactants to the activated complex,which could be attributed to the adsorption of only one surfactant molecule by desorption of more water molecules.44

Table 3 Electrochemical impedance parameters for copper in 0.5 mol·L-1H2SO4solution in the absence and presence of various concentrations of inhibitors at 303 K

Table 4 Electrochemical polarization parameters for copper in 0.5 mol·L-1H2SO4solution in the absence and presence of 5.0 mmol·L-1inhibitors at different temperatures
3.5 Adsorption isotherm
There is a general agreement in the literature that organic inhibitors establish their inhibitionviathe adsorption of the inhibitor molecules on the metal surface and the adsorption process is influenced by various aspect factors such as the chemical structures of organic compounds,the distribution of charge in molecule,the nature and surface charge of metal and the type of aggressive media.45,46Since the adsorption isotherm can provide the basic information on the interaction between the inhibitor and the metal surface.47The surface coverage,θ(η/100),for the different concentrations of the studied inhibitors was used to explain the best adsorption isotherm.Theθvalues are calculated from the following equation:


According to the data obtained from the different techniques,it can be concluded that the best description of the adsorption behavior of these inhibitors can be explained by Langmuir adsorption isotherm.As shown in Fig.7,a straight line was obtained on plottingc/θ versus c(concentration of inhibitors)which suggested that the surface adsorption process of the inhibitors studied on copper follows Langmuir′s adsorption isotherm.Theθvalues obtained from potentiodynamic polariza-tion and EIS measurements are in good agreement and both obey Langmuir adsorption isotherm.
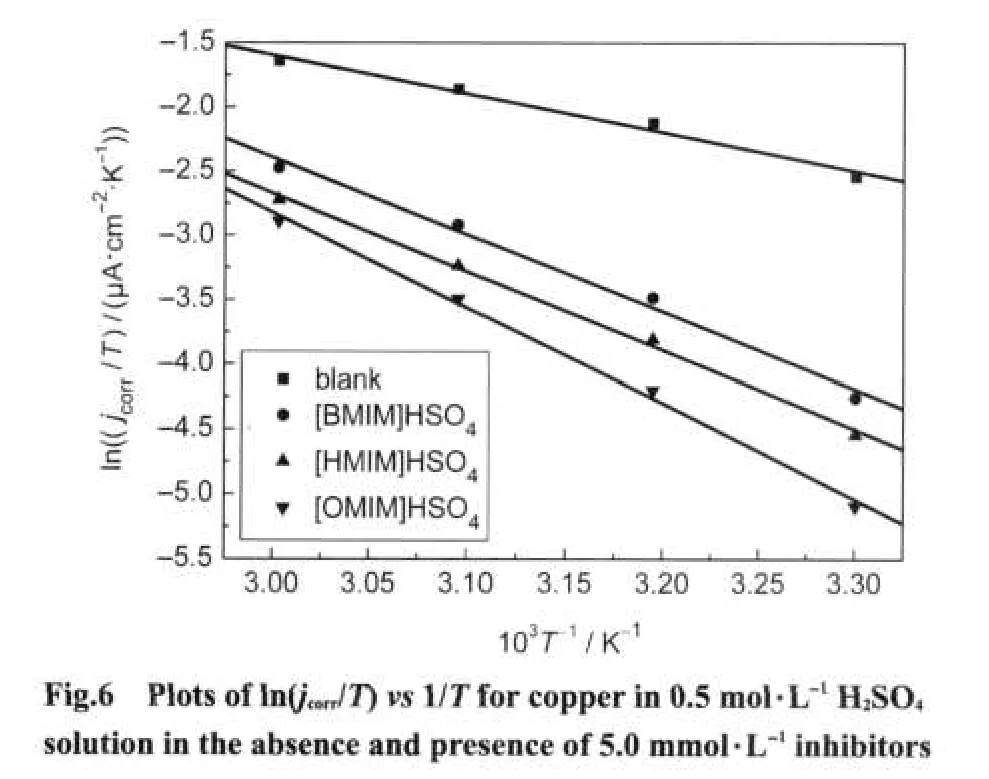

Table 5 Activation parameters for copper in 0.5 mol·L-1H2SO4solution in the presence of 5.0 mmol·L-1inhibitors obtained from electrochemical polarization measurement
The standard free energy of adsorption(ΔGads)at different temperatures is calculated from the equation:48


whereKadsis equilibrium constant and is given by:

whereθis degree of surface coverage of the metal surface andcis the concentration of inhibitors.
The equilibrium constants and standard free energies for copper in 0.5 mol·L-1H2SO4solution in the presence of 5.0 mmol·L-1inhibitors are given in Table 6.
It is generally accepted that the standard free energy of adsorption values of 40 kJ·mol-1or more negative involves charge sharing or transfer from the inhibitor molecules to the metal surface to form a co-ordinate covalent bond(chemical adsorption);those of 20 kJ·mol-1or less negative are associated with an electrostatic interaction between charged molecules and charged metal surface(physical adsorption).49Since the absolute values of standard free energy of adsorption(ΔGads)calculated in the presence of the inhibitors studied are found to be low and less than 30 kJ·mol-1.The results show that the adsorption of the inhibitors on the metal surface is more physical than chemical one and their negative sign indicates a spontaneous interaction of inhibitor molecule with the corroding copper surface.50In addition,theKadsdecreases with increasing temperature,also suggesting that the inhibitors are physically adsorbed on the metal surface and desorption process enhances with elevating temperature.
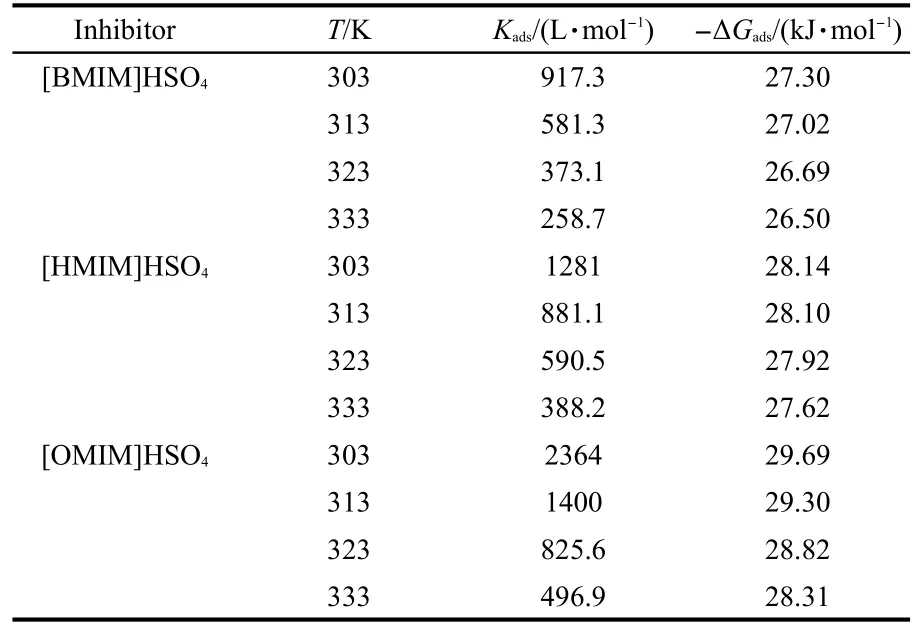
Table 6 Equilibrium constants and standard free energy values for copper in 0.5 mol·L-1H2SO4solution in the presence of 5.0 mmol·L-1alkylimidazolium ionic liquids at different temperatures
3.6 Mechanism of corrosion inhibition
A general mechanism for the dissolution of copper in sulfuric acid solution could be similar to that reported in the literature.51The anodic dissolution of copper proceedsviaa two-step reaction mechanism and can be described as follows:
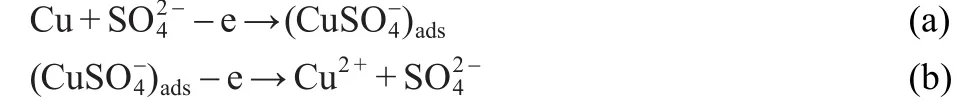
The cathodic corrosion reaction follows the step:

It is assumed that the negative sulphated ions are first adsorbed onto the positively charged metal surface by columbic attraction.Since the imidazolium group as well as nitrogen atom in heteroaromatic ring of imidazolium compounds can be protonated in acidic solutions,53the protonated inhibitor molecules can be adsorbed through electrostatic interactions between the positively charged molecules and the negatively charged metal surface.25These adsorbed imidazolium compound molecules will interact with(CuS)adsions generated from step(a)to form a protective layer(by forming a complex)at active sites,which hiders both mass and charge transfers and blocks the further oxidation reaction of(CuS)adsto Cu2+as shown by step(b).Moreover,the protonated imidazolium molecules also block the transfer of oxygen from the bulk solution to the copper/solution interface that going to reduce the cathodic reaction of oxygen.In this case,this adsorption would have occurred through polar centers as nitrogen atom in the-C=N-group.Meanwhile,the presence of the electron donating group(S)on the imidazolium compounds structure will increase theelectron density on the nitrogen of the-C=N- group.25In particular,this effect is more pronounced with increase in the carbon chain length of the alkyl connecting with N(3)of imidazolium ring due to their electron-releasing ability.Therefore,compound[OMIM]HSO4is the best inhibitor and the η follows the order: [OMIM]HSO4>[HMIM]HSO4>[BMIM]HSO4.Based on the discussion above,it could be deduced that imidazolium molecules,which had a number of active centers(N and S atoms),might form a good protective layer on the copper surface to retard its further corrosion.25,26
4 Conclusions
(1)The three alkylimidazolium based ionic liquids have proved to be excellent inhibitors for the corrosion inhibition of copper in 0.5 mol·L-1H2SO4solution and the inhibiting efficiency of these inhibitors follows the order of[OMIM]HSO4>[HMIM]HSO4>[BMIM]HSO4.
(2)The potentiodynamic polarization curves indicate the alkylimidazolium based inhibitors behave as mixed type inhibitors with a predominantly cathodic action.
(3)The corrosion inhibitive action of these imidazolium based compounds is mainly due to their adsorption on the surface of copper and the adsorption obeys Langmuir adsorption isotherm.
(4)Thermodynamic values obtained from this study reveal that these inhibitors adsorb on copper surface by a physisorption-based mechanism involving a spontaneous process.
(1) Tavakoli,H.;Shahrabi,T.;Hosseini,M.G.Mater.Chem.Phys.2008,109,281.
(2) Elmorsi,M.A.;Hassanein,A.M.Corrosion Sci.1999,41,2337.
(3) Gassa,L.M.;Ribotta,S.B.;Folquer,M.E.;Vilche,J.R.Corrosion 1998,54,179.
(4) Zucchi,F.;Grassi,V.;Frignani,A.;Trabanelli,G.Corrosion Sci.2004,46,2853.
(5) Walker,R.Corrosion 1973,29,290.
(6) Walker,R.Corrosion 1975,31,97.
(7) Kuron,D.;Rother,H.J.;Graefen,H.Werkst.Korros.1981,32,409.
(8) Zhao,Y.S.;Pang,Z.Z.Acta Phys.-Chim.Sin.2003,19,419.[赵永生,庞正智,物理化学学报,2003,19,419.]
(9)Wang,X.Q.;Liu,R.Q.;Zhu,L.Q.;Gong,J.W.Acta Phys.-Chim.Sin.2007,23,21.[王献群,刘瑞泉,朱丽琴,宫建伟.物理化学学报,2007,23,21.]
(10) Scendo,M.;Poddebniak,D.;Malyszko,J.J.Appl.Electrochem.2003,33,287.
(11) Quraishi,M.A.;Ansari,F.A.J.Appl.Electrochem.2006,36,309.
(12) Quraishi,M.A.;Rafiquee,M.Z.A.;Saxena,N.;Khan,S.J.Corrosion Sci.Eng.2006,10,11.
(13)El Rehim,S.S.A.;Hassan,H.H.;Amin,M.A.Mater.Chem.Phys.2003,78,337.
(14) Bentiss,F.;Traisnel,M.;Chaibi,N.;Mernari,B.;Vezin,H.;Lagrenee,M.Corrosion Sci.2002,44,2271.
(15)Lebrini,M.;Lagrenee,M.;Vezin,H.;Gengembre,L.;Bentiss,F.Corrosion Sci.2005,47,485.
(16)Li,S.L.;Wang,Y.G.;Chen,S.H.;Yu,R.;Lei,S.B.;Ma,H.Y.;Liu,D.X.Corrosion Sci.1999,41,1769.
(17) Scendo,M.Corrosion Sci.2008,50,2070.
(18) Stupnišek-Lisac,E.;Gazivoda,A.;Modzarac,M.Electrochim.Acta 2002,47,4189.
(19) Scendo,M.Corrosion Sci.2007,49,2985.
(20) El-Maksoud,S.A.A.Electrochim.Acta 2004,49,4205.
(21) Forsyth,S.A.;Pringle,J.M.;MacFarlane,D.R.Aust.J.Chem.2004,57,113.
(22) Earle,M.J.;Seddon,K.R.Ionic Liquids:Green Solvents for the Future;PureAppl.Chem.ACS Publications:Washington,DC,2000.
(23)Ashassi-Sorkhabi,H.;Eshaghi,M.Mater.Chem.Phys.2009,114,267.
(24)Likhanova,N.V.;Dominguez-Aguilar,M.A.;Olivares-Xometl,O.;Nava-Entzana,N.;Arce,E.;Dorantes,H.Corrosion Sci.2010,52,2088.
(25) Zhang,Q.B.;Hua,Y.X.Electrochim.Acta 2009,54,1881.
(26)Zhang,Q.B.;Hua,Y.X.Mater.Chem.Phys.2010,119,57.
(27) Zhang,Q.B.;Hua,Y.X.J.Appl.Electrochem.2009,39,261.
(28) Zhang,Q.B.;Hua,Y.X.J.Appl.Electrochem.2009,39,1185.
(29) Bentiss,F.;Lagrenee,M.;Traisnel,M.;Mernari,B.;Elattari,H.J.Hetrocycl.Chem.1999,36,149.
(30)Tripathy,B.C.;Das,S.C.;Singh,P.;Hefter,G.T.;Misra,V.N.J.Electroanal.Chem.2004,565,49.
(31) Stupnisek-Lisac,E.;Podbrscek,S.;Soric,T.J.Appl.Electrochem.1994,24,779.
(32)Goncalves,R.S.;Azambuja,D.S.;Lucho,A.M.S.Corrosion Sci.2002,44,467.
(33) Popova,A.;Raicheva,S.;Sokolova,E.;Christov,M.Langmuir 1996,12,2083.
(34) Hsu,C.H.;Mansfeld,F.Corrosion 2001,57,747.
(35) Oquzie,E.E.;Li,Y.;Wang,F.H.J.Colloid Interface Sci.2007,310,90.
(36) Khaled,K.F.;Hackerman,N.Electrochim.Acta 2004,49,485.
(37) Behpour,M.;Ghoreishi,S.M.;Soltani,N.;Salavati-Niasari,M.Corrosion Sci.2009,51,1073.
(38) Hosseini,M.;Mertens,S.F.L.;Ghorbani,M.;Arshadi,M.R.Mater.Chem.Phys.2003,78,800.
(39) Elkadi,L.;Mernari,B.;Traisnel,M.;Bentiss,F.;Lagrenee,M.Corrosion Sci.2000,42,703.
(40)Yan,Y.;Li,W.H.;Cai,L.K.;Hou,B.R.Electrochim.Acta 2008,53,5953.
(41) Ashassi-Sorkhabi,H.;Shaabani,B.;Seifzadeh,D.Appl.Surf.Sci.2005,239,154.
(42)Hermas,A.A.;Morad,M.S.;Wahdan,M.H.J.Appl.Electrochem.2004,34,95.
(43)Abd El Rehim,S.S.;Hassan,H.H.;Amin,M.A.Mater.Chem.Phys.2001,70,64.
(44)Saleh,M.M.Mater.Chem.Phys.2006,98,83.
(45) Saleh,M.R.;Din,A.M.S.E.Corrosion Sci.1972,12,689.
(46) Maayta,A.K.;Al-Rawashdeh,N.A.F.Corrosion Sci.2004,46,1129.
(47) Lagrenée,B.M.;Bouanisb,M.M.;Traisnelc,M.;Bentiss,F.Corrosion Sci.2002,44,573.
(48) Cases,J.M.;Villieras,F.Langmuir 1992,8,1251.
(49)Abiola,O.K.;Oforka,N.C.Mater.Chem.Phys.2004,83,315.(50)Gomma,G.K.;Wahdan,M.H.Mater.Chem.Phys.1995,39,209.
(51)Smyrl,W.H.;Bockris,J.O.M.;Conway,B.E.;Yeager,E.;White,R.E.Comprehensive Treatise of Electrochemistry;Plenum Press:New York,1981,Vol.4.
(52)Ma,H.Y.;Chen,S.H.;Yin,B.S.;Zhao,S.Y.;Liu,X.Q.Corrosion Sci.2003,45,867.
(53) Quraishi,M.A.;Rafiquee,M.Z.A.;Khan,S.;Saxena,N.J.Appl.Electrochem.2007,37,1153.
咪唑离子液体对铜在硫酸溶液中的缓蚀作用
张启波*华一新
(昆明理工大学冶金与能源工程学院,离子液体冶金重点实验室,昆明650093)
采用动电位极化和电化学阻抗谱技术研究了三种新型烷基咪唑离子液体,1-丁基-3-甲基咪唑硫酸氢盐([BMIM]HSO4),1-已基-3-甲基咪唑硫酸氢盐([HMIM]HSO4),1-辛基-3-甲基咪唑硫酸氢盐([OMIM]HSO4),对铜在0.5 mol·L-1H2SO4溶液中的缓蚀作用.实验结果表明:咪唑离子液体能有效抑制铜在硫酸溶液中的腐蚀,相同浓度下的缓蚀效率大小顺序为[OMIM]HSO4>[HMIM]HSO4>[BMIM]HSO4.动电位极化表明三种咪唑化合物的加入对铜的阴阳极腐蚀过程均有抑制作用,属于混合型缓蚀剂.电化学阻抗谱用带两个常相位原件的等效电路对含两个时间常数的体系进行拟合,发现咪唑化合物的添加会引起电荷传递电阻和双电层电容等阻抗参数的变化,表明此类化合物通过吸附于铜电极与溶液界面起到缓蚀作用,且这种吸附符合Langmuir吸附等温关系.吸附过程热力学计算说明咪唑化合物在铜表面发生了自发的物理吸附.
缓蚀剂;咪唑离子液体;铜;动电位极化;电化学阻抗谱
O646
Received:October 20,2010;Revised:December 30,2010;Published on Web:February 21,2011.
∗Corresponding author.Email:qibozhang@yahoo.com.cn;Tel:+86-871-5162008.
The project was supported by the National Natural Science Foundation of China(50864009,50904031)and Research Fund for the Doctoral Program of Higher Education of China(20070674001).
国家自然科学基金(50864009,50904031)及高等学校博士学科点专项科研基金(20070674001)资助项目
- 物理化学学报的其它文章
- Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
- Catalytic Decomposition of Cellulose in Cooperative Ionic Liquids
- Electronic Structures and Optical Properties of Ilmenite-Type Hexagonal ZnTiO3
- Effects of Substrate-Target Distance and Si Co-Doping on the Properties of Al-Doped ZnO Films Deposited by Magnetron Sputtering
- Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
- Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol

