A Comparison of Heterogeneous Reaction Kinetics of Oleic Acid Thin Film and Oleic Acid Coated Flyash with Ozone Using Vacuum FTIR
HE Xiang,LENG Chun-bo,ZHANG Yun-hong
The Institute of Chemical Physics, School of Chemistry, Beijing Institute of Technology, Beijing 100081, China
A Comparison of Heterogeneous Reaction Kinetics of Oleic Acid Thin Film and Oleic Acid Coated Flyash with Ozone Using Vacuum FTIR
HE Xiang,LENG Chun-bo,ZHANG Yun-hong*
The Institute of Chemical Physics, School of Chemistry, Beijing Institute of Technology, Beijing 100081, China

Heterogeneous reaction kinetics; Oleic acid; Flyash; Ozone; Vacuum FTIR
Introduction
As a major component of atmospheric aerosols, organic matter, become a focus of current study due to its complex physical properties and possible impacts on global climate[1-2].Atmospheric organics can undergo chemical aging via heterogeneous reactions with gaseous oxidants such as ozone, lead to form secondary atmospheric aerosols (SOA) with higher hygroscopicities and more complex radiative properties[3].Oleic acid (OA), a representative species of the unsaturated fatty acid, can be oxidized by O3which provides a convenient model system to investigate the effects of aging on organic aerosol properties[4].As micron particles, flyash, the main compositions are Al2O3and SiO2, which come from the emission of coal-fired power plants and can be treated as a carrier for OA reacting with ozone.
In order to investigate the impact of flyash in heterogeneous reaction, two kinds of OA samples were compared to drive the kinetics of ozone oxidation reaction: pure OA thin film and OA coated flyash.The two reactions were measured under unified atmospherically relevant condition by a vacuum FTIR flow reactor.
The experimental setup for this study is shown in Figure 1.The vacuum FTIR spectrometer (Bruker VERTEX 80v)equipped with liquid-nitrogen cooled photovoltaic mercury-cadmium-telluride (MCT) detector was utilized to monitor the ozonolysis reactions under room temperature and dry condition.All the optical elements of the spectrometer were working in a chamber under vacuum condition.Two Si infrared optical windows for infrared penetrating were fixed on both sides of sample cell in the internal sample cabin of vacuum FTIR.Both samples (0.2 μm thickness of OA) overspreaded onto the both Si windows, and were reacted with ozone separately in sample cell.The ozone was generated and diluted by dry air through the PTPE pipe.
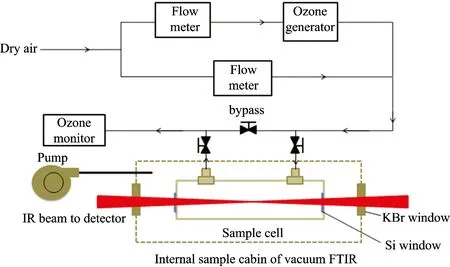
Fig.1 Schematic diagram of vacuum FTIR



Fig.2 FTIR spectrum of OA coated flyash (a) and fresh OA thin film (b) at room temperature

(1)
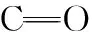
(2)

Fig.3 FTIR spectra of (a) (b) OA thin film and (c) (d) OA coated flyash at different ozone exposure time during the reaction

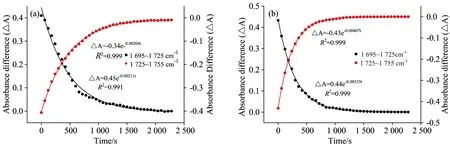
Fig.4 Temporal changes in the infrared spectra focusing on stretching bands of two samples
In this study, vacuum FTIR was utilized to study the comparisons of reaction kinetics between the OA thin film and OA coated flyash for heterogeneous reaction with ozone.Catalytic surface area and catalytic effect are the main factor for the larger reaction rate of the flyash sample compared to the OA thin film.This confirms that surface-active unsaturated organic acids in aerosols like flyash often easily lead to the secondary organic particles when exposure to ozone, which directly affect cloud albedo and form the fog haze weather.Further studies should emphasis on the reaction and catalytic mechanisms for the heterogeneous reactions between ozone and flyash samples.
[1] Rudich Y,Donahue N M,Mentel T F.Annu.Rev.Phys.Chem.,2007, 58:321.
[2] Cassar N,Bender M L,Barnett B A.Science,2007, 317:1067.
[3] Robinson A L,Donahue N M,Shrivastava M K.Science,2007, 315:1259.
[4] Tunved P,Hansson H C,Kerminen V M.Science,2006, 312:261.
[5] Hearn J D,Smith G D.J.Phys.Chem.A, 2004, 108(45):10019.
[6] Zeng G, Holladay S, Langlois D.J.Phys.Chem.A, 2013, 117(9):1963.
[7] Hung H M,Katrib Y,Martin S T.J.Phys.Chem.A,2005, 109(20):4517.
[8] Hung H M, Tang C W.J.Phys.Chem.A,2010, 114(50): 13104.
[9] Segal-Rosenheimer M,Dubowski Y.J.Phys.Chem.C, 2007, 111(31):11682.
[10] Petrick L,Dubowski Y.Indoor Air, 2009, 19(5): 381.
[11] Thornberry T,Abbatt J P D.Phys.Chem.Chem.Phys.,2004, 6(1):84.
[12] Kwamena N O A, Clarke J P, Kahan T F.Atmos.Environ., 2007, 41:37.
[13] Deborah J L, Juan J N, Percival C J.Phys.Chem.Chem.Phys.,2009, 11:8214.
[14] Leng C B, Hiltner J, Pham H.Phys.Chem.Chem.Phys., 2014, 16:4350.
*通讯联系人
O657.3
A
利用真空FTIR比较研究油酸薄膜和油酸包覆粉煤灰分别与臭氧的非均相化学反应动力学
何 翔,冷春波,张韫宏*
北京理工大学化学学院化学物理研究所,北京 100081
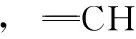
非均相反应动力学; 油酸; 粉煤灰; 臭氧; 真空傅里叶红外光谱仪
2015-01-07,
2015-04-26)
Foundation item:supported by the National Natural Science Foundational of China (41175119, 21373026, 21473009)
10.3964/j.issn.1000-0593(2016)05-1576-05
Received:2015-01-07; accepted:2015-04-26
Biography:HE Xiang, (1987—), graduate student of Beijing Institute of Technology e-mail: hexiang720@163.com *Corresponding author e-mail: yhz@bit.edu.cn

