系统综述与网状Meta分析的PRISMA扩展声明
李志霞,杨俊,叶欣,周凌波译;杨智荣,孙凤,詹思延审校
系统综述与网状Meta分析的PRISMA扩展声明
李志霞1,杨俊1,叶欣1,周凌波1译;杨智荣2,孙凤1,詹思延1审校
PRISMA声明旨在提高系统综述和META分析报告的完整性,该声明已经广泛用于指导系统综述和META分析的报告和发表。原始的PRISMA声明是针对两种干预措施比较的传统的系统综述与META分析而制定的,然而,随着多种干预措施比较的系统综述的发展,实施和报告这一类系统综述面临较大挑战。此时,针对网状META分析的PRISMA扩展声明应运而生,旨在提高网状META分析系统综述的报告质量。PRISMA扩展声明是由专家们通过DELPHI调查、面对面讨论和共识大会而最终确立的。PRISMA扩展声明是在原始PRISMA声明的报告清单的基础上经过修改,最终确定了32个条目,每个条目均与网状META分析报告的内容直接相关。本文对网状META分析的PRISMA扩展声明进行了阐述,对报告清单各条目进行了举例说明,并详细说明了在原始PRISMA声明的基础上新增和修改各条目的理由。此外,PRISMA扩展声明强调了在网状META分析的实际操作中需要重点关注的信息。本文的目标读者包括网状META分析的作者与读者,以及期刊杂志的编辑与同行评审。
系统综述和Meta分析是为临床医生、决策者和患者总结可靠医疗信息的重要工具。系统综述不仅可以给出有关临床干预措施利弊的信息,以帮助制定临床推荐意见,同时还有助于辨明未来研究的方向。为了提高系统综述和Meta分析报告的质量,1999年和2009年分别提出了QUOROM声明[1]和PRISMA声明[2,3]。随着这两份声明的广泛使用,系统综述报告的质量已经有所提高[4,5]。
系统综述和Meta分析常常用于比较干预措施的有效性。但是由于随机对照临床试验同时评估多种干预措施利弊时存在诸多困难;而传统的疗效比较的系统综述即便是纳入了很多个原始研究,也只是对部分干预措施的效果进行比较;而且,传统Meta分析每次只针对两种干预措施进行比较,但实际决策中常常要求基于所有可得的证据进行综合全面的比较。因此出现了同时对多种干预措施进行比较的方法,这些方法被称为网状Meta分析,或者混合治疗比较Meta分析[6-8]。近几年网状Meta分析文章的发表数量有明显增加[9]。2014年已有学者对网状Meta分析报告过程中所存在的问题进行了概述[10],我们也用Delphi法对研究者和杂志编辑进行了调查,结果表明亟需制定一个针对网状Meta分析的报告规范。
本文将对网状Meta分析报告规范的具体内容和制定过程进行描述。
1 PRISMA网状META分析扩展声明的形成
我们根据报告规范的标准制定流程制作了网状Meta的PRISMA扩展声明[11]。首先,我们成立了指导委员会(包括Hutton、Salanti、Moher、Caldwell、Chaimani、Schmid、Thorlund 和Altman),召集了期刊编辑、报告指南的制定者、在系统综述和网状Meta分析领域拥有丰富经验的研究者等17人,对现有的关于网状Meta分析的报告质量的综述进行了总结,并识别出与网状Meta分析报告质量相关的候选条目[10]。此外,我们同时在2013年年中通过Fluid Survey在线软件对网状Meta分析的作者进行了Delphi调查(共邀请了215人,收到反馈114人,应答率53%),以帮助确定哪些条目可以达成一致意见增加到扩展声明中来或进行详细阐述,哪些条目仍需要进一步讨论。
其次,我们举行了为期一天的面对面会议,讨论了扩展声明的结构、需要进一步讨论的内容和出版计划。会后,我们邀请了指导委员会的成员和部分参会者负责完成这份报告规范的特定部分。需要说明的是,所有参与者均对这份声明的初稿进行了审阅。
2 扩展声明的适用范围
本文为作者、同行评审和编辑提供了网状Meta分析的报告规范。它同样可以帮助临床医生、技术评估人员和患者解读网状Meta分析。我们也希望它能够帮助读者更好地理解网状Meta分析的核心概念、术语和相关问题。
考虑到关于网状Meta分析的制作和解读已经有足够多的参考资料[6,12-51],本文就不再赘述。本文主要针对网状Meta分析的结果如何报告提供指南,说明报告时应该包括哪些重要信息。对于那些在原始PRISMA声明基础上修改的条目,我们均辅以实例对这部分内容可能用到的方法进行了说明。但是需要注意的是,除了本文提到的方法以外,也可以用其他改进的方法对这些条目进行报告。
3 如何解读本文
本文主要描述了如何修改原始PRISMA声明中的条目,以满足网状Meta分析的报告要求。同时也对网状Meta分析中新增加的条目进行了描述。网状Meta分析PRISMA扩展声明共包含32个条目,本文根据这32个条目解释说明了如何对网状Meta分析的结果进行清楚透明的报告。该说明(见http://www.prisma-statement.org/documents/ PRISMA%20NMA%20Annals%202015.pdf)不仅对每个条目进行了一一描述,而且对于新增加的和经过修改的条目均辅以实例进行了解释。需要说明的是,新增加的条目均是按照逻辑顺序插入到原始PRISMA声明中的,但是在实际报告的过程中,不一定要按照清单上的顺序进行报告。此外,这份说明还包含了五个信息栏,针对网状Meta分析在方法学方面需要考虑的因素分别进行了阐述(表1)。
表格中列出了网状Meta分析的PRISMA扩展声明,供相关作者在报告时进行参考。扩展声明不仅包含了原始PRISMA的核心条目,还包含了修改的条目以及针对网状Meta分析新增的条目。扩展声明中基于网状Meta分析的特点而新增加的条目被命名为“New Item”,标记为S1~S5。带有“Addition”标记的条目均来源于原有的PRISMA声明,但已针对网状Meta分析的特点进行了修改扩展。在附加的解释说明中,均对这两种类型的条目提供了相关参考案例。
4 什么是网状图?
对多种治疗措施同时进行比较的网状Meta分析,其复杂程度高于仅对两种治疗措施进行比较的传统Meta分析。网状Meta分析用网状图的形式展示各个干预措施的研究数量和纳入的患者数量(图1)。网状图由结点(每一个结点代表一种干预措施)和连线(结点之间的连线表示纳入的研究中,两种干预措施进行了直接比较)组成。网状图中结点的大小和连线的粗细分别代表了对应干预措施纳入的患者数量和直接比较的干预措施的研究数量。有时候会增加附加线条用以区分某两个干预措施的比较是来自某些多臂研究。
网状图还可以帮助读者了解干预措施的网状证据结构特征,包括识别网状图中的闭合环。当三种或以上的干预措施均进行了两两比较,此时就会形成一个闭合环。如图1中的干预措施A、B 和C,它们两两之间均进行过直接比较,形成了闭合环路(AB、AC和BC),这个环可以同时用于直接比较和间接比较(框1中给出了直接比较和间接比较的定义,图2用图示对该定义作了说明)。
5 讨论
在临床研究的整个过程中,如果提出的问题不合理、采用的研究方法不恰当、报告不详实、成果传播不充分等,都将会产生很大的浪费。其中,报告不详实并不是什么难懂的问题,然而它可能会导致对干预效果的估计产生偏倚,进而对病人照护和决策制定产生影响。杂志期刊经常会出版一些关于不充分报告新证据的文章[52]。实践表明,通过提高研究报告的完整性和透明度能有效地减少这种浪费,这也解释了目前越来越多报告规范陆续发布[53,54]和EQUATOR协作网快速发展的原因。
原始PRISMA声明旨在改善传统系统综述和Meta分析的报告质量,它受到了数百个杂志期刊和编辑部的支持。此外,一些PRISMA扩展声明已经发布,包括系统综述摘要的PRISMA扩展声明[55]和关于公平性评价的系统综述的PRISMA扩展声明[56]。另外一些扩展声明还正在制定中,包括个体病例资料Meta分析的PRISMA扩展声明以及安全性评价的PRISMA扩展声明。
本文主要描述了网状Meta分析的PRISMA扩展声明,包括32个条目和流程图。该扩展声明针对网状Meta分析的特点,在原始PRISMA声明的基础上新增了5个条目,并且对其中原有的11个条目进行了修订,多数是一些微调,部分是更全面的修改,例如第20条和21条中要求作者对各个研究的结果及相应的合并结果进行描述。

图1 网状图示例(上图展示了4种干预措施A、B、C和D,用直线相连的两种干预措施表示开展了直接比较;结点的大小代表对应干预措施纳入的患者数量,直线的粗细代表两种干预措施直接比较的研究数量)
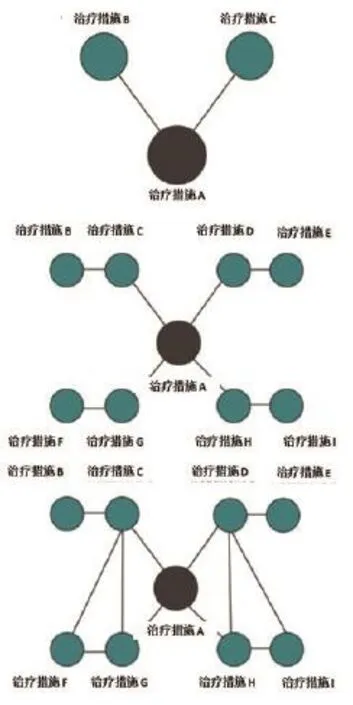
图2 图示网状图涉及的术语(术语详细解释参考框1。第一个图,干预措施B和C均与干预措施A进行了直接比较,B和C通过A构建了间接比较的关系;第二个图,8种干预措施和1个常见对照A组成的网状图,既有干预措施和对照A的直接比较,也有某两个干预措施之间的直接比较;第三个图,在第二个网状图的基础上,增加了几个直接比较,形成了几个闭合环路)

与传统系统综述相比,网状Meta分析包含了更多的干预措施,纳入了更多的原始研究,因此作者在投稿时可能需要准备一些附件作为补充说明材料。杂志编辑应当考虑到这种情况。
对某些条目进行的修改(例如,模型拟合的评估,合并干预措施的基本原理和研究特征的列表展示等)同样适用于传统Meta分析中两两比较的情况。有些修改虽然不一定是必须的,但却是很有意义的,因为这些条目虽然在原始PRISMA声明中没有被明确强调,但在网状Meta分析时可能会经常遇到。参与制定网状Meta分析PRISMA扩展声明的几个共同作者同样是原始PRISMA声明制作团队中的成员,今后对原始PRISMA声明进行更新时,他们也将会对这些修改的条目重新进行考虑。
我们希望杂志期刊会像认可原始PRISMA声明一样认可网状Meta分析PRISMA扩展声明。最好是在杂志期刊中对网状Meta分析的作者提出明确的要求,我们在附录中(见http://www.prismastatement.org/documents/PRISMA%20NMA%20Ann als%202015.pdf)提供了范例文本以供参考。
期刊的认可固然重要,但如果实施不利则毫无意义。最简单的实施方法,就是要求作者填写网状Meta分析的PRISMA扩展声明,如果不提供该声明,杂志可以拒绝发表。然而,有资料表明,某些规模较小的出版社,发表了很多的系统综述[57],对他们来说,接受和实施这一扩展声明可能阻碍了他们接收网状Meta分析的文章。编辑应该认识到推广和实施报告规范是提高所发表文章的完整性和透明度的重要途径[58,59],这也是对Helsinki宣言[60]核心原则的支持。这样做同时可以减少所报告研究中的信息浪费。
近几年网状Meta分析的文章发表数量呈现出急剧上升趋势[8,9],其研究方法也迅速得到发展和关注。为保证网状Meta分析的PRISMA扩展声明尽可能与时偕行和有据可循,我们诚邀读者及时反馈以帮助我们今后对该扩展声明进行更新。
翻译声明:
本文翻译已得到American College of Physicians杂志社和PRISMA官网的授权。本文翻译由北京大学公共卫生学院李志霞、杨俊、叶欣、周凌波合作完成,杨智荣、孙凤、詹思延审校。本翻译的准确性由译者全权负责,原版权单位American College of Physicians(ACP)不对本翻译的准确性负责。
[1] Moher D,Cook DJ,Eastwood S,et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses[J]. Lancet,1999,354(9193):1896-900.
[2] Moher D,Liberati A,Tetzlaff J,et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement[J].Ann Intern Med,2009, 151:264-9.
[3] Liberati A,Altman DG,Tetzlaff J,et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration[J]. Ann Intern Med,2009, 151(4):W65-94.
[4] Panic N,Leoncini E,de Belvis G,et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and metaanalysis (PRISMA) statement on the quality of published systematic review and meta-analyses[J]. PLoS One,2013, 8(12):e83138.
[5] Wen J,Ren Y,Wang L,et al. The reporting quality of meta-analyses improves: a random sampling study[J]. J Clin Epidemiol,2008,61(8):770-5.
[6] Lu G,Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons[J]. Stat Med,2004, 23(20):3105-24.
[7] Ioannidis JP. Integration of evidence from multiple meta-analyses:a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses[J].CMAJ,2009,181(8):488-93.
[8] Lee AW. Review of mixed treatment comparisons in published systematic reviews shows marked increase since 2009[J]. J Clin Epidemiol,2014,67(2):138-43.
[9] Nikolakopoulou A,Chaimani A,Veroniki AA,et al. Characteristics of networks of interventions: a description of a database of 186 published networks[J]. PLoS One,2014, 9(1):e86754.
[10] Hutton B,Salanti G,Chaimani A,et al. The quality of reporting methods and results in network meta-analyses: an overview of reviews and suggestions for improvement[J]. PLoS One,2014,9(3):e92508.
[11] Moher D,Schulz KF,Simera I,et al. Guidance for developers of health research reporting guidelines[J]. PLoS Med,2010,7(2):e1000217.
[12] Ades AE,Mavranezouli I,Dias S,et al. Network meta-analysis with competing risk outcomes[J]. Value Health,2010,13(8):976-83.
[13] Ades AE,Caldwell D,Reken S,et al. NICE DSU Technical Support Document 7: Evidence Synthesis of Treat ment Efficacy in Decision Making: A Reviewer's Checklist. London:National Institute for Health and Care Excellence[J]. 2012.
[14] Caldwell DM,Ades AE,Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence[J].BMJ,2005,331(7521):897-900.
[15] Del GC,Vacchi L,Mavridis D,et al. Network meta-analysis models to account for variability in treatment definitions: application to dose effects[J]. Stat Med,2013, 32(1):25-39.
[16] Dias S,Welton NJ,Caldwell DM,et al. Checking consistency in mixed treatment comparison meta-analysis[J]. Stat Med,2010,29(7-8):932-44.
[17] Dias S,Welton N,Marinho V,et al. Estimation and adjustment of bias in randomised evidence using mixed treatment comparison metaanalysis[J]. J R Stat Soc Ser A,2010,173:613-29.
[18] Dias S,Sutton AJ,Ades AE,et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials[J].Med Decis Making,2013, 33(5):607-17.
[19] Dias S,Welton NJ,Sutton AJ,et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials[J].Med Decis Making,2013, 33(5):641-56.
[20] Dias S,Sutton AJ,WeltonNJ,et al. Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment[J].Med Decis Making,2013, 33(5):618-40.
[21] Higgins JP,Jackson D,Barrett JK,et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies[J]. Res Synth Methods,2012,3(2):98-110.
[22] Jackson D,Barrett JK,Rice S,et al. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects[J]. Stat Med,2014, 33(21):3639-54.
[23] Jansen JP,Cope S. Meta-regression models to address heterogeneity and inconsistency in network meta-analysis of survival outcomes[J]. BMC Med Res Methodol,2012,12:152.
[24] Jansen JP. Network meta-analysis of individual and aggregate level data[J]. Res Synth Methods,2012,3(2):177-90.
[25] Jansen JP,Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers[J]. BMC Med,2013,11:159.
[26] Jones B,Roger J,Lane PW,et al. Statistical approaches for conducting network meta-analysis in drug development[J]. Pharm Stat,2011,10(6):523-31.
[27] Lindsley K,Cameron N,Wormald R,et al. Evaluating the transitivity assumption when constructing network meta-analysis: lumping or splitting? Cochrane Library Supplement. Presented at the 21st Cochrane Colloquium, Quebec, Canada, 23 September 2013.[J].
[28] Lu G,Ades A. Assessing evidence inconsistency in mixed treat ment comparisons[J]. J Am Stat Assoc,2006,101:447-59.
[29] Lu G,Ades A. Modeling between-trial variance structure in mixed treatment comparisons[J].Biostatistics,2009, 10(4):792-805.
[30] Mills EJ,Bansback N,Ghement I,et al. Multiple treatment comparison meta-analyses: a step forward into complexity[J]. Clin Epidemiol,2011, 3:193-202.
[31] Thorlund K,Mills E. Stability of additive treatment effects in multiple treatment comparison meta-analysis: a simulation study[J]. Clin Epidemiol,2012,4:75-85.
[32] Mills EJ,Kanters S,Thorlund K,et al. The effects of excluding treatments from network meta-analyses: survey[J]. BMJ,2013,347:f5195.
[33] Salanti G,Kavvoura FK,Ioannidis JP. Exploring the geometry of treatment networks[J].Ann Intern Med,2008,148(7):544-53.
[34] Salanti G,Marinho V,Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered[J]. J Clin Epidemiol,2009,62(8):857-64.
[35] Salanti G,Dias S,Welton NJ,et al. Evaluating novel agent effects in multiple-treatments meta-regression[J]. Stat Med,2010,29(23):2369-83.
[36] Salanti G,Ades AE,Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment metaanalysis: an overview and tutorial[J]. J Clin Epidemiol,2011,64(2):163-71.
[37] Sutton A,Ades AE,Cooper N,et al. Use of indirect and mixed treatment comparisons for technology assessment[J]. Pharmacoeconomics,2008,26(9):753-767.
[38] Thorlund K,Mills EJ. Sample size and power considerations in network meta-analysis[J]. Syst Rev,2012,1:41.
[39] Thorlund K,Thabane L,Mills EJ. Modelling heterogeneity variances in multiple treatment comparison meta-analysis--are informative priors the better solution?[J]. BMC Med Res Methodol,2013,13:2.
[40] Veroniki AA,Vasiliadis HS,Higgins JP,et al. Evaluation of inconsistency in networks of interventions[J]. Int J Epidemiol,2013,42(1):332-45.
[41] Mills EJ,Ioannidis JP,Thorlund K,et al. How to use an article reporting a multiple treatment comparison meta-analysis[J]. JAMA,2012, 308(12):1246-53.
[42] Cipriani A,Higgins JP,Geddes JR,et al. Conceptual and technical challenges in network meta-analysis[J]. Ann Intern Med,2013,159(2):130-7.
[43] Woods BS,Hawkins N,Scott DA. Network meta-analysis on the loghazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial[J]. BMC Med Res Methodol,2010,10:54.
[44] Cooper NJ,Sutton AJ,Morris D,et al. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons:Application to stroke prevention treatments in individuals with nonrheumatic atrial fibrillation[J]. Stat Med,2009, 28(14):1861-81.
[45] Donegan S,Williamson P,D'alessandro U,et al. Assessing key assumptions of network meta-analysis: a review of methods[J]. Res Synth Methods,2013, 4(4):291-323.
[46] Achana FA,Cooper NJ,Dias S,et al. Extending methods for investigating the relationship between treatment effect and baseline risk from pairwise meta-analysis to network meta-analysis[J]. Stat Med,2013,32(5):752-771.
[47] Jansen JP,Fleurence R,Devine B,et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1[J]. Value Health,2011,14(4):417-28.
[48] Hoaglin DC,Hawkins N,Jansen JP,et al. Conducting indirecttreatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2[J]. Value Health,2011,14(4):429-37.
[49] Jansen JP,Trikalinos T,Cappelleri JC,et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making:an ISPOR-AMCP-NPC Good Practice Task Force report[J]. Value Health,2014,17(2):157-73.
[50] Chaimani A,Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions[J]. Res Synth Methods,2012,3(2):161-76.
[51] Salanti G,Del GC,Chaimani A,et al. Evaluating the quality of evidence from a network meta-analysis[J]. PLoS One,2014,9(7):e99682.
[52] Glasziou P,Meats E,Heneghan C,et al. What is missing from descriptions of treatment in trials and reviews?[J]. BMJ,2008,336(7659):1472-4.
[53] Simera I,Moher D,Hoey J,et al. A catalogue of reporting guidelines for health research[J]. Eur J Clin Invest,2010,40(1):35-53.
[54] Moher D,Weeks L,Ocampo M,et al. Describing reporting guidelines for health research: a systematic review[J]. J Clin Epidemiol,2011,64(7):718-42.
[55] Hopewell S,Clarke M,Moher D,et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts:explanation and elaboration[J]. PLoS Med,2008, 5(1):e20.
[56] Welch V,Petticrew M,Tugwell P,et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity[J]. PLoS Med,2012,9(10):e1001333.
[57] Moher D,Tetzlaff J,Tricco AC,et al. Epidemiology and reporting characteristics of systematic reviews[J]. PLoS Med,2007,4(3):e78.
[58] Turner L,Shamseer L,Altman DG,et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals[J]. Cochrane Database Syst Rev,2012,11:Mr000030.
[59] Turner EH,Matthews AM,Linardatos E,et al. Selective publication of antidepressant trials and its influence on apparent efficacy[J]. N Engl J Med,2008,358:252-60.
[60] Association WM. Declaration of Helsinki—ethical principles for medical research involving human subjects[J]. 2008.Accessed at wwwwmanet/en/30publications/10policies/b3/index html on 31 August 2014.
本文编辑:翁鸿,田国祥
·循证理论与实践·论著·
·循证理论与实践·论著·
The PRISMA extension statement for reporting of systematic reviews incorporating network metaanalyses of health care interventions: checklist and explanations
Brian Hutton, PhD, MSc; Georgia Salanti, PhD; Deborah M. Caldwell, PhD, MA, BA; Anna Chaimani,PhD;Christopher H. Schmid, PhD; Chris Cameron, MSc; John P.A. Ioannidis, MD, DSc; Sharon Straus, MD, MSc; Kristian Thorlund, PhD;Jeroen P. Jansen, PhD; Cynthia Mulrow, MD, MSc; Ferrán Catalá-López, PhD, MPH, PharmD; Peter C. Gozsche, MD, MSc;Kay Dickersin, PhD, MA; Isabelle Boutron, MD, PhD; Douglas G. Altman, DSc; and David Moher, PhD
The PRISMA statement is a reporting guideline designed to improve the completeness of reporting of systematic reviews and meta-analyses. Authors have used this guideline worldwide to prepare their reviews for publication. In the past, these reports typically compared 2 treatment alternatives. With the evolution of systematic reviews that compare multiple treatments, some of them only indirectly, authors face novel challenges for conducting and reporting their reviews. This extension of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement was developed specifically to improve the reporting of systematic reviews incorporating network meta-analyses. A group of experts participated in a systematic review, Delphi survey, and face-to-face discussion and consensus meeting to establish new checklist items for this extension statement. Current PRISMA items were also clarified. A modified, 32-item PRISMA extension checklist was developed to address what the group considered to be immediately relevant to the reporting of network meta-analyses. This document presents the extension and provides examples of good reporting, as well as elaborations regarding the rationale for new checklist items and the modification of previously existing items from the PRISMA statement. It also highlights educational information related to key considerations in the practice of network meta-analysis. The target audience includes authors and readers of network meta-analyses, as well as journal editors and peer reviewers.
R4
A
1674-4055(2016)06-0656-05
国家自然科学青年基金项目支持(81302508)
译者单位:1100191 北京,北京大学公共卫生学院;2剑桥大学初级医疗中心
原文刊载于Ann Intern Med, 2015, 162: 777-784.
URL: http://annals.org/article.aspx?articleid=2299856.
© 2016 American College of Physicians www.acponline.org.
10.3969/j.issn.1674-4055.2016.06.05

