Syntheses,Crystal Structures and Theoretical Calculations of Nickel/Cobalt Supramolecular Coordination Compounds
WANG Qing-WeiSUI WeiWANG Ya-NanLI Xiu-MeiLIU Bo
(1Key Laboratory of Preparation and Applications of Environmental Friendly Materials, Ministry of Education,Jilin Normal University,Siping,Jilin 136000,China)
(2Faculty of Chemistry,Tonghua Normal University,Tonghua,Jilin 134002,China)
Syntheses,Crystal Structures and Theoretical Calculations of Nickel/Cobalt Supramolecular Coordination Compounds
WANG Qing-Wei*,1SUI Wei1WANG Ya-Nan1LI Xiu-Mei2LIU Bo1
(1Key Laboratory of Preparation and Applications of Environmental Friendly Materials, Ministry of Education,Jilin Normal University,Siping,Jilin 136000,China)
(2Faculty of Chemistry,Tonghua Normal University,Tonghua,Jilin 134002,China)
Two new metal-organic supramolecular coordination compounds[Ni(eoba)(phen)(H2O)2]·0.58H2O(1) and[Co(eoba)(phen)(H2O)2]·H2O(2)(H2eoba=4,4′-(ethane-1,2-diyldioxy)dibenzoicacid,phen=phenanthroline) have been hydrothermally synthesized and structurally characterized by elemental analysis,IR spectrum,TG and single-crystal X-ray diffraction.Complexes 1 and 2 are isomorphous.Each is six-coordinated by two nitrogen atoms from phen molecule,two carboxylate oxygen atoms from eoba ligand and two coordinated water molecules. In addition,natural bond orbital(NBO)analysis of 1 was performed by the PBE0/LANL2DZ method in Gaussian 03 Program.The calculation results show obvious covalent interaction between the coordinated atoms and Ni(Ⅱ) atom.CCDC:1417662,1;1417663,2.
hydrothermal synthesis;crystal structure;nickel complex;cobalt complex;natural bond orbital
0 Introduction
Recently,there has been much interest in the construction of coordination compounds due to their versatile structures and interesting topologies[1-4]as well as their potential applications as functional materialsinthefieldsofmolecularmagnetism, catalysis,gas sorption and optoelectronic devices[5-9].The most useful building blocks for constructing organic-inorganic hybrid coordination compounds are carboxylate and N-donor ligands.Aromatic carboxylate ligands have been extensively employed in the constructionofvariousdimensionalcoordination compounds because of their abundant coordination modes and high structural stability for functional materials applications[10-16].
In this aspect,the chelting ligand phenanthroline (phen)have been widely used in the construction of metal-organic coordination polymers[17].In addition,as a versatile ligand,4,4′-(ethane-1,2-diyldioxy)dibenzoic acid(H2eoba)has been hardly used to construct coordination compounds containing transition metals[18].
To explore the combination effects of this neutral ligandphenandanionicligand,wesynthesize coordination compounds of Co(Ⅱ)and Ni(Ⅱ)containing these ligands.Herein,we report the synthesis and characterizations of two new coordination compounds, namely,[Ni(eoba)(phen)(H2O)2]·0.58H2O(1)and [Co(eoba)(phen)(H2O)2]·H2O(2),which exhibit twodimensional(2D)supramolecular network structures.
1 Experimental
1.1 General procedures
All reagents were purchased commercially and used without further purification.Elemental analyses (C,H and N)were measured on a Perkin-Elmer 2400 CHN Elemental Analyzer.IR spectrum was recorded in the range of 4 000~400 cm-1on a Nicolet 6700 spectrometer using a KBr pellet.TG studies were performed on a Perkin-Elmer TGA7 analyzer.Powder X-ray diffraction(PXRD)patterns were obtained on Bruker D8 Advance X-ray diffractmeter with Cu Kα radiation(λ=0.154 056 nm)at room temperature.
1.2 Synthesis
[Ni(eoba)(phen)(H2O)2]·0.58H2O(1):A mixture of Ni(OAc)2·4H2O(0.050 g,0.2 mmol),H2eoba(0.045 g, 0.15 mmol),phen(0.027 g,0.15 mmol),H2O(10 mL) and C2H5OH(1 mL)was put in a 30 mL Teflon-lined stainless steel vessel,and then the vessel was sealed and heated at 150℃for 5 days.After the reaction mixture was slowly cooled down to room temperature at the rate of 5℃·h-1,green block crystals were collected by filtration and washed with distilled water in 43%yield(based on Ni).Anal.Calcd.for C28H25.16N2NiO8.58(%):C,57.42;H,4.33;N,4.78.Found (%):C,56.92;H,4.09;N,4.07.IR(cm-1):3 071w,1 606 m,1 588m,1 509m,1 425s,1 383m,1 244s,1 167w, 1 069w,937w,849m,789w,670w.
[Co(eoba)(phen)(H2O)2]·H2O(2):A mixture of Co(OAc)2·4H2O(0.053 g,0.2 mmol),H2eoba(0.045 g, 0.15 mmol),phen(0.027 g,0.15 mmol)and H2O(10 mL)was put in a 30 mL Teflon-lined stainless steel vessel,and then the vessel was sealed and heated at 150℃for 5 days.After the reaction mixture was slowly cooled down to room temperature at the rate of 5℃·h-1,pink block crystals were collected by filtration and washed with distilled water in 42%yield (based on Co).Anal.Calcd.for C28H26CoN2O9(%):C, 56.67;H,4.42;N,4.72.Found(%):C,56.18;H,4.11; N,4.16.IR(cm-1):3 401w,1 606m,1 589m,1 513m, 1 426s,1 387m,1 244s,1 167w,1 141w,1 068w,941 w,850m,788w.
1.3 Structure determination
Single-crystal diffraction data of 1 and 2 were respectively collected on a Bruker SMART APEX-CCD diffractometer equipped with a graphite-monochromatic Mo Kα(λ=0.071 073 nm)radiation at room temperature.The structure was solved by direct methods with SHELXS-97 program[19]and refined by full-matrix least-squares techniques on F2with SHELXL-97[20]. All non-hydrogen atoms were refined anisotropically and the hydrogen atoms of organic ligands were generated geometrically.The details of the crystal parameters,data collection and refinement for 1 and 2 aresummarizedinTable1,andselectedbond parameters are given in Table 2.
CCDC:1417662,1;1417663,2.
2 Results and discussion
2.1 IR spectrum
For complex 1,the carboxylates are coordinated with its asymmetric and symmetric stretching appearing at 1 606(ν(OCO)assym)and 1 425 cm-1(ν(OCO)sym)[21], respectively.TheΔν(ν(OCO)assym-ν(OCO)sym)is 181cm-1,showing the presence of bidentate linkage of carboxylates in the dianions.Thus the carboxylates coordinate to the metal as bidentate ligands via the carboxylate groups[22].
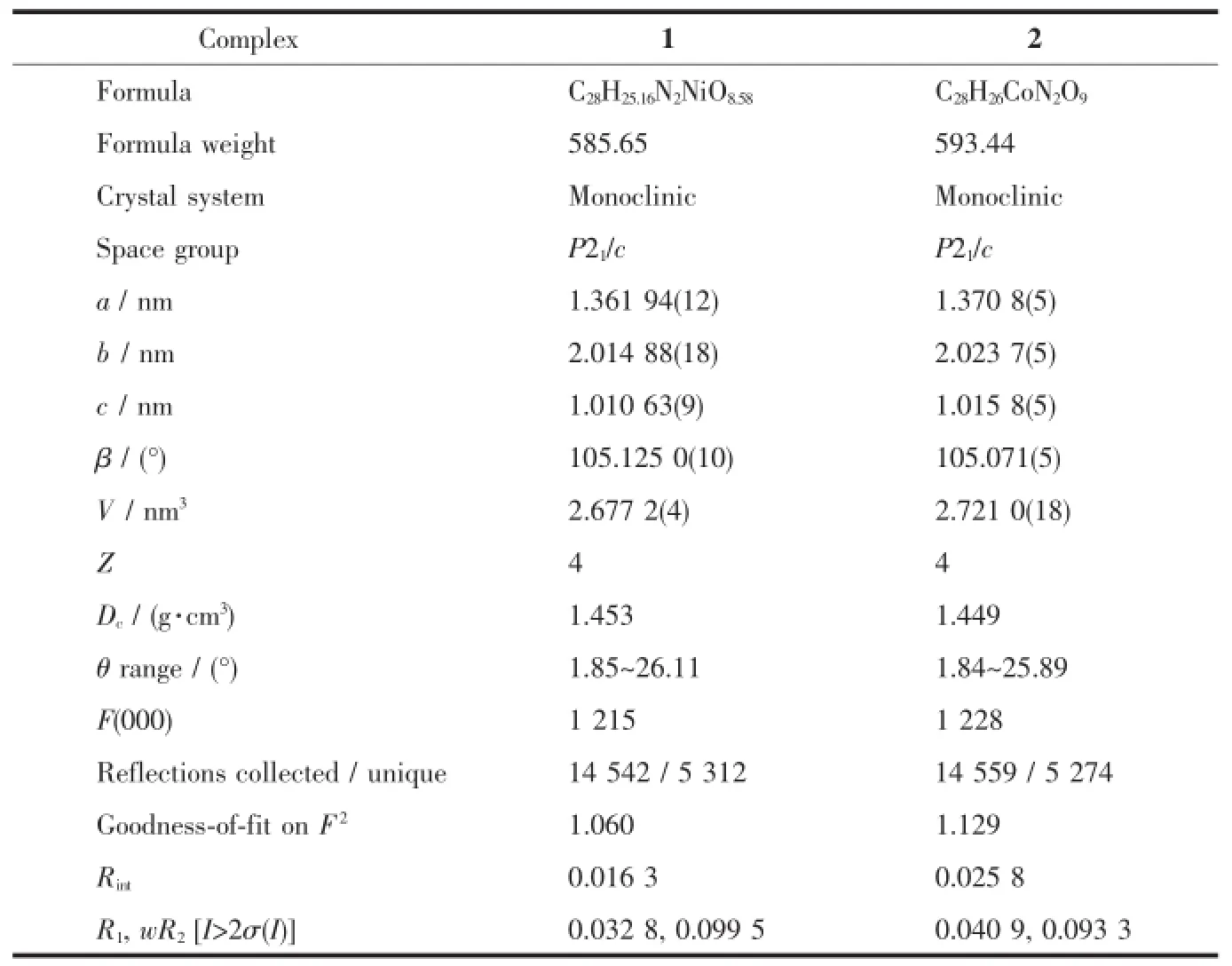
Table 1Crystal data and structure refinement for 1 and 2
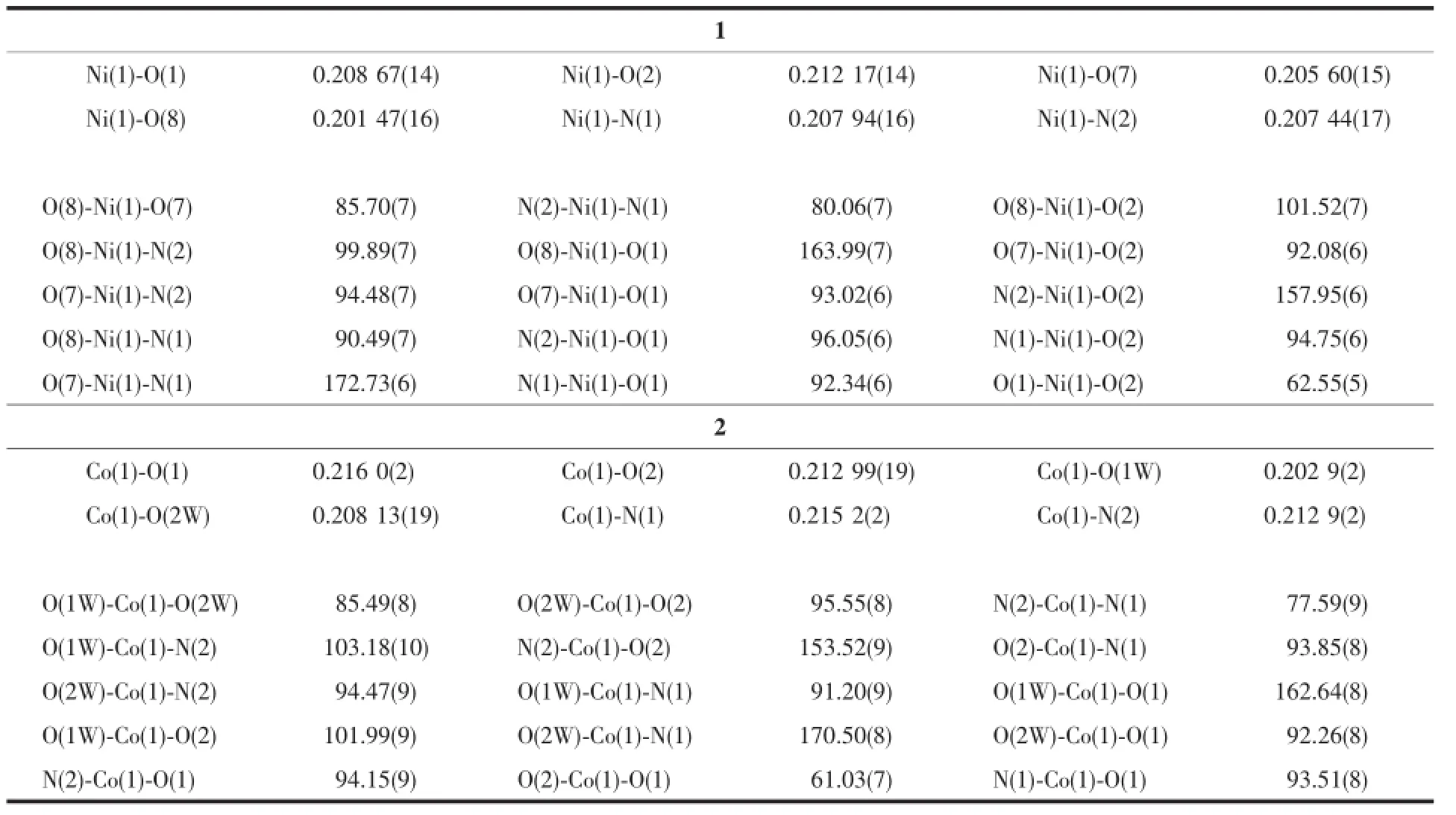
Table 2Selected bond lengths(nm)and bond angles(°)for 1 and 2
The IR spectrum of complex 2 is similar to complex 1 with the Δν(ν(OCO)assym-ν(OCO)sym)of 180 cm-1showing the presence of bidentate linkages of carboxylates in the dianions.
2.2 Description of the structure
A single-crystal X-ray diffraction study reveals that complexes 1 and 2 are isostructral.They all crystallizeinmonoclinicspacegroupP21/cand feature a 2D network structure.Here,we only descript the complex 1.The coordination environment of Ni(Ⅱ)in complex 1 is shown in Fig.1.There are one Ni(Ⅱ) ion,one eoba ligand,one phen ligand,two coordinated water molecules and 0.58 lattice water molecules in the asymmetric unit.Each Ni(Ⅱ)ion is six-coordinated by two nitrogen atoms(N(1),N(2))from one phen molecule,two carboxylate oxygen atoms(O(1),O(2)) from one eoba ligand and two coordinated water molecules,showing a distorted octahedral geometry. The bond distances of Ni-O in complex 1 fall in the range of 0.201 47(16)~0.212 17(14)nm,and those of Ni-N in 0.207 94(16)~0.207 44(17)nm.The coordination angles around the Ni atom vary from 62.55(5)° to 172.73(6)°.In the coordination environment,the two carboxylate oxygen atoms(O(1),O(2)),one nitrogen atom(N(2))and one coordinated water molecules(O(8)) are located in the basal plane,whereas the other coordinated water molecules(O(7))and nitrogen atom (N(1))occupy the axial positions from the opposite directions.
In 1,the phen ligand adopts typical cheltingconformation,whileeacheobaligandadoptsμ2coordination mode.Further investigation of the crystal packingofcomplex1suggeststhatthereare persistent O-H…O hydrogen bonding interactions (Fig.2)between carboxylate oxygen atoms and water molecules of eoba ligands(Table 3)and are usually importantinthesynthesisofsupramolecular architecture[23].Moreover,there are π-π interactions in complex 1 between pyridine rings of phen ligands and between benzene rings of eoba ligands(Table 4). Therefore,throughhydrogenbondsandπ-π interactions,complex 1 is further extended into a twodimensional supramolecular network framework.
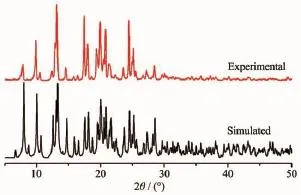
Fig.3PXRD analysis of complex 1
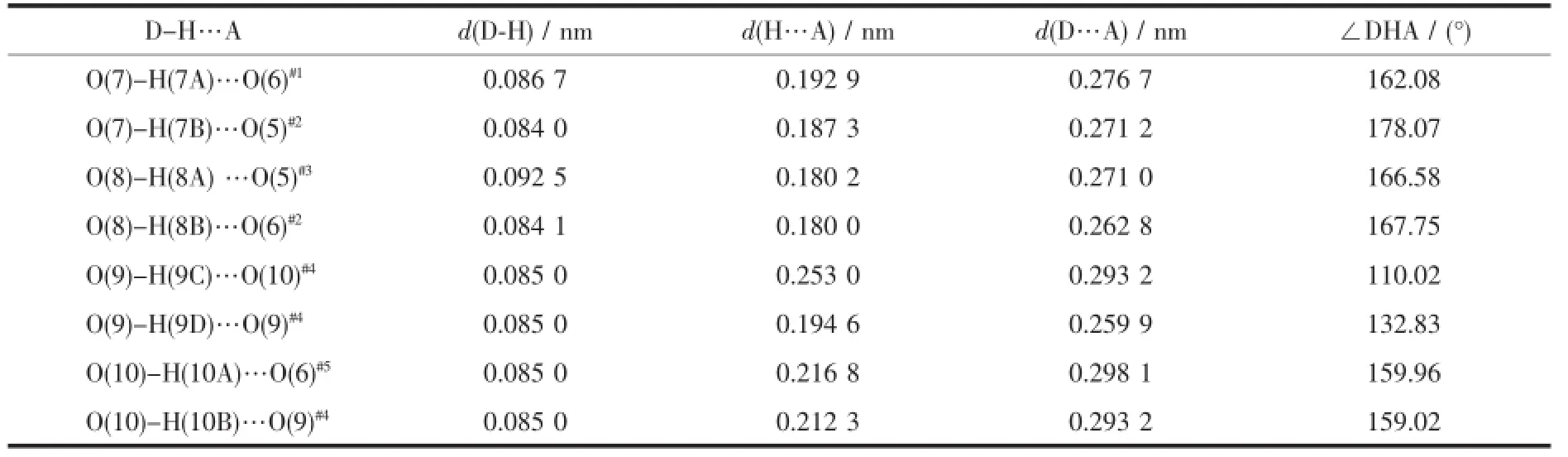
Table 3Hydrogen bonds for complex 1

Table 4Parameters between the planes
Toinvestigatewhethertheanalyzedcrystal structure is truly representative of the bulk materials, X-ray powder diffraction(PXRD)technology has been performed for the complex at room temperature(Fig.3). Themainpeakpositionsobservedareingood agreement with the simulated ones.Although minor differences can be found in the positions,widths,and intensitiesofsomepeaks,thebulksynthesized materials and analyzed crystal can still be considered as homogeneous.The differences may be due to the preferred orientation of the powder samples[24-25].
2.3 Thermal analysis
To study the thermal stability of 1 and 2, thermogravimetric(TG)analyses were performed on polycrystalline samples under a nitrogen atmosphere from 20 to 1 000℃at a heating rate of 10℃·min-1. For compound 1,the TG curve(Fig.4)reveals that the first weight loss of 10.3%from21 to 120℃corresponds to the removal of water molecules(Calcd. 7.9%).Upon further heating,an obvious weight loss (79.6%)occurs in the temperature range of 355~795℃,corresponding to the release of eoba and phen ligands(Calcd.82.0%).After 795℃,no weight loss is observed,which means the complete decomposition of 1.For compound 2,the TG curve is similar to 1. The first weight loss of 11.2%from 21 to 90℃corresponds to the removal of water molecules(Calcd. 12.2%),and an obvious weight loss(67.6%)occurs in the temperature range of 350~796℃corresponds to the release of eoba and phen ligands(Calcd.69.0%).
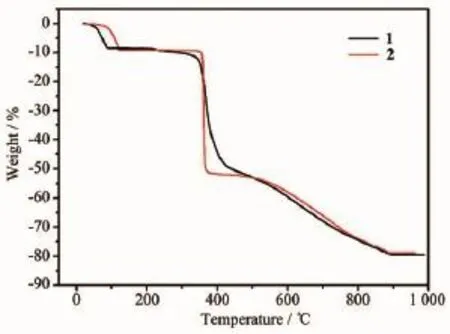
Fig.4TG curves of complexes 1 and 2
3 Theoretical calculation
All calculations in this work were carried out with the Gaussian09 program[26].The parameters of the molecular structure for calculation were all from the experimental data ofthecomplex.Naturalbond orbital(NBO)analysis was performed by density functional theory(DFT)[27]with the PBE0[28]hybrid functional and the LANL2DZ basis set[29].
The selected natural atomic charges,natural electron configuration,wiberg bond indices and NBO bond orders for the complex are shown in Table 5.It is indicated that the electronic configurations of Ni(Ⅱ) ion,N and O atoms are 4s0.293d8.324p0.44,2s1.312p4.20and 2s1.60~1.712p4.99~5.20,respectively.Basedontheaboveresults, one can conclude that the Ni(Ⅱ)ion coordination with N and O atoms is mainly on 4s,3d,and 4p orbitals. N atoms form coordination bonds with Ni(Ⅱ)ion using 2s and 2p orbitals.All O atoms supply electrons of 2s and 2p to Ni(Ⅱ)ion and form the coordination bonds. Therefore,the Ni(Ⅱ)ion obtained some electrons from two N atoms of phen ligand,two O atoms of eoba ligand and two coordinated water molecules[28-29].Thus, according to valence-bond theory,the atomic net charge distribution and the NBO bond orders of the complex 1(Table 5)shows the obvious covalent interaction between the coordinated atoms and Ni(Ⅱ) ion.The differences of the NBO bond orders for Ni-O and Ni-N bonds make their bond lengths be different[30],which is in good agreement with the X-ray crystal structural data of complex 1.
AscanbeseenfromtheFig.5,lowest unoccupied molecular orbital(LUMO)is mainlycomposed of eoba ligand,whereas highest occupied molecular orbital(HOMO)mainly consists of phen ligand.So,the charge transfer from ligand to ligand may be inferred from some contours of molecular orbital of complex 1.
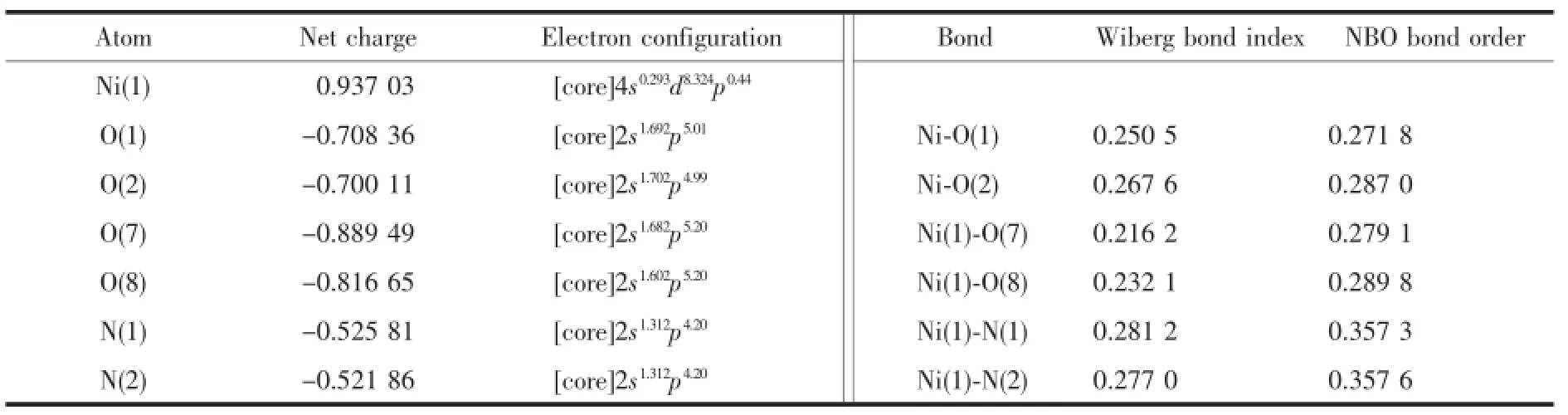
Table 5Natural atomic charges,natural valence electron configurations,wiberg bond indexes and NBO bond orders for the complex 1
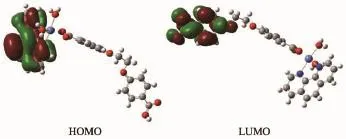
Fig.5Frontier molecular orbital of complex 1
[1]Hill R J,Long D L,Champness N R,et al.Acc.Chem.Res., 2005,38:335-348
[2]Friedrichs O D,O′Keeffe M,Yaghi O M.Acta Crystallogr. A,2003,59:22-27
[3]Ye B H,Tong M L,Chen X M.Coord.Chem.Rev.,2005, 249:545-565
[4]Inoue K,Imai H,Ghalsasi P S,et al.Angew.Chem.,Int. Ed.,2001,40:4242-4245
[5]Evans O R,Lin W B.Acc.Chem.Res.,2002,35:511-522
[6]Janiak C.Dalton Trans.,2003:2781-2804
[7]Seo J S,Whang D,Lee H,et al.Nature,2000,404:982-986
[8]Sato O,Lyoda T,Fujishima A,et al.Science,1996,271:49-51
[9]Lin W,Evans O R,Xiong R G,et al.J.Am.Chem.Soc., 1998,120:13272-13273
[10]Anokhina V,Vougo-Zanda M,Wang X Q,et al.J.Am. Chem.Soc.,2005,127:15000-15001
[11]Wang X Q,Liu L M,Jacobson A J.Angew.Chem.Int.Ed., 2006,39:6499-6503
[12]Chen W,Wang J Y,Chen C,et al.Inorg.Chem.,2003,42: 944-946
[13]Pan L,Parker B,Huang X Y,et al.J.Am.Chem.Soc., 2006,128:4180-4181
[14]Reineke T M,Eddaoudi M,Fehr M,et al.J.Am.Chem. Soc.,1999,121:1651-1657
[15]He J H,Yu J H,Zhang Y T,et al.Inorg.Chem.,2005,4: 9279-9282
[16]Go Y B,Wang X Q,Anokhina E V,et al.Inorg.Chem., 2005,44:8265-8271
[17](a)Li X M,Wang Q W,Liu B,et al.Chin.J.Struct.Chem., 2009,28(10):1257-1260 (b)Li X M,Wang Q W,Cui Y C,et al.Chin.J.Struct.Chem., 2009,28(10):1317-1320 (c)LI Xiu-Mei(李秀梅),WANG Qing-Wei(王庆伟),LIU Bo (刘博),et al.Chinese J.Inorg.Chem.(无机化学学报), 2011,27(6):1207-1211 (d)LI Xiu-Mei(李秀梅),JI Jian-Ye(纪建业),NIU Yan-Ling (牛艳玲),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29(6):1302-1306 (e)Liu B,Guo J,Zhou S,et al.Chin.J.Struct.Chem.,2013, 29(2):199-204
[18]Wang Q W,Wang Y N,Qi X F,et al.Chin.J.Struct.Chem., 2014,33:1629-1635
[19]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[20]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[21]Devereux M,Shea D O,Kellett A,et al.Inorg.Biochem., 2007,101:881-892
[22]Bellamy L J.The Infrared Spectra of Complex Molecules. New York:Wiley,1958.
[23]Krische M J,Lehn J M.Struct.Bonding,2000,96:3-29
[24]Gilbert A,Baggott J.Essentials of Molecular Photochemistry. Boca Raton:CRC Press,1991.
[25]Han Z B,He Y K,Ge C H,et al.Dalton Trans.,2007,46: 3020-3024
[26]Frisch M J,Trucks G W,Schlegel H B,et al.Gaussian 09,Revision B.09,Gaussian,Inc.,Pittsburgh,PA,2009.
[27]Parr R G,Yang W.Density Functional Theory of Atoms and Molecules.Oxford:Oxford University Press,1989.
[28](a)Ernzerhof M,Scuseria G E.J.Chem.Phys.,1999,110: 5029-5036 (b)Adamo C,Barone V.J.Chem.Phys.,1999,110:6158-6170
[29]Wang L,Zhao J,Ni L,et al.J.Inorg.Gen.Chem.,2012, 638:224-230
[30]LI Zhang-Peng(李章朋),XING Yong-Heng(邢永恒),ZHANG Yuan-Hong(张元红),et al.Acta Phys.-Chim.Sin.(物理化学学报),2009,25:741-746
镍、钴超分子配合物的合成、晶体结构及理论计算
王庆伟*,1隋薇1王亚男1李秀梅2刘博1
(1吉林师范大学,环境友好材料制备与应用省部共建教育部重点实验室,四平136000)
(2通化师范学院化学学院,通化134002)
通过水热法合成了2个新的金属-有机超分子配合物[Ni(eoba)(phen)(H2O)2]·0.58H2O(1)和[Co(eoba)(phen)]2·H2O(2)(H2boba =4,4′-(乙烷-1,2-二氧基)-二苯甲酸,phen=菲咯啉),并对其进行了元素分析、红外光谱、热重和X射线单晶衍射测定。配合物1和2是同构的,每个配合物都是六配位的,菲咯啉分子上的2个氮原子、4,4′-(乙烷-1,2-二氧基)-二苯甲酸配体上的2个氧原子和2个配位水分子与金属配位。此外,还用高斯09程序PBE0/LANL2DZ方法对配合物1进行了自然键轨道(NBO)分析,计算结果表明配位原子与Ni原子之间存在着共价作用。
水热合成;晶体结构;镍配合物;钴配合物;自然键轨道
O614.81+3;O614.81+2
A
1001-4861(2016)06-1120-07
2016-02-26。收修改稿日期:2016-04-15。
10.11862/CJIC.2016.133
吉林省科技发展计划(No.201205080)资助项目。
*通信联系人。E-mail:wqw611223@163.com

