A Crystalline Pb(Ⅱ)Coordination Polymer:Synthesis,Crystal Structure, Exfoliation and Luminescent Property
WANG Peng-FeiWU Xiao-ShuoLU Peng-Peng
(School of Chemistry and Material Engineering,Chizhou College,Chizhou,Anhui 247000,China)
A Crystalline Pb(Ⅱ)Coordination Polymer:Synthesis,Crystal Structure, Exfoliation and Luminescent Property
WANG Peng-Fei*WU Xiao-ShuoLU Peng-Peng
(School of Chemistry and Material Engineering,Chizhou College,Chizhou,Anhui 247000,China)
A novel coordination polymer,[Pb(4-NPA)(H2O)]n(1)(4-H2NPA=4-nitrophthalic acid)with 2D layered structure was obtained via a hydrothermal reaction.Compound 1 has been characterized by IR spectroscopy, elemental analysis,thermogravimetric analysis,single-crystal and powder X-ray diffraction analysis.In 1,the 4-NPA2-anion connects four Pb(Ⅱ)ions with its carboxylate groups in a(κ1-κ1)(κ2-κ2)-μ4-bridging mode,forming a one-dimensional chain structure running along the a axis through the oxygen atoms of the carboxylate groups.The neighboring chains are connected through the O-C-O bridges,resulting in an undulating inorganic layered structure in the ab plane.The layers of 1 can be exfoliated in the methanol using a“top-down”approach.The bulk sample of 1,and the exfoliated sample of 1 as well as its dispersion solution show luminescent properties. CCDC:1408043.
nanosheet;exfoliation;Pb(Ⅱ)coordination polymer;luminescence
0 Introduction
Recently,nanosheets with two-dimensional(2D) structures have attracted significant interest due to their fascinating range of structures and widely variety ofphysicalpropertiesdifferentfromtheirbulk materials[1-5].Most 2D nanosheets are pure inorganic materials(metal oxides,metal hydroxides,metal sulfides),graphene,covalent organic frameworks and so on[6-10].As a class of important hybrid materials,coordination polymers(CPs)have attracted much attention owing to their catalytic,gas storage and separation,photoluminescenceandmagnetism properties.Most of them show different structures from zero-dimensional(0D)unit/clustertothreedimensional(3D)framework structures.Many of the researches on CPs have focused on the syntheses of singlecrystalsandpolycrystallinepowderswith micron-sized grains.Most of the reported CPs feature two-dimensional layered structures packed through the weak inter-layer interactions.Significant attention has been drawn to the layered structures to be exfoliated into nanosheets by top-down delamination,which often exhibit intriguing physical and chemical properties that are rare present in bulk materials[11-13].The topdown approach provides an effective and readily available route for preparing nanosheets materials that are typically 10 to 100 nm thick.
Oneofthepotentstrategiestoconstruct inorganic-organic hybrid materials is the introduction of the aromatic multi-caboxylate ligands because of their various coordination geometries and modes.As a class of dicarboxylate ligand,4-nitrophthalic acid(4-H2NPA)may be a useful block to construct many novel coordination polymers,in which a secondary N-donor ligands such as 1,2-bi(4-pyridyl)ethane,1,3-bis (4-pyridyl)propane,1,4-bis(1-imidazoly)benzene,4,4-bipyridine have been introduced.Subsequently,the organic-inorganic hybrid materials with 2D layer and 3D framework structures have been obtained[14-20]. Interestingly,compound{[Mn(NPTA)(4,4-bpy)(H2O)]· (H2O)2}nshows a 2D homochiral inorganic-organic framework exhibiting a spin-flop transition[17].On the other hand,less attention has been paid to the preparation of the heavy p-block Pb(Ⅱ)coordination polymers[21-22].With this in mind,we use the 4-H2NPA as the organic building block to construct the Pbbased coordination polymer.Herein,we report the preparation,crystal structure,and exfoliation of a novel layered Pb(Ⅱ)coordination polymer,[Pb(4-NPA) (H2O)]n(1).We also report the photoluminescence of the bulk and exfoliated samples as well as the dispersion solution.
1 Experimental
1.1 Materials and methods
Allreagentsemployedwerecommercially availableandusedwithoutfurtherpurification. Elemental analyses(C,H,and N)were carried out on a Perkin-Elmer 240C elemental analyzer.The infrared spectra(KBr pellets)was taken on a Nicolet iS10 spectrometer.Powder X-ray diffraction(PXRD)data werecollectedonaRigakuUltimaⅣX-ray diffractometer with Cu Kα(λ=0.154 056 nm)radiation over the 2θ range of 5°~50°at room temperature.The XRD accelerating voltage and emission current were 30 kV and 50 mA,respectively.Thermogravimetric analysis(TGA)wasperformedonaDTG-60H instrumentunderflowingN2atmospherewitha heating rate of 10℃·min-1from room temperature to 800℃.Scanning electron microscopy(SEM)measurements were performed on a Hitachi S-4800 SEM instrument operated at an acceleration voltage of 5.0 kV.Fluorescence spectra were recorded using a Jobin Yvon Inc.FLUOROLOG-3-TAU spectrfluorometer at room temperature.
1.2 Synthesis of[Pb(4-NPA)(H2O)]n(1)
The mixture of Pb(NO3)2(0.033 1 g,0.1 mmol), 4-H2NPA(0.021 1 g,0.1 mmol),and H2O(10 mL)was stirred at an ambient temperature for 30 min,and then the resulting hydrous gel solution was treated in a 30 mL Telflon-lined stainless steel vessel at 120℃for 72 h.After being slowly cooled to the room temperature,colorlessblockcrystalsof1were obtained and washed by ethanol and H2O and dried in air(Yield:about 40%based on Pb).Elemental analysis Calcd.for C8H5NO7Pb(%):C 22.12,H 1.15,N 3.23. Found(%):C 22.33,H 1.21,N 3.31.IR(KBr,cm-1): 3 472(s),3 070(w),1 604(vs),1 568(s),1 480(m), 1 414(s),1 348(vs),1 245(w),1 157(w),1 120(w), 1 068(m),917(m),848(s),829(s),810(w),787(w), 752(m),731(s),710(s),668(m),591(m).
1.3 Crystallographic analysis
Single crystal X-ray structure analysis of 1 was performedonaBrukerSMARTAPEXⅡdiffractometer(Mo Kα radiation,λ=0.071 073 nm,graphite monochromator)at 296 K.The data were integrated using the Siemens SAINT program[23],which also was used for the intensity corrections for Lorentz polarization,and air absorption.The structure was solved by the direct method using SHELXS-97[24]and refined by full-matrix least-squares on F2using the SHELXL-97 program[25].All non-hydrogen atoms were refined anisotropically and all the hydrogen atoms except those attaching to water molecule were put in calculated positions.The hydrogen atoms of water molecule were found from the Fourier maps.All hydrogen atoms were refined isotropically with the isotropic vibration parameters related to the nonhydrogenatomstowhichtheybonded. Crystallographic and refinement details are listed in Table 1.Selected bond lengths and angles are given in Table 2.
CCDC:1408043.

Table 1Crystallographic data and structure refinement for 1
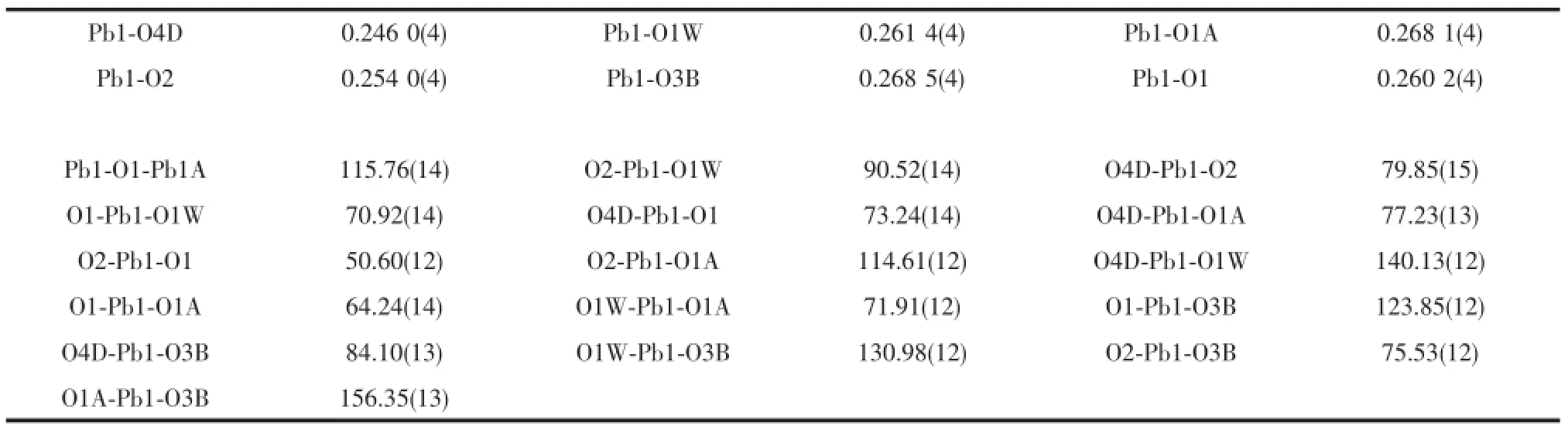
Table 2Selected bond lengths(nm)and angles(°)for 1
2 Results and discussion
2.1 Crystal structure
Compound 1 crystallizes in the triclinic crystal system with the P1 space group.In the asymmetric unit, there is one unique Pb(Ⅱ)ion,one independent 4-NPA2-anion,and one coordination water molecule(Fig.1). Each Pb(Ⅱ)ion was connected to six carboxylate oxygen atoms(O1,O1A,O2,O2B,O3B,O4D)from four different 4-NPA2-anions and one water molecule(OW1) (Pb-O 0.246 0~0.276 3 nm),generating a seven coordination sphere for the Pb(Ⅱ)ion,comparable with those observed in the other Pb(Ⅱ)coordination polymers[26-29].It is noticeable that the Pb1-O1A distance(0.276 3 nm)from the carboxylate group of the 4-NPA2-anion suggested a non-negligible interaction[30].The 4-NPA2-anion presents one kind of coordination mode(Scheme 1),and adopts a μ4-bridging mode to connect four Pb(Ⅱ)ions with its carboxylate groups in a(κ1-κ1)(κ2-κ2)-μ4-bridging mode,forming an one-dimensional chain structure running along the a axis through the oxygen atoms of the carboxylate groups.The neighboring chains are connected through the O-C-O bridges,resulting in an undulating inorganic layered structure in the ab plane(Fig.2a).The Pb…Pb distances are 0.437 87 nm and 0.447 6 nm through the oxygen atom bridges, respectively.The Pb…Pb distance over the O-C-O bridge is 0.549 0 nm.The phenyl groups are grafted on the two sides of the inorganic layer.The layers are further packing along the c axis through the van der Waals interactions(Fig.2b).The interlayer distance is ca.0.95 nm.
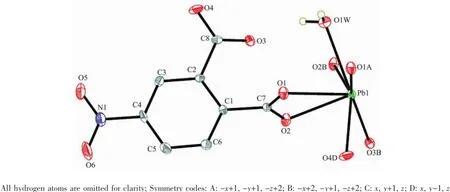
Fig.1Asymmetric unit of 1 with 30%probability ellipsoids

Scheme 1Coordination mode of the 4-NPA2-anion in 1
It is noticeable that a compound[Pb3(4-NPA)2(OH)2]based the same ligand 4-nitrophthalic acid has been reported by Tian et al.under the hydrothermal condition[31].The compound has been obtained under the reaction temperature 140℃and the auxiliary ligand 2,2-bipyridine was added to the start system.In that compound,the two Pb(Ⅱ)ions are six-coordinate, Pb1 with distorted octahedral coordination and Pb2 with a pentagonal pyramid,forming an infinite 2D Pb-O-Pb(3,6-net)honeycomblayerstructure.The structures of compounds 1 and[Pb3(4-NPA)2(OH)2]are completely different,which could be attributed to the different reaction temperature and the second ligand 2,2-bipyridine.

Fig.2(a)Layer of 1 viewed along the c axis;(b)Packing diagram of 1 viewed along the b axis
2.2 Exfoliation of 1
Since each layer of 1 is held only by the interlayer weak interaction,it is possible to exfoliate the layers via a conventional“top-down”approach. The bulk sample of 1(1.2 mg)was ultrasonicated in methanol(5 mL)for a period of 2 hours at room temperature to obtain a milky colloidal suspension. The resulting mixture was left undisturbed in air for 24 h.Apart from methanol,we have also tried other solvents as the exfoliation agent,including N,N-dimethylformamide(DMF),water,propanol,ethanol, hexane,andtetrahydrofuran(THF).Methanol, however,was found to be the most efficient for exfoliating the layers.As shown in Fig.3a,the Tyndall effect is clearly observed in the supernatant.The colloidal solution was centrifuged and the precipitate of nanosheets was collected,and the PXRD patterns of them matches well with that of the as-prepared bulkcrystalline sample of 1(Fig.3b).Scanning electron microscope(SEM)measurements were also carried out for the supernatant of the ultrasonicated sample of 1 and the bulk sample of 1(Fig.4).The SEM image of the bulk sample of 1 shows clearly the pillared structure with a lateral dimension of 5~20 μm.The SEM images of the supernatant show the dispersion of the nanosheets which are incompletely exfoliated.In otherwords,thebulkcrystalsamplecouldbe successfully exfoliated into nanosheets with a few layer thickness.

Fig.3(a)Tyndall effect of the supernatant in colloidal suspensions comprising nanosheets of 1 in methanol solvent; (b)PXRD patterns of the experimental,simulated and exfoliated samples of 1

Fig.4(a)Scanning electron microscopy(SEM)image of as-synthesized 1 crystals;(b)SEM image of the exfoliated sample of 1 after ultrasonication
2.3 Thermal analysis
In order to characterizes the thermal stability of compound 1,thermal gravimetric(TG)analysis was carried out from room temperature to 800℃under N2atmosphere.As shown in Fig.5,compound 1 is stable up to about 130℃,and a weight loss of ca.4.18%in the temperature range of 130~180℃corresponds to thelossofcoordinatedwatermolecules(Calcd. 4.15%).Then,no significant weight loss is observed until the decomposition of the compound occurs at 330℃.Between the temperature range of 330~370℃,a quick weigh loss is observed,which means the compound begins to decompose with the collapsion of the structure.Above 370 up to 800℃,continuous weight loss is also found.The final residue might be PbO(Found 44.65%;Calcd.51.38%).
2.4 Luminescent property

Fig.5TG curve of 1
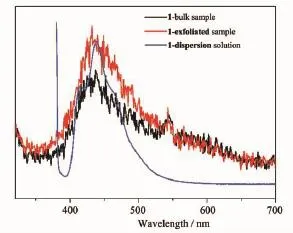
Fig.6Emission spectra of 1,1-exfoliated and 1-dispersion solution under excitation wavelength 300,300, and 372 nm,respectively
3 Conclusions
Inconclusion,anewlayeredPb-based coordination polymer[Pb(4-NPA)(H2O)]n(1)based on4-nitrophthalic acid(4-H2NPA)has been hydrothermal synthesized and characterized.The hybrid material can be successfully exfoliated into nanosheets simply byultrasonicationinthemethanolsolution. Furthermore,the bulk sample,the exfoliated sample and the dispersion solution of 1 show the similar luminescence.
[1]Peng Y,Li Y S,Yang W S,et al.Science,2014,346:1356 -1359
[2]Nie W X,Bao S S,Zheng L M,et al.Chem.Commun., 2014,50:10622-10625
[3]Tan J C,Saines P J,Bithell E G,et al.ACS Nano,2012,6: 615-621
[4]Hermosa C,Horrocks B R,Martínez J I,et al.Chem.Sci., 2015,6:2553-2558
[5]Gallego A,Hermosa C,Castillo O,et al.Adv.Mater.,2013, 25:2141-2146
[6]Zhang X D,Xie Y.Chem.Soc.Rev.,2013,42:8187-8199
[7]Li G H,Wang X W,Ding H Y,et al.RSC Adv.,2012,2: 13018-13023
硫酸钾整体高位守稳,需求支撑尚可。福建与四川烟草招标公告陆续发布,云南等地区烟草招标公告待发布,南方需求支撑明显回暖,后期仍有一定潜在需求。目前市场价格高位坚挺,曼海姆硫酸钾50%粉主流出厂价2850-2950元/吨。青海水盐体系粉状硫酸钾主流到站报价2750-2800元/吨,开工较低,交投维稳,实际成交可谈。罗钾处于检修期,价格目前坚挺守稳,地区市场仍有部分前期货源,51%粉状3000元/吨,实际成交可议。
[8]Xie J F,Zhang J J,Xie Y.et al.J.Am.Chem.Soc.,2013, 135:17881-17888
[9]Geim A K,Novoselov K S.Nat.Mater.,2007,6:183-191
[10]Chandra S,Kandambeth S,Banerjee R,et al.J.Am.Chem. Soc.,2013,135:17853-17861
[11]Amo-Ochoa P,Welte L,González-Prieto R,et al.Chem. Commun.,2010,46:3262-3264
[12]Li P Z,Maeda Y,Xu Q.Chem.Commun.,2011,47:8436-8438
[13]Saines P J,Tan J C,Cheetham A K,et al.Dalton Trans., 2012,41:8585-8593
[14]Li G L,Yin W D,Wang L Y,et al.J.Solid State Chem., 2014,220:1-8
[15]Li G L,Liu G Z,Huang L L,et al.Chin.J.Struct.Chem., 2014,33(6):942-946
[16]Li G L,Liu G Z,Huang L L,et al.J.Inorg.Organomet. Polym.,2014,24:617-623
[17]Li W,Barton P T,Cheetham A K,et al.Dalton Trans., 2011,40:7147-7152
[18]Li G L,Liu G Z,Wang L Y,et al.Chem.Commun.,2014, 50:2615-2617
[19]Wang P F,Wu S,He S Y,et al.J.Inorg.Organomet. Polym.,2014,24:608-616
[20]Wang P F,Wu S,He S Y,et al.J.Inorg.Organomet. Polym.,2015,25:1160-1168
[21]Santra A,Bharadwaj P K.Cryst.Growth Des.,2014,14: 1476-1485
[22]Chen S C,Zhang Z H,Du M,et al.Cryst.Growth Des., 2011,11:4190-4197
[23]SAINT,ProgramforDataExtractionandReduction, Siemens Analytical X-ray Instruments,Madison,WI,1994-1996.
[24]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of Göttingen,Göttingen,Germany,1997.
[25]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,UniversityofGöttingen,Göttingen,Germany,1997.
[26]XU Zhan-Lin(徐占林),HE Yu(贺宇),PANG Jin-Hui (逄金慧),et al.Chinese J.Inorg.Chem.(无机化学学报), 2011,27(11):2279-2282
[27]Bo Q B,Pang J J,Wang H Y,et al.New J.Chem.,2015,39: 431-438
[28]Wu K F,Jiang F,Bai X L,et al.J.Inorg.Organomet. Polym.,2015,25:879-885
[29]LIU Jing(刘晶),JIA Xiao-Yan(贾晓燕),CHENG Mei-Ling (程美令),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(9):2165-2173
[30]Song J L,Lei C,Sun Y Q,et al.J.Solid State Chem., 2004,177:2557-2564
[31]Sun Z,Tian L J,Zhnag X T,et al.J.Coord.Chem., 2012,65:1847-1857
[32]Dutta S K,Perkovic M W.Inorg.Chem.,2002,41:6938-6940
[33]Yang E C,Li J,Ding B,et al.CrystEngComm,2008,10:158 -161
[34]Li X Q,Zhang H B,Wu S T,et al.CrystEngComm,2012, 14:936-944
一个晶态铅配位聚合物的合成、晶体结构、剥离及荧光性质
汪鹏飞*吴小说路朋朋
(池州学院化学与材料工程学院,池州247000)
通过水热反应合成了1个二维层状结构的铅配位聚合物,[Pb(4-NPA)(H2O)]n(1)(4-H2NPA=4-硝基邻苯二甲酸)。对化合物1进行了红外光谱、元素分析、热重性质以及单晶、粉末X射线衍射表征。在化合物1中,有机配体4-NPA2-阴离子采用(κ1-κ1)(κ2-κ2) -μ4桥连方式连接了4个Pb(Ⅱ)离子形成一维链状结构,该链状结构再通过1个羧酸基团形成一个二维无机层结构。利用“自上而下”的方法将该化合物在甲醇中进行了剥离实验。此外,我们还研究了化合物1的块状样品、剥离样品以及分散在甲醇中的样品的荧光性质。
纳米片;剥离;铅配位聚合物;荧光
O614.43+3
A
1001-4861(2016)06-1071-07
2015-10-07。收修改稿日期:2016-05-02。
10.11862/CJIC.2016.137
国家自然科学基金(No.21101019)、安徽省高校优秀青年人才支持计划重点项目(No.gxyqZD2016371)和池州学院自然科学研究项目(No.2015ZR001)资助。
*通信联系人。E-mail:wangpfchem@gmail.com;njuwangpf@163.com

