Changes of Protective Enzyme Activity and MDA Contentin Leaves of Agropyron cristatum Under Grazing Stress
Hua XIU
Institute of Grassland Farming of Tibetan Academy of Agriculture and Animal Husbandry,Tibet 850009,China
Changes of Protective Enzyme Activity and MDA Contentin Leaves of Agropyron cristatum Under Grazing Stress
Hua XIU*
Institute of Grassland Farming of Tibetan Academy of Agriculture and Animal Husbandry,Tibet 850009,China
[Objective]This study was conducted to explore the mechanism of physiological adaptation of Agropyron cristatum under grazing pressure,and to provide a theoretical basis for the protection and rational utilization of forage resources,breeding and introduction of high-quality forage materials.[Method]The mature leaves of upper parts of A.cristatum plants in grazed and non-grazed areas were collected at tillering(late May),heading(late June),flowering(late July)and seed maturity(late August)stages for determination of superoxide dismutase(SOD),peroxidase(POD) activities and malondialdehyde(MDA)content.[Result]The MDA content of grazing population was higher than that of non-grazing population throughout the whole growth period of A.cristatum.The SOD activity was significantly increased from heading to flowering stage but was rapidly reduced at seed maturity stage,with no significant difference between grazing and non-grazing populations of A.cristatum. The POD activity kept increasing from heading stage,and it was higher of grazing population than that of non-grazing population.[Conclusion]The MDA content in leaves of A.cristatum was increased under grazing pressure,leading to increased membrane lipid oxidation;POD played an important role in removal of the free radicals those accumulated in A.cristatum under grazing pressure.
Grazing stress;SOD;MDA;POD
T he intake and trample of animals greatly affect the growth and tolerance of plants in grazed grassland areas.So,creeping and tillering grasses have better adaptability in grazed areas.The study of Archer and Tieszen[1]showed that the survival,growth and propagation of a certain plant in of a population depend on its tolerance to grazing pressure. Generally,there are a variety of adaptation mechanisms help a plantto co-exist with non-biological environment and animals,and to compete with other plantspecies.
Agropyron cristatum(L.)Gaertn is a perennialherb of Agropyron in family Gramineae.It is the favorite food of domestic animals due to its rich nutrients and good palatability.In addition, it is tolerant to animal trample and drought stress,so it is an important forage source.Recent studies about A.cristatum are mostly focused on its ecological and biological characteristics,geographical distribution,and artificialcultivation,etc.Due to long-term overgrazing and irrational land reclamation activities,grassland degradation is getting worse,grassland ecosystem is destructed and its self-repair ability is weakened,the A.cristatum population is decreasing too.So far, much progress has been made on the researches about degradation and restoration succession mechanisms of Agropyron plants,while their physiological adaptation mechanisms under grazing pressure have not been reported.Therefore,the leaf protective enzyme activities and malondialdehyde(MDA)contentatdifferentgrowth stages of A.cristatum under long-term grazing pressure were measured tounderstand its physiological adaptation mechanism,and to provide a theoreticalbasis for the protection and rational utilization of forage resources, breeding and introduction of high-quality forage materials.
Materials and Methods
Overview of sampling site
The A.cristatum plants were sampled from long-term grazed and nongrazed(over two decades)areas at the Grassland Ecological Research Station of Chinese Academy of Science in Baiyinxile Grassland Ranch, XilingolLeague,Inner Mongolia,which located at 116°04′E-117°05′E, 43°26′N-44°08′N,and an altitude of 1 250-1 280 m,belonging to typical chestnut soil subzone of temperate grasslands.The average annual temperature there was-0.4℃,and the average temperature in January was -23℃in January,and 17.9℃in July. The annualrainfall was 350 mm,and the rainfall from June to August accounted for 76.26%of the annualprecipitation.Three replicates were prepared for each population.
Methods
The mature leaves of upper parts of A.cristatum plants were collected from 50 random plants of each population at tillering(late May),heading (late June),flowering(late July)and seed maturity(late August)stages and broughtback in ice boxes to laboratory for the determination of superoxide dismutase(SOD),peroxidase(POD) activities and malondialdehyde(MDA) content.
Leaf sample(0.5 g)was precisely weighed and milled to homogenate on ice by adding phosphate buffer(PBS, pH=7.8),centrifuged at 4℃and 8 000 r/min for 15 min to collect the supernatant for analysis.
Determination of SOD activityThe SOD activity was measured by nitroblue tetrazolium(NBT)test.In detail, 0.05 ml of the enzyme solution to be tested and 0.3 ml of 20μmol/L riboflavin solution were added to 2.65 ml of reaction solution(0.05 mol/L phosphate buffer,130 mmol/L methionine solution,750μmol/L NBT solution,100μmol/L EDTA-Na2solution, distilled water,pH 7.8).In the maximum photochemical reduction tube, the enzyme solution to be tested was replaced by phosphate buffer,and the tube of blank control was filled with 3 ml of phosphate buffer.The reaction was carried out in light4 000 lux for 10 min.After that the absorbance of the reaction solutions were measured at a wavelength of 560 nm.One unit of SOD activity was defined as the quantity of enzyme required to inhibit the reduction of NBT by 50%in the reaction mixture.The SOD activity was calculated using the formula as follows:
Wherein,ACKis the absorbance of blank control;AEis the absorbance of the sample to be tested;VTstands for the total volume of sample solution (ml);V1is the volume of sample solution tested in the reaction(ml);W is the fresh weightofsample(g).
Determination of POD activityThe POD(peroxidase)activity was measured using guaiacolsubstrate.In detail,1 ml of the enzyme solution to be tested was mixed with 3 mlof reaction mixture(50 ml of 100 mol/L,phosphate buffer,28μlof guaiacolsolution, 19μl of 30%H2O2solution,pH 6.0), then,the absorbance of the reaction solution was read at470 nm once every minute.The enzyme solution was replaced by 1 mlofphosphate buffer in blank control.The unit of POD activity was defined as the changes in absorbance every minute.
Wherein,△A470stands for the change in absorbance during the reaction;W is the fresh weightofsample (g);VTstands for the total volume of sample solution(ml);V1is the volume of sample solution tested in the reac
tion(ml);t is the reaction time(min).
Determination of MDA content
The MDA content was measured by thiobarbituric acid(TBA)reaction.In detail,2 mlof the sample solution was mixed with 2 mlof 0.6%TBA solution, incubated in a boiling waterbath for10 min.The reaction solution cooled before centrifugation at 3 000 r/min for 15 min.The supernatant was collected and its volume was measured.The absorbance of the supernatant was read at532,600 and 450 nm,respectively,using 0.6%TBA solution as blank control.The MDA content was calculated using the formula as follows:
MDA content(μmol/g)=MDA concentration(μmol/L)×Extract volume (ml)/[Sample weight(g)×1 000],wherein,the MDA concentration=6.45× (OD532-OD600)-0.56×OD450.
Results and Analysis
Changes in SOD activity
SOD,a ubiquitous metal-containing enzyme that catalyzes the removal of reactive oxygen or other peroxide radicals in cooperation with peroxidase and catalase to slow down cellular aging.Oxygen radicals O2-which is generated during the reduction of oxygen to water in aerobic organisms,is toxic to cells and may also induce the generation ofharmfulsubstances such as H2O2and hydroxyl radicals.SOD catalyzes the disproportionation reaction of superoxide to produce hydrogen peroxide,and hydrogen peroxide is then converted to harmless molecules oxygen and water under the action of peroxidase[2].Dynamic equilibrium exists between the production and clearance ofintracellular free radicalunder normal circumstances,but it is broken under stresses.In both populations of A.cristatum,the SOD activity changed slightly at tillering and heading stages,but was increased sharply and peaked at flowering stage because the grassland areas were attacked by drought and then a large amount of reactive oxygen species was accumulated in A.cristatum.With the relieving of drought stress at seed maturity stage,SOD activity returned to the levelas at heading stage.As the changes in SOD activity were consistent between the grazing and nongrazing populations of A.cristatum,it could be concluded that grazing pressure had no significant influence on plantSODactivity.
Changes in POD activity
POD,an enzyme able to catalyze reduction of hydrogen peroxide in plants is closely related to the respiration,photosynthesis and growth hormone oxidation.Its activity keeps changing during plant growth and development.As shown in Fig.2,the changes in grazing and non-grazing populations of A.cristatum showed similar trends during the entire phenology.In detail,POD activity was decreased before heading stage and was increased from heading to seed maturity stage.Besides,POD activity of grazing population was higher than of non-grazing population at tillering, flowering and seed maturity stages, but the difference was not significant (Table 1,P>0.05).
Changes in MDA content
As the boundary between the cell’s internal and external environments,cellmembrane is inevitably affected by environmental stress and it may be the initial site of injury caused by drought stress to plants[3].MDA, one of the products of lipid peroxidation of cell membranes,is commonly used to evaluate the damage and degeneration of the membrane system caused by oxidation.Its content is an important indicator of membrane damage.Under adverse stresses such as drought,some physiological and biochemical processes in plants are affected,the ability to scavenge free radicals reduces and thus MDA contentincreases[4].As shown in Fig.3,the changes of MDA content in grazing and non-grazing populations of A.cristatum exhibited similar trends.It changed slightly from tillering to heading stage,but was sharply increased from heading to seed maturity stage. The MDA content changed in a trend similar to SOD activity from tillering to heading stage,butin a trend similar to POD activity from flowering to seed maturity stage.The reason could be that SOD played an more important role in scavenging offree radicals from tillering to heading stage,but POD played an more important role from flowering to maturity stage of A.cristatum.Besides,MDA content of grazing population was slightly higher than that of non-grazing population of A.cristatum throughout its growth period,but the difference was not significant(Table 1,P>0.05),indicating that grazing pressure affected membrane lipid peroxidation in plants by a certain degree.
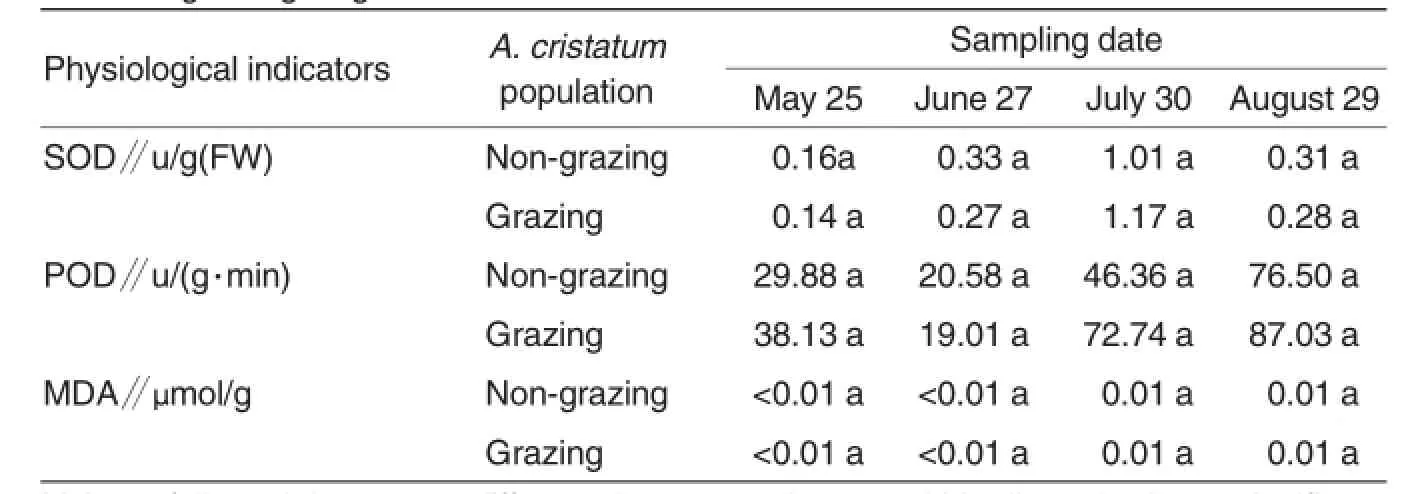
Table 1 Variance in SOD,POD activities and MDA content of A.cristatum at different growing stages
Conclusion and Discussion
When plants are subjected to adverse stresses such as cold,drought, salt,disease,pests and rays,some enzymes in vivo such as POD,catalase (CAT),SOD and other isoenzymes play important roles for scavenging free radicals,reducing plant damage and slowing down cellular aging. Therefore,free radicalgeneration and related protective systems(including enzymatic and non-enzymatic protective systems)become an hot issue in study of biological resistance.Excessive active oxygen in plants under stress induces or aggravates membrane lipid peroxidation,causing the oxidation of unsaturated fatty acid in cell membranes,and thus increasing the content of final product MDA. Then,the reduced membrane mobility affects normalfunction ofmembranes, such as material and information exchange,metabolic regulation and selective permeability,which can even lead to celldeath[4].The study of Ge et al.[4]revealed that the leaf and root SOD,CAT and POD activities were increased in early and middle growth stage but were decreased in late growth stage ofmaize under long-term drought stress.The study of Yan et al.[5]showed thatin pine seedlings the MDA content and membrane permeability were rapidly increased on the 3rd d ofdrought stress,while the autooxidation rate of tissues returned to pre-treatment level after an increase; the activities of SOD,POD and CAT activity were also significantly increased.Wang et al.[6]studied the effects of osmotic stress on protective enzyme system and found thatosmotic stress increased the activity of protective enzymes to avoid damage caused by stress to cells.
The present study revealed that MDA content of grazing populationwas higher than that of non-grazing population throughout the growth period of A.cristatum,proving thatgrazing stress damaged A.cristatum population and exacerbated membrane lipid peroxidation.SOD activity was significantly increased from heading to flowering stage,butwas rapidly decreased at seed maturity stage,but the difference between grazing and non-grazing populations was not significant,indicating that SOD activity changed little under grazing stress,and the increased SOD activity could be a result of drought and other environmental stresses.POD activity was increased from heading stage,and it was higher in grazing population than in nongrazing population,suggesting that POD played an important role for clearing reactive oxygen and maintaining cellstability of A.cristatum under droughtstress.
SOD activity changed significantly among the differentgrowth stages,but had no obvious response to grazing pressure.POD activity in A.cristatum was increased under grazing stress. The soluble sugar content of grazing population was higherthan thatofnongrazing population of A.cristatum at tillering stage,and the difference was significant(P<0.05).MDA content of grazing population was higher than that of non-grazing population during throughout the whole growth period of A.cristatum,indicating that grazing stress caused the accumulation of free radicals and exacerbated membrane lipid peroxidation of A.cristatum.POD played an important role for relieving the damage caused by grazing stress and clearing the harmful substances of A.cristatum.
[1]ARCHER SR,TIESZEN LL.Plant response to defoliation:Hierarchical considerations[A].In O.Gccdmundsson (ed.)Grazing research at Aorthern latitudes[C].New York:Plenum Press, 1996.
[2]XU YY(许月英),ZHENG R(征荣), YANG TQ(杨体强),et al.The influence of electric field treatment on enzyme activities of gaertn in seeding stage(电场处理冰草种子对幼苗酶活性的影响) [J].Journalof Jishou University(Natural Science Edition)(吉首大学学报:自然科学版),2003,24(4):21-23.
[3]PAN RC(潘瑞炽),DOU ZJ(豆志杰),YE QS(叶庆生).Effects of methyl jasmonate on SOD activity and membrane lipid peroxidation of peanut seedlings under water stress(茉莉酸甲酯对水分胁迫下花生幼苗SOD活性和膜脂过氧化作⒚的影响)[J].Journal of Plant Physiology and Molecular Biology(植物生理学报),1995,21(3):221-228.
[4]ZHANG XZ(张宪政),SU ZS(苏正淑).A review on the damage caused by water deficitto crops(作物水分亏缺伤害生理研究概况)[J].JournalofShenyang AgriculturalUniversity(沈阳农业大学学报), 1996,27(1):85-91.
[5]ZHAO ML(赵萌莉),LIN P(林鹏). Molecular markers and its use in mangrove genetic diversity(分子标记技术在红树植物遗传多样性研究中的应⒚). Journal of Inner Mongolia Agricultural University(内蒙占农业大学学报), 2002,23(1):112-114.
[6]WANG RH(王荣华),SHIL(石雷),TANG GG(汤庚国),et al.Effect of osmotic stress on activities of protective enzymes system in Agropyron mongolicum seedling(渗透胁迫对蒙古冰草幼苗保护酶系统的影响)[J].Bulletin of Botany(植物学通报),2003,20(3):330-335.
Responsible editor:Qingqing YIN
Responsible proofreader:Xiaoyan WU
放牧胁迫下冰草叶片内保护酶活性及丙二醛含量的变化
秀 花*
(西藏农牧科学院畜牧兽医研究所,西藏拉萨850009)
[目的]探索冰草在放牧胁迫下的生理适应机理,为牧草资源的保护㈦合理利⒚、优质牧草的选育㈦引进提供理论依据。[方法]分别在植物分蘖期(5月下旬)、抽穗期(6月下旬)、开花期(7月下旬)、种子成熟期(8月下旬),随机采集放牧和无放牧区冰草植株上层同龄新生成熟叶片,装入冰盒带回实验室测定超氧化物岐化酶(SOD)、过氧化物酶(POD)酶活性以及丙二醛(MDA)含量。[结果]放牧冰草种群MDA含量在整个物候期都高于无放牧冰草种群;SOD活性抽穗期到开花期明显增加,到种子成熟期又急速下降,但无放牧㈦放牧种群之间的差异极小;POD活性从抽穗期开始一直升高,而且放牧冰草种群的活性高于无放牧冰草种群。[结论]放牧胁迫使冰草体内MDA含量增加,膜脂氧化程度加剧,在清除放牧胁迫所积累的自由基过程中POD起重要作⒚。
放牧胁迫;SOD;MDA;POD
公益性行业(农业)科研专项(201003023)。
秀花(1977-),女,蒙古族,内蒙古兴安人,硕士,助理研究员,主要从事草地生态和管理研究工作,E-mail:xiuhua621@163.com。*通讯作者。
2014-10-25
Supported by Special Fund for Agro-scientific Research in the Public Interest (201003023).
*Corresponding author.E-mail:xiuhua621@163.com
Received:October25,2014 Accepted:December10,2014
修回日期2014-12-10
 Agricultural Science & Technology2015年1期
Agricultural Science & Technology2015年1期
- Agricultural Science & Technology的其它文章
- Technology Research of Plantlets Rooting and Transplanting on Tissue Culture of Ilex centrochinensis
- Establishmentand Comparison of Two TaqMan Real-time PCR Methods for PCV2
- Analysis on Expression Patterns of NAC1 Gene in Tomato Induced by Low Temperature
- Characteristics and High-yielding Cultivation Technology of HuaimaiNo.29
- Study on Sensitivities of16 Rice Varieties to Acetochlor
- ELISA Detection of Antibody against Canine Distemper Virus in Fox
