急性肾损伤时水通道蛋白2及钠钾泵的表达
尹永杰 张京晓 宋德彪


DOI:10.3760/cma.j.issn.1671-0282.2014.08.005基金項目:吉林省自然科学基金项目(201015170)
作者单位:130041长春,吉林大学白求恩第二医院急救医学科
通信作者:尹永杰,Email:yinyongjie2003@sina.cn
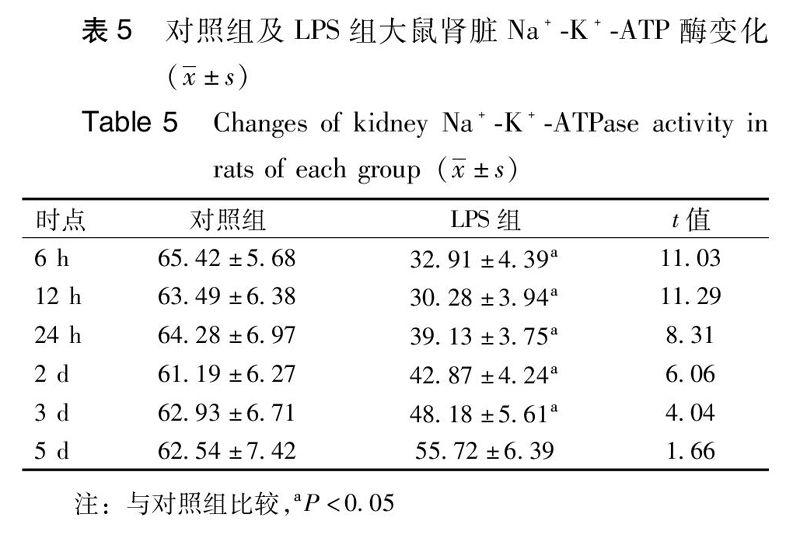
【摘要】目的 探讨水通道蛋白2的表达及钠-钾-三磷酸腺苷酶在多器官功能障碍肾损伤发病机制中的作用,研究大鼠多器官功能障碍肾损伤与水通道蛋白2及钠-钾-三磷酸腺苷酶的表达。方法 选取健康大鼠72只,随机(随机数字法)分为对照组24只,脂多糖组48只,采用大鼠腹腔注射脂多糖 5 mg/kg造成内毒素致多器官功能障碍动物模型,对照组仅作假手术处理。造模成功后6 h、12 h、24 h、2 d、3 d、5 d分别处死动物,留取血液、尿量进行生化检测,并应用免疫组化法和RT-PCR技术检测肾组织内水通道蛋白2 mRNA及蛋白的表达水平。应用试剂盒测定钠-钾-三磷酸腺苷酶的含量及活性。结果 造模成功后致伤组大鼠尿量迅速减少,48 h后尿量增多。尿素氮、肌酐逐渐增高,48 h达高峰,此后逐渐下降。水通道蛋白2 mRNA及蛋白表达迅速减少,48 h降至最低,此后逐渐增多。钠-钾-三磷酸腺苷酶含量差异无统计学意义,但其活性在造模成功后明显降低,此后逐渐增高,但仍低于对照组。结论 大鼠多器官功能障碍综合征肾损伤模型中,水通道蛋白2是肾脏重吸收功能恢复的结构基础,钠-钾-三磷酸腺苷酶则直接参与或间接反映肾脏的能量代谢状态,只有在水通道蛋白2及能量代谢恢复后,大鼠的肾脏功能才能逐渐好转。
【关键词】多器官功能障碍综合征;肾损伤;水通道蛋白;钠-钾-三磷酸腺苷酶;内毒素;炎症;脂多糖;能量代谢
The expression of water channel protein 2 and Na+-K+-ATPase in acute kidney injury Yin Yongjie,Zhang Jingxiao,Song Debiao. Emergency Department,The Second Hospital of Jilin University ,Changchun 130041,China
Corresponding author: Yin Yongjie, Email: yinyongjie2003@sina.cn
【Abstract】Objective To investigate the role of AQP2 and Na+-K+-ATPasein the pathogenesis of kidney injury with multiple organ dysfunction syndrome, and try to find the express characteristics of them . Methods A total of 72 healthy rats were randomly(random number) divided intotwo groups:control group(n=24)and Lipopolysaccharidegroup(n=48) .TheLipopolysaccharidegroupratswere injected with 5 mg/kg lipopolysaccharide at the beginning while the control group was 0.9% sodium chloride. After the modelwas succeeded , the rats were putto death at6 h, 12 h, 24 h, 2 days,3 days and 5 daysequally. The urineand blood were collected. Blood wereused biochemical tests to check.kidney AQP2 protein and mRNA expression level in the organization wereapplied the immune organized and RT - PCR technique to detect. Applied kit for determining the content and activity of sodium/potassium - atpase. Results The volume of urine in LPS group decrease quicklyat 12 h and 24 h, butincreased after 2 days . Urea nitrogen and creatinine increased gradually, and peaked at 48 h, after then gradually decline. AQP2 mRNA and protein expression decreased, and minimize at 48 h. The content of Na+-K+- ATP ase has no obvious difference, but the activity significantly decreased at the beginning, then increased gradually, but it was still lower than the control group. Conclusions In renal injury rats model with multiple organ dysfunction syndrome, AQP2 is the structure ofrenal reabsorption function ,while Na+-K+-ATPasedirectly involved in or indirectly reflected the state of kidney energy metabolism. Recovery of AQP2 protein and energy metabolism, before the rat kidney function improved.
LPS组大鼠肾脏病理切片可见明显的肾小管上皮细胞肿胀,肾小管管腔变小。肾小球细胞肿胀,炎症细胞浸润。
2.4 肾脏组织AQP2 mRNA及蛋白表达
LPS组大鼠肾小管上皮细胞AQP2 mRNA表达在6 h时增高,但随后迅速降低,在48 h降至最低,随后逐渐增高。LPS组大鼠肾脏组织在实验12 h出现肾小管上皮细胞AQP2蛋白表达减少,在48 h肾脏AQP2蛋白表达减少最为明显,此后逐渐增加。见表3和表4。
3 讨论
目前认为,多器官功能障碍综合征主要是因机体炎症反应失控所导致的多器官功能序贯性损伤,多种炎症介质的参与是其发病的关键,其中肾脏是最易受损伤的器官之一。早期人们认为脓毒症导致的肾损伤是由肾脏缺血及再灌注损伤时造成,随着研究的进展发现,脓毒症患者肾脏皮质血流未见明显下降时已经出现肾损伤[8],因此炎症介质对肾损伤的作用受到了广泛关注[9-10]。
近年来研究发现,水通道蛋白是在全身广泛分布的一组蛋白,其表达的异常与多种疾病的发生及发展密切相关。目前已经发现在肾脏表达的水通道蛋白为AQP1、AQP2、AQP3、AQP4、AQP6、AQP7、AQP11。其中,AQP1主要在肾脏的近曲小管顶端和基底膜表达,在ADH调节有关的集合管几乎没有分布,主要与肾脏水分子的基础重吸收功能有关[11]。AQP2在肾脏含量最多,在肾脏集合管的大多数细胞表面都有表达,是受ADH调节的水通道蛋白。基础状态下,AQP2储存在囊泡中,但是一旦有ADH刺激,通过蛋白激酶A途径使AQP2磷酸化,AQP2迅速翻转到细胞膜的表面,成为浓缩尿液的水通道[12-13]。AQP3与AQP4在肾脏集合管的基底外侧表达,帮助水分子穿过集合管[14-15]。AQP6主要在集合管的能够分泌酸的细胞中表达,可能与肾脏的泌酸功能有关[16-17]。AQP2是目前研究发现与肾脏急性水代谢障碍关系最为密切的水通道蛋白。而Na+-K+-ATPase则通过直接参与或间接反映肾脏细胞的能量代谢水平,与临床许多疾病密切相关。
本实验通过腹腔注射脂多糖复制大鼠MODS模型诱导肾损伤,与临床急性炎症反应导致的MODS肾损伤的病理生理过程类似。本实验发现:造模成功大鼠尿量早期明显减少,尿素氮、肌酐水平增高,APQ2表达明显减少,考虑与肾小管上皮细胞肿胀、功能障碍甚至凋亡有关。而肾功能恢复阶段,尿量的多少与AQP2的表达呈负相关。实验过程中Na+-K+-ATPase含量在实验组及对照组各时间点差异无统计学意义,但Na+-K+-ATPase活性在造模成功后迅速下降,在AQP2及肾脏功能恢复之前就已经开始逐渐增高。需要特别指出,LPS组大鼠在3d及5d时尽管AQP2的表达已经高于对照组,但Na+-K+-ATPase活性較对照组仍偏低,这可能是导致LPS组大鼠3 d及5 d时尿量有减少趋势但仍较对照组增多的原因。AQP2是肾脏重吸收功能恢复的结构基础,Na+-K+-ATPase则可能通过直接参与或间接反映的方式提示肾脏的能量代谢状态,只有在AQP2蛋白表达及能量代谢状态接近正常水平后患者的肾脏功能才逐渐好转。
炎症介质在多器官功能障碍肾损伤的发病机制中发挥了重要作用,研究其对肾脏水通道蛋白的表达及Na+-K+-ATPase的影响为临床脓毒症肾损伤提供分子学基础。水通道蛋白和Na+-K+-ATPase可能是炎症介质作用的共同通路,干预水通道蛋白及Na+-K+-ATPase的表达可能是调节多器官功能障碍肾损伤水平衡的主要作用靶点,通过对其作用机制的研究对进一步探索炎症致肾损伤的治疗具有重要意义。
参考文献
[1]Pinto CF, Watanabe M, da Fonseca CD, et al . The sepsis as cause of acute kidney injury: an experimental model [J]. Rev Esc Enferm USP,2012,46:86-90.
[2]Poukkanen M, Vaara ST, PettilV, et al. Acute kidney injury in patients with severe sepsis in Finnish Intensive Care Units[J]. Acta Anaesthesiol Scand,2013,57(7):863-872.
[3]Shah PR, Gireesh MS, Kute VB, et al . Renal involvement in sepsis: a prospective single-center study of 136 cases[J].Saudi J Kidney Dis Transpl, 2013,24(3):620-629.
[4]李国民,田蜜,纪木火,等.氢水对脓毒血症大鼠急性肾损伤的保护作用[J].中华急诊医学杂志,2013, 22(5): 472-475.
[5]Verkman AS. Dissecting the roles of aquaporins in renal pathophysiology using transgenic mice[J]. Semin Nephrol,2008,28(3):217-226.
[6]Hamilton KL, Devor DC. Basolateral membrane K+ channels in renal epithelial cells[J]. Am J Physiol Renal Physiol,2012,302(9):F1069-1081.
[7]Taub M, Springate JE, Cutuli F.Targeting of renal proximal tubule Na,K-ATPase by salt-inducible kinase[J]. Biochem Biophys Res Commun,2010,393(3):339-344.
[8]Dagher PC, Herget-Rosenthal S, Ruehm SG,et al. Newly developed techniques to study and diagnose acute renal failure[J]. J Am Soc Nephrol,2003, 14(8):2188-2198.
[9]Cantaluppi V, Quercia AD, Dellepiane S, et al. New mechanisms and recent insights in the pathogenesis of acute kidney injury (AKI) [J]. G Ital Nefrol,2012,29(5):535-47.
[10]亢翠翠,吴铁军,张保军.脓毒血症患者外周血中Th17细胞检测及意义[J].中华急诊医学杂志,2012, 21(4): 378-380.
[11]Zhang J, An Y, Gao J, et al. Aquaporin-1 translocation and degradation mediates the water transportation mechanism of acetazolamide[J].PLoS One, 2012,7(9):e45976.
[12]Takata K, Matsuzaki T, Tajika Y, et al. Localization and trafficking of aquaporin 2 in the kidney[J]. Histochem Cell Biol,2008 ,130(2):197-209.
[13]Zhao W, Xu AG, Wu J,et al. Effects of imidapril treatment on aquaporin-2 expression in the kidneys and excretion in the urine of hypertensive rats[J]. Exp Ther Med, 2013,5(5):1327-1331.
[14]Arnspang EC, Sundbye S, Nelson WJ, et al.Aquaporin-3 and aquaporin-4 are sorted differently and separately in the trans-Golgi network[J].PLoS One,2013,8(9):e73977.
[15]Lee JY, Shin JH, Song KH,et al .Expression of aquaporin-3 in ipsilateral rat kidney with unilateral partial ureteral obstruction[J]. Korean J Urol, 2013,54(4):266-270.
[16]Nagase H, Agren J, Saito A, et al. Molecular cloning and characterization of mouse aquaporin 6[J]. Biochem Biophys Res Commun,2007,352(1):12-16.
[17]Agarwal SK,Gupta A. Aquaporins: The renal water channels[J]. Indian J Nephrol,2008,18(3):95-100.
(收稿日期:2014-02-22)
(本文編辑:何小军)
P852-856

