舟山群岛海域沉积物厌氧氨氧化细菌多样性
张东声, 刘镇盛, 张海峰, 王小谷, 王春生,2
1 国家海洋局海洋生态系统与生物地球化学重点实验室, 杭州 310012 2 卫星海洋环境动力学国家重点实验室, 杭州 310012
舟山群岛海域沉积物厌氧氨氧化细菌多样性
张东声1,*, 刘镇盛1, 张海峰1, 王小谷1, 王春生1,2
1 国家海洋局海洋生态系统与生物地球化学重点实验室, 杭州 310012 2 卫星海洋环境动力学国家重点实验室, 杭州 310012
通过构建16S rRNA基因文库和克隆测序研究了舟山海域沉积物中厌氧氨氧化细菌(AAOB)的多样性。从5个克隆文库中共获得297条16S rRNA基因序列,包含16个操作分类单元(OTUs)。离岸距离较近的3个站具有相似的群落结构,且与离岸较远的2个站具有明显差异。系统发育结果显示,Scalindua属是该海域AAOB的优势类群,95.3%的序列与Scalindua属AAOB具有较近的亲缘关系;1条序列与Kuenenia属具有较近的亲缘关系;此外还有15条序列与已知的AAOB相似性较低。相关性分析表明沉积物有机碳含量与多样性指数具有显著正相关,可能是该海域AAOB多样性变化的重要影响因子。
厌氧氨氧化细菌(AAOB); 舟山群岛; 海洋沉积物; 多样性
微生物的硝化作用能够将环境中的铵盐和亚硝酸盐转化成硝酸盐[1],因而对保持河口等生境的水质和生态健康具有重要作用。然而,近年来河口生态系统的季节性缺氧现象越发严重,其出现的频率、范围、持续时间和强度都呈现持续上升的趋势[2]。低氧容易导致好氧的硝化作用中断,使具有生物毒性的亚硝酸盐大量积聚,进而引发一系列生态和环境问题[3]。
厌氧氨氧化(Anaerobic ammonium oxidation, anammox)是由一类微生物主导,在缺氧条件下以亚硝酸盐为底物进行氨氧化并产生氮气的过程[4]。当硝化作用受到阻碍时,厌氧氨氧化能够有效地降低亚硝酸盐的浓度,有助于保持水质,维持生态系统的健康。厌氧氨氧化作用最初在废水处理装置中发现[5],主要受浮霉菌门的一类化能自养细菌调控,这类细菌很难从自然环境中分离纯化[6],目前已有描述的包括5个属(Brocadia,Kuenenia,Scalindua,Anammoxoglobus和Jettenia)[7-9]。厌氧氨氧化细菌(AAOB)在自然环境中的分布非常广泛,在淡水沉积物[10-11]、海水沉积物[12]和低氧海水[13]都有发现,厌氧氨氧化作用是水生环境中维持氮平衡的一个重要途径。
舟山群岛位于长江口外海域,受海洋和长江径流的影响,属于咸淡水生态系统的交错群落,具有独特的氮元素生物地球化学循环特征,是厌氧氨氧化作用的重要发生场所。近年来长江口外夏季低氧现象愈加严重,在夏季整个舟山渔场几乎都被低氧区所覆盖[14-15]。低氧现象严重危害该海域的渔业资源和生态系统健康、破坏该海域的海洋经济[2]。Dang等人曾对该海域沉积物中的氨氧化古菌进行过报道[16],但目前还未有关于舟山群岛海域低氧区AAOB的研究。本文采用16S rRNA基因文库构建和克隆测序法,研究夏季舟山海域沉积物中的AAOB多样性,旨在初步了解其种类组成和分布情况,为揭示海洋沉积环境中AAOB对低氧的响应机制提供科学依据。
1 材料和方法
1.1 样品采集
2012年6月在舟山以东海域采集沉积物表层泥样和底层水样,采样站位信息见表1。沉积物泥样使用抓斗采泥器采集,取少量沉积物样品装入预灭菌过的微生物采样杯,-20℃冷冻保存,带回实验室分析;底层水样使用Niskin采水器采集,水样经GF/F滤膜过滤,于-20℃冷冻保存。
1.2 环境参数分析
水样温度和盐度使用HydroLab多参数分析仪现场测定,水样营养盐和溶解氧浓度按照《海洋调查规范》[17]使用分光光度仪测定,沉积物有机碳、总碳和总氮含量参照《海洋监测规范》[18]使用元素分析仪测定。
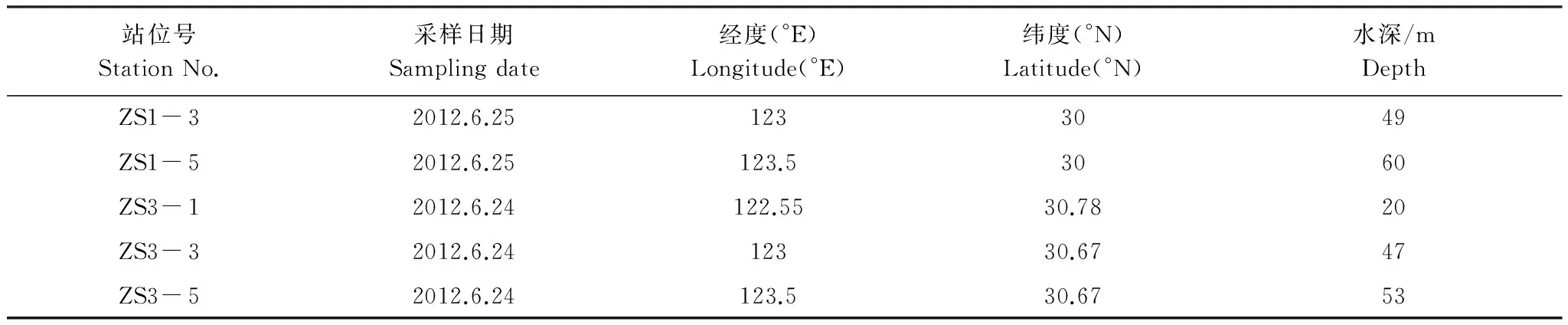
表1 采样站位信息
1.3 DNA的提取和扩增
取少量(1—2 g)沉积物样品,使用Fast DNA@SPIN Kit for soil(MP,美国)提取沉积物总DNA。使用AAOB的16S rRNA基因特异引物[19]进行DNA扩增:Amx368F,5′-TTCGCAATGCCCGAAAGG-3′和Amx820R,5′-AAAACCCCTCTACTTAGTGCCC-3′, 50 μl PCR反应体系包含:5 μL 10×PCR buffer、200nmol/L dNTPs、前后引物各0.25 μmol/L、1 U Taq酶和1 μL DNA提取物。PCR反应条件:94 ℃ 4 min;94 ℃ 30 s,56 ℃ 30 s,72 ℃ 60 s,30个循环;72 ℃ 7 min。1%琼脂糖凝胶电泳检测PCR扩增结果,并用QIAquick Gel Extraction Kit(QIAGEN,美国)纯化、回收DNA目的扩增产物。
1.4 TA克隆与测序
PCR回收产物与pMD20-T载体(TaKaRa)连接,转化到感受态细胞E.coliDH5α(TaKaRa),涂布于LB平板(含Amp、IPTG和X-Gal),37℃培养过夜,筛选阳性克隆构建基因克隆文库。将所获的阳性克隆送生工生物工程(上海)测序。
1.5 AAOB 16S rRNA基因序列分析
测序获得的16S rRNA 基因序列在NCBI 数据库中进行BLAST 比对(http://www.ncbi.him.nih.gov),下载相似性最高的序列作为参比序列,应用Clustal X进行匹配比对。用DOTUR软件包将相似性≥97%的序列定义为一个分类单元(OTU)[20],并构建稀释度曲线。用MEGA4 软件构建系统发育树[21]。研究获得序列已提交GenBank,注册号为KF029766-KF030062。
1.6 多样性分析
用DOTUR软件分析各个克隆文库的生物多样性指标(Shannon和Chao 1),根据OTUs分析结果计算各个克隆文库的覆盖度,计算公式如下[22]:
Good =[1-(n/N)]×100
式中,n代表单克隆OUT的数量,N代表文库中克隆总数量。AAOB群落的生态分布特征使用Fast UniFrac进行分析[23]。
2 结果
2.1 环境特征
研究海域5个采样站位根据其环境特征可分为河口和海洋两类(表2)。EZ3-1站距离长江口最近,受长江径流影响底层海水温度较高、盐度较低,硝酸盐和硅酸盐浓度是研究站位中最高,沉积物中碳、氮含量也是研究站位中最高。其它4个站位环境特征较为均匀,盐度较高、营养盐浓度较低,属典型的海洋环境特征,但EZ1-3和EZ3-3两个站营养盐浓度和沉积物碳、氮含量略高于EZ1-5和EZ3-5,表明长江径流等对距离较近的站位仍有一定的影响。研究海域底层水的溶解氧浓度较低,其中EZ3-3和EZ3-5站位于长江口外低氧区的核心区内[14],其溶解氧低于其它3个站(<4 mg/L)。
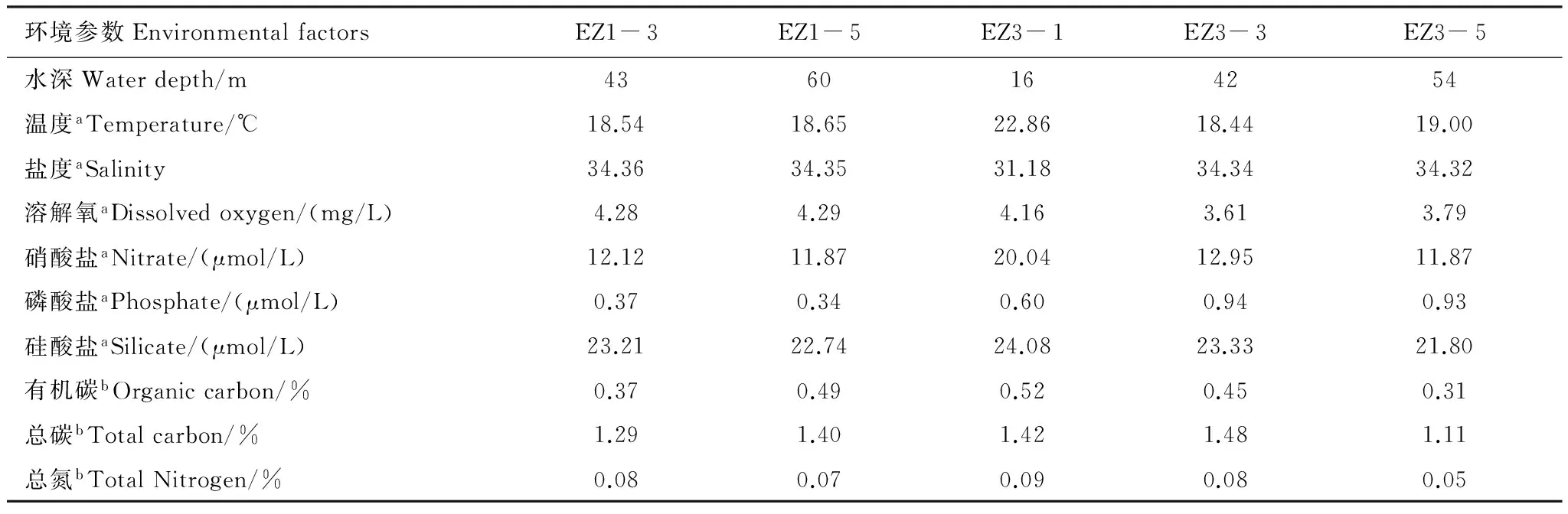
表2 各采样站位环境参数
2.2 克隆文库与多样性分析
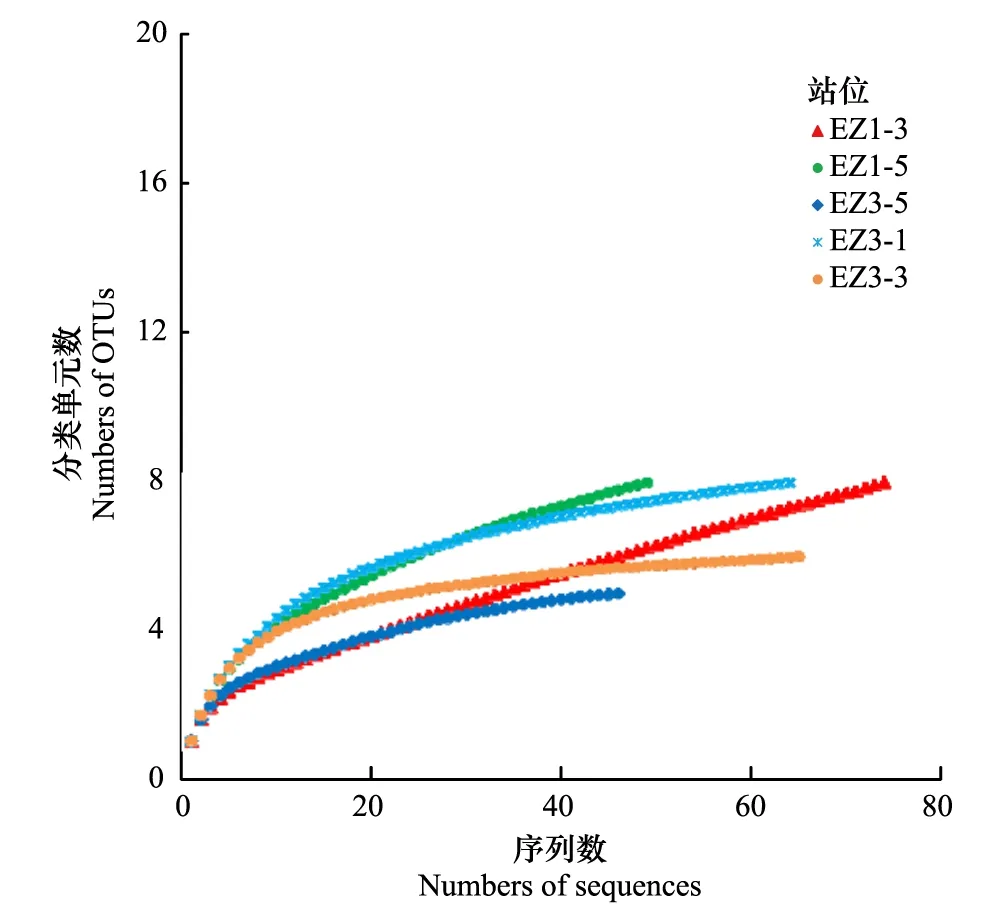
图1 厌氧氨氧化细菌16S rRNA基因稀释度曲线Fig.1 Rarefaction curves of AAOB 16S rRNA libraries
针对5个站沉积物样品分别构建克隆文库,总共获得297条AAOB 16S rRNA基因序列。根据基因序列相似性≥97%归为一个OTU,共获得16个OTUs,5个文库共有的OTUs有5个,文库各自的OUT数在5—8个之间(表3)。稀释度曲线显示5个基因文库用于测序的克隆数量达到或接近饱和(图1)。5个克隆文库的覆盖度较高,均大于80%(表3),表明本研究所构建的文库基本涵盖研究海域沉积物中的AAOB多样性。各个文库的香浓指数和Chao 1指数之间有一定差异(表3),结果显示EZ1-5、EZ3-1和EZ3-3文库的多样性较高,EZ1-3和EZ3-5文库的多样性较低,而EZ1-3和EZ1-5文库的物种数量较高,EZ3-1、EZ3-3和EZ3-5文库的物种数量较低。
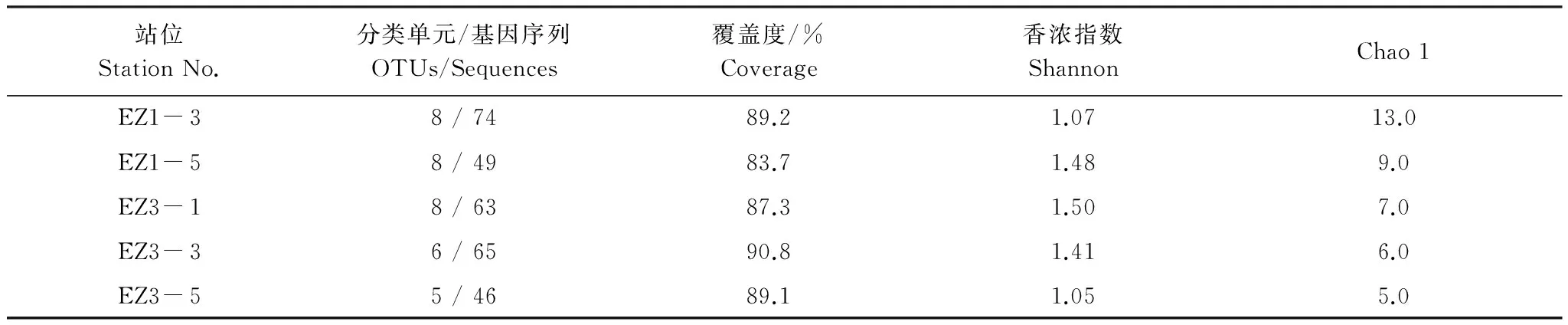
表3 厌氧氨氧化细菌基因文库的多样性指数
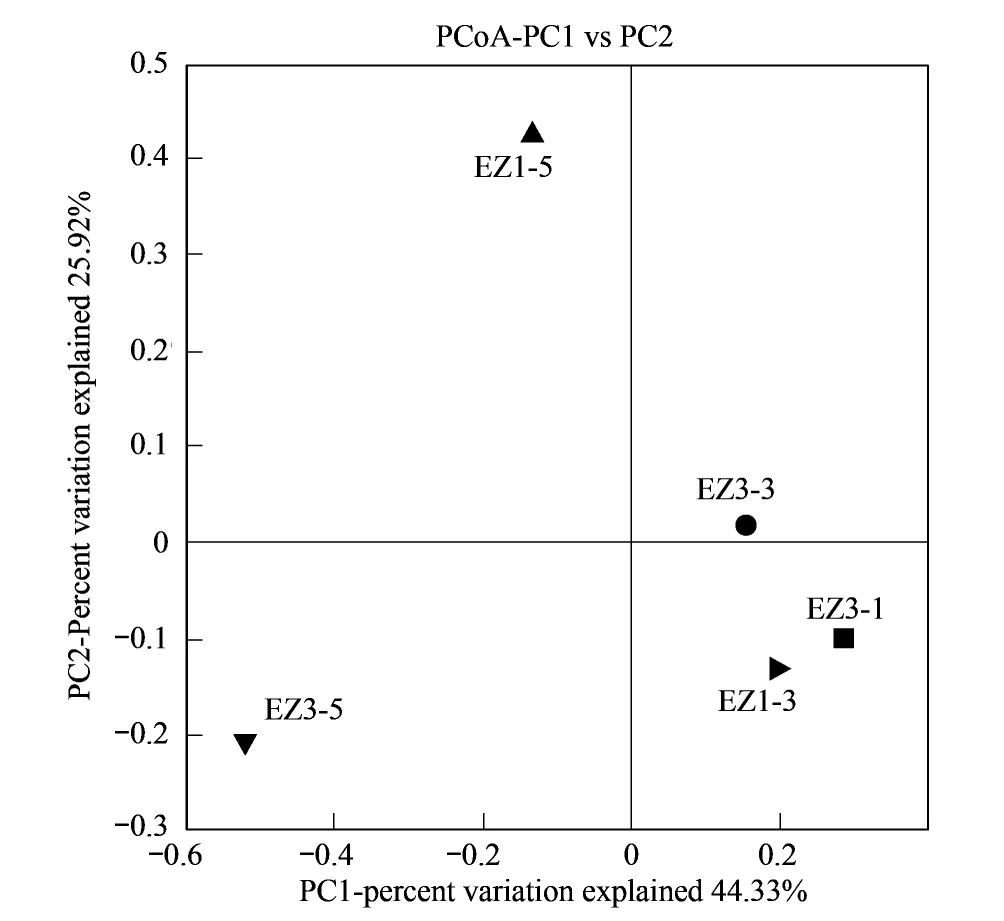
图2 基于16S rRNA基因的厌氧氨氧化细菌群落PCoA分析 Fig.2 The Fast UniFrac weighted PCoA analysis of the anammox communities using 16S rRNA gene sequences
使用FastUniFrac PCoA分析了研究海域5个站位AAOB群落组成的空间分布特征。图2显示,PCoA的前两个主坐标轴(PC1和PC2)能够解释5个采样站位之间AAOB群落差异的70.25%,根据AAOB的群落结构可以把5个站分成3类,EZ1-3、EZ3-1和EZ3-3三个站位具有相似的AAOB群落结构,EZ1-5和EZ3-5两个站AAOB的群落结构与上述3个站具有明显的差别。
2.3 系统发育分析
对从舟山海域沉积物样品中构建的5个AAOB的16S rRNA基因文库进行了系统发育分析,结果显示,本研究获得的绝大部份序列(283条)属于Scalindua属(图3),占总序列数的95.3%。由图3可以看出,来自Scalindua属的序列主要聚类于Brodae和Wagneri两个分支。Brodae分支包含4个OUT,245条序列,占Scalindua属序列数的86.6%,与CandidatusScalinduabrodae的亲缘关系较近。其中,OTU1包含47条序列,与CandidatusS.brodae的相似性在96.0%—97.7%之间,主要与来自河口沉积物[24]、深海热液喷口沉积物[25]的AAOB非培克隆具有较近的亲缘关系;OTU2包含53条序列,与CandidatusS.brodae的相似性在96.8%—97.9%之间,与来自河口沉积物中[24]的AAOB非培克隆具有较近的亲缘关系;OTU3包含26条序列,与CandidatusS.brodae(AY254883)的相似性最高(>98%),与来自秘鲁上升流和纳米比亚上升流区的氧最小层(OMZ)中[26-27]的AAOB非培克隆具有较近的亲缘关系;OTU4包含119条序列,与CandidatusS.brodae的相似性在95.1%—95.6%之间,主要与来自长江口、南海和黄海的AAOB非培克隆[28-30]具有较近的亲缘关系。Wagneri分支包含4个OTU,共36条序列,占Scalindua属序列总数的12.7%,与CandidatusScalinduawagneri的亲缘关系较近。其中OTU10的10条序列与CandidatusS.wagneri的相似性较高(>97%),其他3个OTU的26条序列与CandidatusS.wagneri的相似性在94.5%—96.6%之间,与来自潮间带沉积物、河口沉积物、和近岸低氧沉积物等环境中的AAOB非培克隆[24,31,32]具有较近的亲缘关系。此外,2条来自EZ3-1文库的序列EZ3-1-8(OTU5)和EZ3-1-49(OTU6)也聚类于Scalindua分支内,但与已知CandidatusScalinduabrodae和CandidatusScalinduawagneri的相似性均较低,分别为91%和93%左右,它们可能代表了该属未知的AAOB菌株。
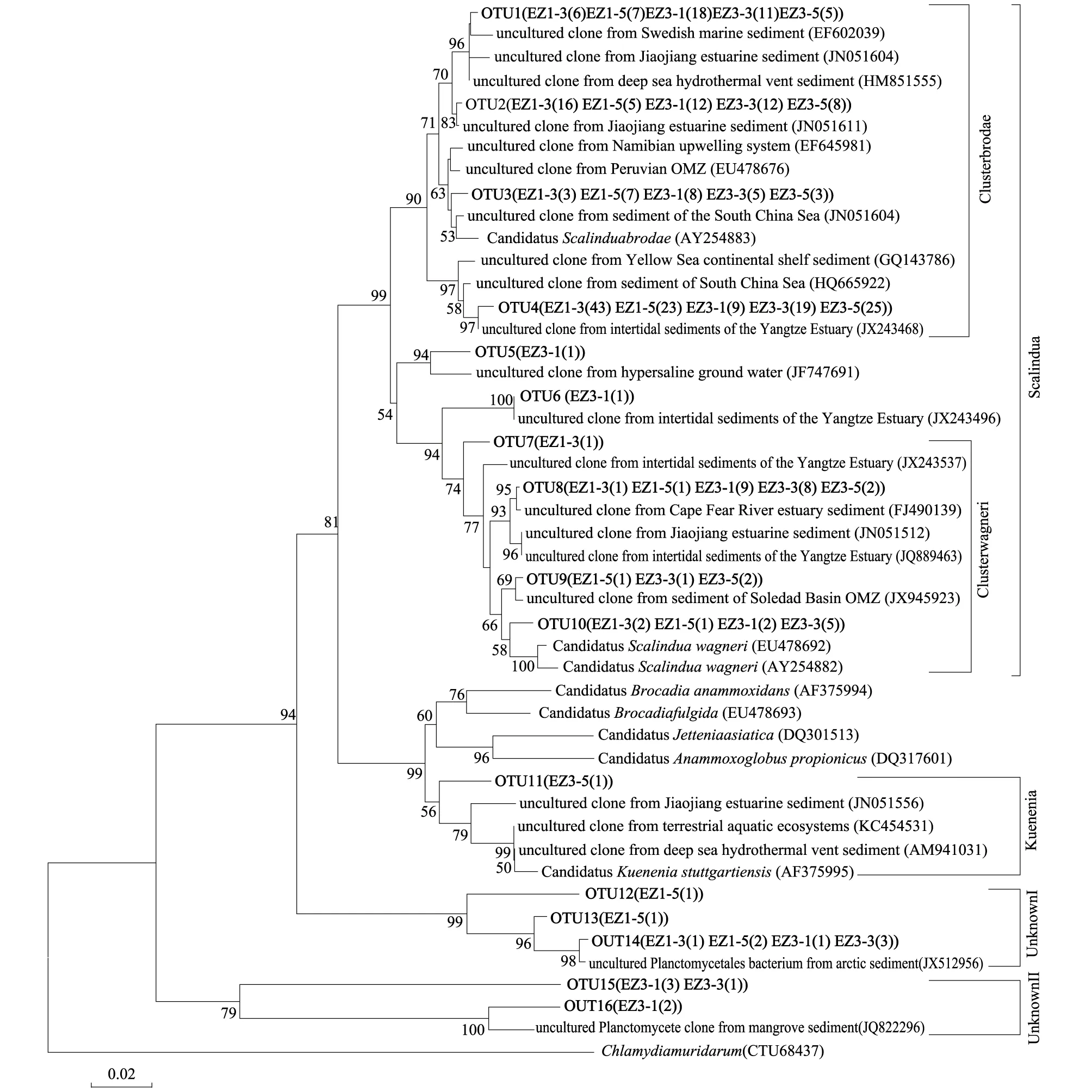
图3 AAOB 16S rRNA基因系统发育树Fig.3 Neighbor-joining phylogenetic tree of anammox bacteria-related 16S rRNA gene fragments from the study areaClong names include the sample name and the number of times a sequence among all of the sequenced clones of samples; Bootstrap values represent 1000 replications and only values above 50% are shown; Branch lengths correspond to sequence differences as indicated by the scale bar; Numbers in parentheses refer to the number of clones were assigned to an OUT
EZ3-5文库中有一条序列EZ3-5-46(OTU11)聚类于Kuenenia分支,与Kuenenia属的其代表种CandidatusKueneniastuttgartiensis的相似性为94.6%,与来自河口、陆源淡水和深海热液喷口的AAOB[24,33]具有较近的亲缘关系。此外,还有5个OTU共15条序列与已知AAOB相似性较低,这些序列可分为2个分支,Unknown I分支包含3个OTU,9条序列,与来自极地海洋沉积物中的非培克隆[34]具有较近的亲缘关系;Unknown II分支包含2个OUT,6条序列,与来自红树林沉积物中的非培养细菌具有较近的亲缘关系(图3)。
3 讨论
厌氧氨氧化细菌(AAOB)广泛分布在河流、湖泊、海洋等不同的环境中,群落组成在不同环境中具有明显的差异性。Scalindua属是海洋环境中AAOB的优势类群[35],它们具有较高的盐耐受性,在多种海洋环境中,特别是上升流区水柱的OMZ和一些次低氧水体中[35-36]都有报道。本文研究结果表明舟山群岛海域的AAOB是典型的海洋环境群落,Scalindua属是研究海域沉积物中AAOB的优势类群,来自于该属的序列占总序列数的95.3%。本文研究海域还获得了1条与Kuenenia属AAOB 亲缘关系较近的16S rRNA基因序列。该属主要分布在淡水或陆源生境中[36-37],在河口等咸淡水交汇的低盐环境中也有分布[24,38]。但本文在距离陆地较远的EZ3-5站获得了Kuenenia属序列,该站位主要受外海水团控制,盐度较高(34.32),营养盐浓度较低,而在盐度相对较低的EZ3-1站未发现Kuenenia属序列。近期Byrne等在北大西洋中脊的深海热液区的贻贝和烟囱体中也获得了与Kuenenia属亲缘关系较近的序列[33],表明Kuenenia属的部分AAOB对盐度具有较强的耐受能力。本文和Byrne等的研究结果表明来自Kuenenia属AAOB的分布范围可能超出我们原来的认识,一方面这可能受到采样和研究不足的限制,另一方面需要更深水平的二代测序研究证实。此外,除了已知的AAOB类群,本研究还发现了一些与来自海洋环境的浮霉菌门非培养克隆相似性较低(<85%)的序列(Unkown I和Unknown II)。有研究表明,海洋环境中可能存在除浮霉菌门以外的其它厌氧氨氧化微生物,如β-和γ-变形菌中的Nitrosospira、Nitrosomonas和Nitrosococcus[39-40],甚至在某些海洋环境中存在具有厌氧氨氧化能力的古菌[41]。因此,这些序列可能代表了某些未知门类的厌氧氨氧化微生物。
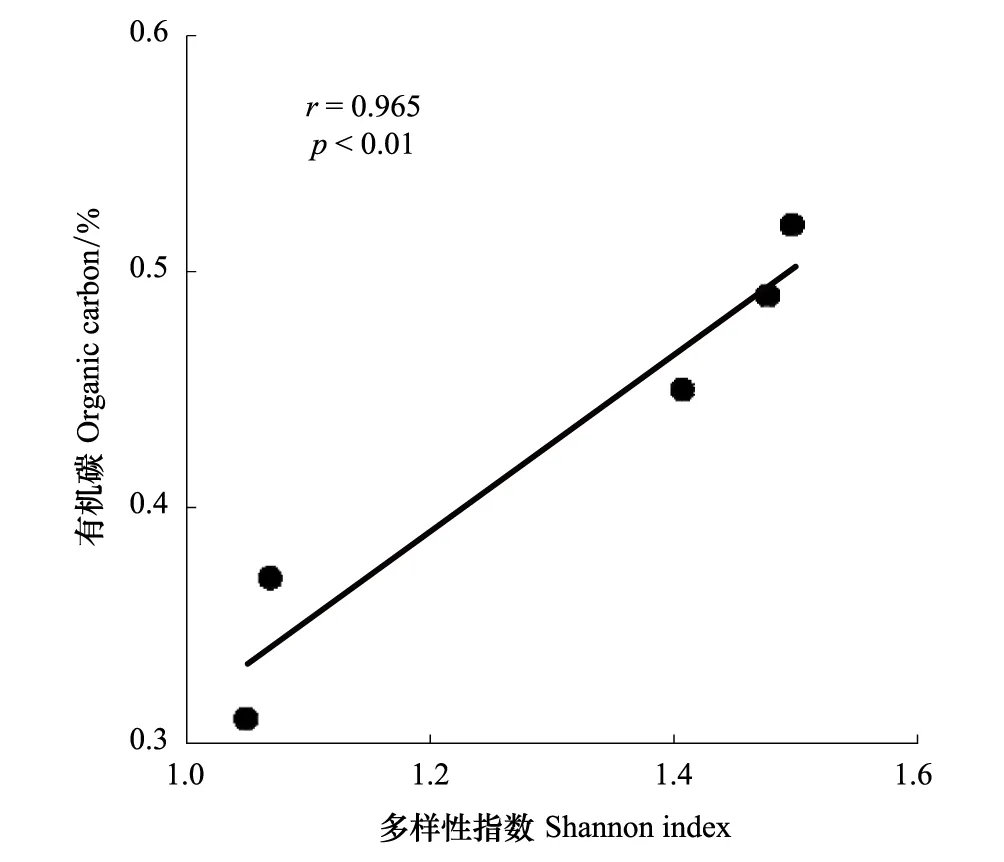
图4 有机碳与多样性指数相关性 Fig.4 Correlation between organic carbon and Shannon index of the five sampling sites
AAOB多样性和群落结构的分布特征与环境因子有着密不可分的关系。Dale等认为盐度是影响河口生态系统中AAOB群落结构的重要因子[31],但本文采样站位之间盐度差别不大,除EZ3-1略低外,其它站位的盐度十分接近。本文中EZ3-1、EZ3-3和EZ1-3三个站的群落结构较为接近,而与EZ1-5和EZ3-5站不同(图2),表明离岸距离和水深可能是影响表层沉积物AAOB群落组成的重要因素[30]。另一方面,各站位的多样性指数却呈现出不同的分布特征,EZ3-1、EZ3-3和EZ1-5三个站的多样性指数高于EZ1-3和EZ3-5站(表2)。相关分析显示沉积物有机碳含量与多样性指数具有显著正相关关系(图4)。Hou等研究认为沉积物有机碳含量是河口生态系统中影响AAOB多样性的重要环境因子[29],高有机碳含量能够促进硝酸盐还原作用,进而提高亚硝酸盐浓度,亚硝酸盐是厌氧氨氧化过程中的电子受体,高浓度的亚硝酸盐有利于促进厌氧条件下的氨氧化作用[42]。Hu等人在椒江口海域的研究表明AAOB多样性指数与沉积物有机碳含量呈正比[24],Li等人在南海北部也有类似结果[30]。
[1] Ward B B. Nitrification and the marine nitrogen cycle // Kirchman D L. Microbial Ecology of the Oceans New York: John Wiley and Sons, 2000: 427-453.
[2] 陈春辉, 王春生, 许学伟, 刘镇盛. 河口缺氧生物效应研究进展. 生态学报, 2009, 29(5): 2595-2602.
[3] Alonso A, Camargo J A. Toxicity of nitrite to three species of freshwater invertebrates. Environmental Toxicology, 2006, 21(1): 90-94.
[4] Jetten M S M, Logemann S, Muyzer G, Robertson L A, Vries S, van Loosdrecht M C M, Kuenen J G. Novel principles in the microbial conversion of nitrogen compounds. Antonie Van Leeuwenhoek, 1997, 71(1/2): 75-93.
[5] Mulder A, van de Graff A A, Robertson L A, Kuenen J G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology, 1995, 16(3): 177-183.
[6] Strous M, Jetten M S M. Anaerobic oxidation of methane and ammonium. Annual Review of Microbiology, 2004, 58: 99-117.
[7] Kartal B, Rattray J, van Niftrik LA, van de vossenberg J, Schmid M C, Webb R I, Schouten S, Fuerst J A, Damste J S, Jetten M S M, Strous M.Candidatus"Anammoxglobuspropionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Systematic and Applied Microbiology, 2007, 30(1): 39-49.
[8] Schmid M, Walsh K, Webb R, Rijpstra W I, van de Pas-Schoonen K, Verbruggen M J, Hill T, Moffett B, Fuerst J, Schouten S, Damste J S, Harris J, Shaw P, Jetten M S M, Strous M.Candidatus"ScalinduaBrodae", sp. nov.,Candidatus"ScalinduaWagneri", sp. nov., two new species of anaerobic ammonium oxidizing bacteria. Systematic and Applied Microbiology, 2003, 26(4): 529-538.
[9] Schmid M C, Maas B, Dapena A, van de Pas-Schoonen K, van de Vossenberg J, Kartal B, van Niftrik L, Schmidt I, Cirpus I, Kuenen J G, Wagner M, Damste J S, Kuypers M, Revsbech N P, Mendez R, Jetten M S M, Strous M. Biomarkers for the in situ detection of anaerobic ammonium oxidizing (anammox) bacteria. Applied and Environmental Microbiology, 2005, 71(4): 1677-1684.
[10] Hu B L, Shen L D, Zheng P, Hu A H, Chen T T, Cai C, Liu S, Lou L P. Distribution and diversity of anaerobic ammonium-oxidizing bacteria in the sediments of the Qiantang River. Environmental Microbiology Reports, 2012, 4(5): 540-547.
[11] Zhu GB, Wang S Y, Wang W D, Wang Y, Zhou L L, Jiang B, Huub J M, Camp O, Risgaard-Petersen N, Schwark L, Peng Y Z, Hefting M M, Jetten M S M, Yin C Q. Hotspots of anaerobic ammonium oxidation at land freshwater interfaces. Nature Geoscience, 2013, 6(2): 103-107.
[12] Dang H Y, Chen R P, Wang L, Guo L Z, Chen P P, Tang Z W. Environmental factors shape sediment anammox bacterial communities in hyper nitrified Jiaozhou Bay, China. Applied and Environmental Microbiology, 2010, 76(21): 7036-7047.
[13] Lam P, Jensen M M, Lavik G, McGinnis D F, Müller B, Schubert C J, Amann R, Thamdrup B, Kuypers M M M. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea, Proceedings of the National Academy of the Sciences of the United States of America, 2007, 104(17): 7104-7109.
[14] Li D J, Zhang J, Huang D J, Wu Y, Liang J. Oxygen depletion off the Changjiang (Yangtze River) Estuary. Science in China (Series D), 2002, 45(12): 1137-1146.
[15] Chen C C, Gong G C, Shiah F K. Hypoxia in the East China Sea: One of the largest coastal low-oxygen areas in the world. Marine Environmental Research, 2007, 64(4): 399-408.
[16] Dang H Y, Zhang X X, Sun J, Li T G, Zhang Z N, Yang G P. Diversity and spatial distribution of sediment ammonia-oxidizing crenarchaeota in response to estuarine and environmental gradients in the Changjiang Estuary and East China Sea. Microbiology, 2008, 154(7): 2084-2095.
[17] GB/T 12763. 4-2007, 海洋调查规范第4部分: 海水化学要素调查. 北京: 中国标准出版社, 2008.
[18] GB/T 17378. 5-2007, 海洋监测规范第5部分: 沉积物分析. 北京: 中国标准出版社, 2008.
[19] Amano T, Yoshinaga I, Okada K, Yamagishi T, Ueda S, Obuchi A, Sako Y, Suwa Y. Detection of anammox activity and diversity of anammox bacteria-related 16S rRNA genes in coastal marine sediment in Japan. Microbes and Environment, 2007, 22(3): 232-242.
[20] Schloss P D, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Applied and Environmental Microbiology, 2005, 71(3): 1501-1506.
[21] Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4. 0. Molecular Biology and Evolution, 2007, 24(8): 1596-1599.
[22] Good I J. The population frequencies of species and the estimation of population parameters. Biometrika, 1953, 40(3/4): 237-264.
[23] Sagaram U S, De Angelis K M, Trivedi P, Andersen G L, Lu S E, Wang N. Bacterial diversity analysis of Huanglongbing pathogen-infected citrus, using PhyloChip arrays and 16S rRNA gene clone library sequencing. Applied and Environmental Microbiology, 2009, 75(6): 1566-1574.
[24] Hu B L, Shen L D, Du P, Zheng P, Xu X Y, Zeng J N. The influence of intense chemical pollution on the community composition, diversity and abundance of anammox bacteria in the Jiaojiang Estuary (China). PLoS ONE, 2012, 7(3): e33826.
[25] Hirsch M D, Long Z T, Song B. Anammox bacterial diversity in various aquatic ecosystems based on the detection of hydrazine oxidase genes (hzoA/hzoB). Microbial Ecology, 2011, 61(2): 264-276.
[26] Woebken D, Fuchs BM, Kuypers MMM, Amann R. Potential interactions of particles-associated anammox bacteria with bacterial and archaeal partners in the Nambian upwelling system. Applied and Environmental Microbiology, 2007, 73(14): 4648-4657.
[27] Woebken D, Lam P, Kuypers M M M, Naqvi S W A, Kartal B, Strous M, Jetten M S M, Fuchs B M, Amann R. A microdiversity study of anammox bacteria reveals a novelCandidatusScalindua phylotype in marine oxygen minimum zones. Environmental Microbiology, 2008, 10(11): 3106-3119.
[28] Hong J K and Cho J C. High level of bacterial diversity and novel taxa in continental shelf sediment. Journal of Microbiology and Biotechnology, 2012, 22(6): 771-779.
[29] Hou L J, Zheng Y L, Liu M, Gong J, Zhang X L, Yin G Y, You L. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. Journal of Geophysical Research: Biogeosciences, 2013, 118(3): 1237-1246.
[30] Li M, Hong Y G, Cao H L, Gu J D. Community structures and distribution of anaerobic ammonium oxidizing andnirS-encoding nitrite-reducing bacteria in surface sediments of the South China Sea. Microbial Ecology, 2013, 66(2): 281-296.
[31] Dale O R, Tobias C R, Song B. Biogeographic distribution of diverse anaerobic ammonium oxidizing (anammox) bacteria in Cape Fear River Estuary. Environmental Microbiology, 2009, 11(5): 1194-1207.
[32] Prokepenko M G, Hirst M B, De Brabandere L, Lawrence D J P, Berelson W M, Granger J, Chang B X, Dawson S, Crane III E J, Chong L, Thamdrup B, Townsend-Small A, Sigman D M. Nitrogen losses in anoxic marine sediments driven byThioploca-anammox bacterial consortia. Nature, 2013, 500(7461): 194-198.
[33] Byrne N, Strous M, Crepeau V, Kartal B, Birrien J L, Schmid M, Lesongeur F, Schouten S, Jaeschke A, Jetten M, Prieur D, Godfroy A. Presence and activity of anaerobic ammonium-oxidizing bacteria at deep-sea hydrothermal vents. International Symposium on Microbial Ecology Journal, 2009, 3(1): 117-123.
[34] Tait K, Laverock B, Widdicombe S. Response of an Arctic sediment nitrogen cycling community to increased CO2. Estuaries and Coasts, 2014, 37(3): 724-735.
[35] Schmid M C, Risgaard-Petersen N, van de Vossenberg J, Kuypers M M, Lavik G, Petersen J, Hulth S, Thamdrup B, Canfield D, Dalsgaard T, Rysgarrd S, Sejr M K, Strous M, den Camp H J, Jetten M S. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environmental Microbiology, 2007, 9(6): 1476-1484.
[36] Schubert C J, Durisch-Kaiser E, Wehrli B, Thamdrup B, Lam P, Kuypers M M M. Anaerobic ammonium oxidation in a tropical freshwater system (LakeTanganyika). Environmental Microbiology, 2006, 8(10): 1857-1863.
[37] Penton C R, Devol A H, Tiedje J M. Molecular evidence for the broad distribution of anaerobic ammonium-oxidizing bacteria in freshwater and marine sediments. Applied and Environmental Microbiology, 2006, 72(10): 6829-6832.
[38] Jetten M S M, Sliekers A O, Kuypers M, Dalsgaard T, van Niftrik L, Cirpus I, van de Pas-Schoonen K, Lavik G, Thamdrup B, Le Paslier D, Op den Camp H J, Hulth S, Nielsen L P, Abma W, Third K, Engstrom P, Kuenen J G, Jorgensen B B, canfield D E, Sinninghe-Damste J S, Revsbech N P, Fuerst J, Weissenbach J, Wagner M, Schmidt I, Schmid M, Strous M. Anaerobic ammonium oxidation by marine and freshwater Planctomycete-like bacteria. Applied Microbiology and Biotechnology, 2003, 63(2): 107-114.
[39] Freitag T E, Prosser J I. Community structure of ammonia-oxidizing bacteria within anoxic marine sediments. Applied and Environmental Microbiology, 2003, 69(3): 1359-1371.
[40] O′Mullan G D, Ward B B. Relationship of temporal and spatial variability of ammonia-oxidizing bacteria to nitrification rates in Monterey Bay, California. Applied and Environmental Microbiology, 2005, 71(2): 697-705.
[41] Wuchter C, Abbas B, Coolen M J, Herfort L, vanBleijswijk J, Timmers P, Strous M, Teira E, Herndl G J, Middelburg J J, Schouten S, Sinninghe Damste J S. Archaeal nitrification in the ocean. The Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(3): 12317-12322.
[42] Lam P, Lavik G, Jensen M M, van de Vossenberg J, Schmid M C, Woebken D, Gutierrez D, Amann R, Jetten M S M, Kuypers M M M. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. The Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(12): 4752-4757.
Diversity of anaerobic ammonium oxidizing bacteria in marine sediments from the Zhoushan Islands
ZHANG Dongsheng1,*, LIU Zhensheng1, ZHANG Haifeng1, WANG Xiaogu1, WANG Chunsheng1,2
1LaboratoryofMarineEcosystemandBiogeochemistry,StateOceanicAdministration,Hangzhou310012,China2StateKeyLaboratoryofSatelliteOceanEnvironmentDynamics,Hangzhou310012,China
Anaerobic ammonium oxidation (anammox) is an important process regulating the balance of marine nitrogen and ecosystem health, particularly under anoxic conditions. The Zhoushan Islands are located east of the Changjiang river estuary, and collect a high load of anthropogenic nitrogen, which leads to severe eutrophication and seasonal hypoxia. Therefore, bacteria that mediate the anammox process are of major interest in this area. Although the importance of anammox-mediating bacteria is known, few studies on these bacteria have been conducted in the East China Sea. To the best of our knowledge, this study is the first to report the diversity, community composition, and distribution of anammox bacteria in the Zhoushan Islands. Field surveys were conducted in June 2012; triplicate surface sediment samples were collected at each site and stored in sterile plastic bags at -80℃ for subsequent DNA extraction and molecular analysis. Total genomic DNA was extracted using the Fast DNA® SPIN Kit for soil. Environmental DNA extracted from sediment samples was used as the template for PCR amplification of anammox 16S rRNA genes using primers Amx368f—Amx820r. The purified fragments were cloned and sequenced for phylogenetic and statistical analyses. In total, 297 sequences belonging to 16 operational taxonomic units (OTUs) were obtained from five 16S rRNA gene libraries. The biodiversity of anammox bacteria was examined using rarefaction analysis of the 16S rRNA genes, the Chao1 estimator, and Shannon index calculations. EZ3-1, EZ3-3, and EZ1-5 exhibited higher diversity than EZ1-3 and EZ3-5. A significant positive correlation between Shannon index and organic carbon content indicate that sediment organic carbon content plays an important role in modulating anammox bacterial diversity in the Zhoushan Island area. Weighted UniFrac PCoA analysis of the 16S rRNA genes demonstrated spatial heterogeneity in the community composition of anammox bacteria; the anammox bacteria in the study area could be divided into three distinct groups. EZ3-1, EZ3-3, and EZ1-3 exhibited similar community composition, while EZ1-5 and EZ3-5 clustered separately. The composition might be affected by distance from land and water depth. Phylogenetic analysis indicated that anammox bacterial communities were dominated by the genusScalindua(283 of 297 sequences). TheScalinduacluster comprised of 245Scalinduasequences with 95.4%—98.5% sequence similarity toCandidatusScalinduabrodae, and 36Scalinduasequences with 94.5%—97.6% sequence identity to the 16S rRNA gene ofCandidatusScalinduawagneri. In addition, twoScalinduasequences that grouped in theScalinduacluster were distantly related to knownScalinduaspecies, indicating that they might represent unidentified species ofScalindua. One sequence recovered from the EZ3-5 library was closely related to genusKuenenia, which is traditionally considered an anammox bacterial genus present in freshwater or low-salinity environments. Our results suggest that members of the genusKueneniamay possess the ability to survive in high-salinity marine environments. Additionally, two clusters of unknown sequences (unknown cluster I and II) were not classified under any known anammox bacterial genus, but were most closely related to 16S rRNA gene sequences recovered from arctic sediment and mangrove sediment, respectively.
anaerobic ammonium oxidation bacteria (AAOB); Zhoushan islands; marine sediments; diversity
浙江省自然科学基金(Y5110185, Y5110171); 国家自然科学基金青年基金项目(41206104, 41203085)
2014-02-21; < class="emphasis_bold">网络出版日期:
日期:2014-12-04
10.5846/stxb201402210303
*通讯作者Corresponding author.E-mail: dszhang7@163.com
张东声, 刘镇盛, 张海峰, 王小谷, 王春生.舟山群岛海域沉积物厌氧氨氧化细菌多样性.生态学报,2015,35(19):6250-6258.
Zhang D S, Liu Z S, Zhang H F, Wang X G, Wang C S.Diversity of anaerobic ammonium oxidizing bacteria in marine sediments from the Zhoushan Islands.Acta Ecologica Sinica,2015,35(19):6250-6258.

