肿瘤代谢靶点用于癌症治疗的研究进展
温时媛,姜苗苗
(天津中医药大学天津市现代中药重点实验室,天津300193)
肿瘤代谢靶点用于癌症治疗的研究进展
温时媛,姜苗苗
(天津中医药大学天津市现代中药重点实验室,天津300193)
肿瘤细胞可以改变糖酵解和谷氨酰胺代谢等代谢途径,产生其快速增殖所需的原料,从而使自身增殖和生存。因此,研究肿瘤代谢途径的改变,有利于找到治疗癌症疾病的靶点。本文综述了肿瘤细胞中有氧糖酵解、谷氨酰胺代谢、三羧酸循环和合成代谢的代谢特征,并详细介绍了这些代谢途径中用于癌症治疗的代谢靶点和相应的治疗药物,探讨了可以成为癌症治疗靶点的潜在标志物和肿瘤代谢靶向治疗所面临的挑战。
肿瘤代谢;有氧糖酵解;谷氨酰胺
过去,癌症的治疗方式主要是采用具有细胞毒作用的药物,但其对正常细胞组织也有损伤。过去的几十年,研究重点主要集中在通过识别肿瘤细胞的独特特征更精准地锁定肿瘤细胞,于是产生了更高效的靶向治疗且改善了患者的预后。近年来的研究致力于利用肿瘤细胞的代谢特征来区别正常细胞。癌细胞能快速持久地生长、增殖并耐缺氧,基于这些特征寻找不同于正常细胞代谢特征的代谢途径。研究肿瘤代谢途径的主要目的是使癌细胞在治疗窗内被抑制而正常细胞不受影响。
1 肿瘤的代谢特征
癌症是一种复杂的疾病,不同类型的癌症存在较大的差异,同一类型癌症不同患者间也存在较大的差异,甚至同一个肿瘤中不同癌细胞间也有差异性。肿瘤代谢也表现了相当大的区别,肿瘤在不同微环境中采取不同的代谢途径。然而,肿瘤某些重要部位的代谢也存在一些共性。肿瘤代谢的共同特征包括葡萄糖、谷氨酰胺和线粒体代谢中的变化,这些共同特征或许可以成为治疗癌症的新的发展方向。某些代谢途径的基本性质提供了一个统一的研究方向,以避免和克服肿瘤的遗传差异性。
2 肿瘤的有氧糖酵解
葡萄糖首先在不需氧的情况下分解为丙酮酸,然后在线粒体中进行三羧酸(tricarboxylic acid,TCA)循环,产生大量的ATP。然而,20世纪50年代,Warburg[1]报道了肿瘤细胞中葡萄糖分解的丙酮酸在有氧的情况下转变为乳酸,而不是在线粒体中进行氧化。这种代谢不同于普通模式的代谢。一般认为只有在进行糖酵解时才产生乳酸,而这种代谢类似于克勒勃屈利效应(Crabtree effect)。这种效应是如果葡萄糖水平很高,即使有氧气的存在,呼吸也会被抑制。所以Warburg认为,这是肿瘤细胞有氧呼吸的代谢特点,并猜测这种情况是由于癌细胞的线粒体存在缺陷,而导致其自身不能利用氧进行代谢。迄今,发现大部分肿瘤细胞的线粒体是完整的,大量的致癌基因突变导致肿瘤细胞代谢由氧化磷酸化转变为糖酵解[2]。这种转变的原因依然不清楚,但一致认为葡萄糖的糖酵解使肿瘤细胞产生了一种利于其生长和增殖的生物中间体,同时也避免了氧化磷酸化产生有害的活性氧簇。
Warburg的肿瘤细胞有氧糖酵解的研究发现了一些值得深思的问题。如肿瘤细胞对这种有氧糖酵解代谢的依赖程度,抑制这种有氧糖酵解是否可以减少肿瘤细胞的增殖。肿瘤细胞利用葡萄糖及其衍生物的代谢途径,产生其生物合成所需的原料,维持良好的氧化还原环境,满足其所需的能量。重要的核心代谢途径几乎存在于所有细胞中,所以研究肿瘤细胞代谢的特异性和正常细胞的毒性显得至关重要。
糖酵解过程会产生大量相关的蛋白质,它们被认为是潜在的药物靶点。糖酵解的早期阶段,葡萄糖被携带进入癌细胞并在细胞中被磷酸化;糖酵解的后期阶段,葡萄糖转变为丙酮酸并以乙酰辅酶A的形式进入TCA循环或转变为乳酸移出细胞。糖酵解的前2步是葡萄糖转运体将葡萄糖转运至细胞内,随后被己糖激酶磷酸化。在许多类型的癌症中,葡萄糖转运体和己糖激酶的各种亚型会过度表达[3],它们可以成为药物抑制的靶点[4]。在B细胞急性淋巴细胞白血病小鼠模型中,葡萄糖转运蛋白1(glucose transporter 1,Glut1)基因的缺失大大减缓了细胞增殖并减轻了疾病[5]。同样地,在一些癌症疾病中,将Glut作为抑制对象进行研究。例如,抑制小分子Glut1可以减缓非小细胞肺癌的增长[6],对肾癌也有效[2]。一些抑制逆转录病毒蛋白酶的药物,通常用于治疗HIV感染,但也发现其具有抑制Glut1和Glut4的作用[7]。利托那韦就是其中的一种,在多发性骨髓瘤小鼠模型中,可抑制葡萄糖进入细胞从而具有抗增殖作用[8]。想要将Glut1作为人类癌症患者的治疗靶点,就必须了解其毒性作用。例如,Glut1在血脑屏障中大量表达,所以将Glut1基因抑制后可能影响脑部神经,即Glut1缺陷综合征[9]。尽管如此,根据Glut1抑制剂的临床跟踪记录也说明存在一些具有疗效的葡萄糖摄取抑制剂,如利托那韦。
在肿瘤代谢中,选择性抑制己糖激酶亚型或许也可成为一个治疗靶点。很多研究表明,己糖激酶Ⅱ在几种不同类型的肿瘤细胞中过度表达,而在大部分正常细胞中不表达。己糖激酶Ⅱ基因缺陷是有益的,能减缓癌症进展,减少癌细胞存活率,如肺癌、乳腺癌[10]和脑癌[11]。有趣的是,己糖激酶Ⅱ基因片段缺失虽然是早期致死因子,但将其全基因敲除的成年小鼠却能很好地耐受[10-11],说明癌细胞可能选择性地依赖这种基因片段,所以己糖激酶Ⅱ可以成为一个很好的治疗靶点。已经证明己糖激酶的小分子抑制物在体外具有抗癌活性[12],如2-脱氧葡萄糖(2-deoxyglucose,2-DG),不过2-DG在体内作为单剂量使用时效果一般[5]。然而,对于一个独特的己糖激酶亚型来说,这类化合物不具有特异性,而抑制癌症中己糖激酶亚型过度表达的小分子物质,可以通过不断改变或许具有特异性。
糖酵解中重要的一步是6-磷酸果糖激酶1(6-phosphofructo-1-kinase,PFK1)将果糖-6-磷酸转变为果糖-1,6-二磷酸。在糖酵解中,这是必需的一步,在多种癌症类型中,由于PFK1的活性增强,使得大量的葡萄糖进入糖酵解[13]。肿瘤细胞中增强PFK1活性的机制依赖于PFK1变构激活因子的生成。致瘤信号增强了PFK2亚型FB3(6-phospho⁃fructo-2-kinase/fructose-2,6-bisphosphatase-3,PFKFB3)[14],它在许多恶性肿瘤中高表达,是多种肿瘤细胞存活的必需因子,受到缺氧诱导因子1α亚型(hypoxia-inducible factor-1α,HIF-1α)、蛋白激酶B(protein kinase B,Akt)和人第10号染色体缺失的磷酸酶的调节[15]。通过对HIF-1α的调节,降低其在肿瘤细胞中的表达,弱化HIF-1α对肿瘤细胞糖酵解的增强作用,从而减少肿瘤细胞的能量摄入,降低肿瘤细胞转移能力[16]。PFKFB3表达的增强导致产生了果糖-2,6-二磷酸,它是一种PFK1变构激活因子[17]。研究表明,PFKFB3抑制剂利用遗传途径[18]和小分子抑制剂[19]来减少肿瘤细胞糖酵解并减缓肿瘤细胞生长。PFKFB3小分子抑制剂目前正处于临床试验的早期阶段[20]。
葡萄糖代谢中另一个关键是糖酵解生成的丙酮酸具有不同代谢途径,一种是被转运至线粒体内进行TCA循环,一种是在胞液中转变为乳酸。丙酮酸脱氢酶(pyruvate dehydrogenase,PDH)是丙酮酸的关键调节剂,在线粒体中将丙酮酸转变为乙酰辅酶A。PDH活性的重要调节剂是PDH激酶(pyru⁃vate dehydrogenase kinase,PDK),通过促使PDH特定丝氨酸位点的磷酸化促使PDH失活[21],导致丙酮酸进入线粒体的量减少,而生成大量的乳酸[22]。一些PDK的亚型在各种肿瘤细胞中过度表达[23],并且对维持肿瘤有氧糖酵解起到重要作用。大量研究表明,RNA干扰(RNAi)或小分子抑制剂二氯乙酸(dichloroacetate,DCA)通过抑制PDK抑制剂可使体外肿瘤细胞死亡,也可改善疾病的体内模型[24-25]。DCA可以改变肿瘤细胞的能量平衡,促进葡萄糖氧化并由此产生活性氧[24-25]。临床上,DCA用于治疗乳酸性酸中毒[26],并且一些临床试验将其作为抗癌剂进行研究。在一个小的临床试验中,一些患者采用DCA治疗,发现其与多形性成胶质细胞瘤的影像学回归有关,减少癌细胞增殖,增加癌细胞凋亡[41]。然而,仍不清楚DCA显著的抗癌活性是否使小鼠肿瘤模型的代谢正常化[24-27]。
乳酸脱氢酶(lactate dehydrogenase,LDH)是丙酮酸的另一重要调节剂,在各种类型癌症中LDH的表达增多和活性增强,在胞液中将丙酮酸转变为乳酸[28-29]。LDH的2种亚型形成了混合组成的四聚体[28],LDHa亚型在癌细胞中常牵涉有氧糖酵解[28-29],且对丙酮酸有最佳亲和力,对酶的活性有最高的Vmax。所以,LDHa能够迅速将丙酮酸转为乳酸而完成有氧糖酵解。LDHa催化烟酰胺腺嘌呤二核苷酸((nicotinamide adenine dinucleotide,NAD+)产生的反应,NAD+是维持糖酵解途径中酶活性的关键,如3-磷酸甘油醛脱氢酶。NAD+也是维持肿瘤细胞中良好氧化还原环境的关键。小分子抑制剂或遗传方式抑制LDHa,可以减缓多种肿瘤细胞生长,增加凋亡,包括肝癌和乳腺癌[30-31]。一些早期临床试验评价了LDH的非特异性抑制剂,观察结果不一[32]。对LDHa具有更高特异性的抑制剂的临床前开发目前正在研究中[33]。
3 肿瘤中的谷氨酰胺代谢
肿瘤细胞除了改变葡萄糖代谢,还增加对谷氨酰胺的使用和依赖,以利于肿瘤细胞生长和生存。谷氨酰胺的急剧增加与肿瘤中致癌基因c-Myc信号相关的一种代谢有关[34],也与其他的致癌基因突变有关,如致癌基因K-ras[35]。谷氨酰胺代谢的第一步是谷氨酰胺通过转运体进入细胞,这种能够携带谷氨酰胺的转运体在恶性肿瘤中被普遍上调,如SLC1A5(ASCT2)〔中性氨基酸转运蛋白B(0)/ ASC氨基酸转运蛋白2〕和LAT1(大分子中性氨基酸转运蛋白)。在胞浆内,谷氨酰胺可以作为蛋白质、嘌呤和嘧啶从头合成的一种底物[36],或者可以被谷氨酰胺酶(glutaminases,GLS)转变为谷氨酸。肿瘤细胞可以利用谷氨酰胺衍生后的谷氨酸进行各种活动[37]。肿瘤细胞通过谷氨酰胺还原代谢增加乙酰辅酶A生成,通过HIF-1上调脂肪酸合成酶表达,这都利于脂肪酸的大量合成,脂肪酸一方面合成磷脂利于细胞膜构筑,另一方面合成甘油三酯利于能量的储存和信号的传导,这都与肿瘤形成及进展密切相关[39]。HIF-1和c-Myc协同作用可使肿瘤细胞蛋白合成、细胞周期进程和代谢程序重组,从而精细地调控肿瘤细胞在低氧环境下的代谢适应性反应[39]。谷氨酸经转氨基作用生成非必需氨基酸,利于肿瘤细胞的生长和增殖,并且在肿瘤细胞TCA中是碳供体,经谷氨酸脱氢酶(glutamate dehydrogenase,GDH)转化为α-酮戊二酸而再回流到TCA中,它在TCA循环中用来支持氧化磷酸化,脂类生成或补充的关键中间体。谷氨酸在细胞中还可以被用于生成还原剂,被转化为还原型谷胱甘肽或在苹果酸酶作用下生成还原型烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleo⁃tide phosphate,NADPH)。肿瘤细胞依赖谷氨酰胺可能是基于癌基因激活及抑癌基因失活[40],如Ras基因通过转录调节天冬氨酸转氨酶催化谷氨酰胺来源的底物的转氨基作用,回补三羧酸中间代谢物,提供代谢大分子原料[41]。
一些癌症对谷氨酰胺代谢的依赖性提供了其治疗方向。研究者们开始寻找谷氨酰胺转运体的抑制剂,限制谷氨酰胺进入肿瘤细胞。小分子抑制剂2-氨基-(2,2,1)-庚基-2-羧酸可以抑制LAT1活性,用其对肿瘤细胞进行治疗或者LAT1基因敲除都可以减缓肿瘤细胞的增殖和肿瘤的生长[42-43]。RNAi或者小分子L-谷氨酰基-4-硝基苯胺抑制ASCT2活性,可以减弱哺乳动物西罗莫司(雷帕霉素)靶蛋白〔mammalian target of sirolimus(rapamycin),mTOR〕信号通路并诱导肿瘤细胞自我吞噬[44]。另一研究表明,抑制ASCT2可以降低肺癌细胞某些亚型的生长和存活率,这主要是由于降低了mTOR信号通路的活性[45]。在谷氨酰胺代谢中另一个有效的治疗靶点是GLS,GLS有很多小分子抑制剂,其中BPTES在一些癌细胞系中就能成功抑制GLS活性,从而减缓癌细胞生长,促进癌细胞凋亡[46]。还可以通过抑制GDH来阻止谷氨酰胺进入TCA循环,到目前为止仍未发现抑制GDH的特异性小分子[37]。不过,GDH的非特异性抑制剂对肿瘤细胞具有毒性并且可以减缓移植瘤的生长[47]。
4 肿瘤中的TCA循环
近年来发现,肿瘤细胞的增殖和生存除了利用有氧糖酵解和谷氨酰胺代谢外,一些肿瘤细胞还会很大程度地改变正常的TCA循环。TCA循环通常被认为用于支持线粒体氧化反应,但它还被用于细胞的增殖。在TCA循环中,乙酰辅酶A产生的柠檬酸被转运出线粒体,然后转变为合成脂肪酸所需的原料,脂肪酸是细胞增殖不可或缺的。TCA循环在还原羧基化反应中被逆转,使α-酮戊二酸转变为异柠檬酸,然后再转变为脂质合成所需的柠檬酸。TCA循环中的某些酶导致肿瘤的突变和发展。迄今,某些突变发生在TCA循环酶上,包括琥珀酸脱氢酶(succinate dehydrogenase,SDH)、延胡索酸水合酶(fumarate hydratase,FH)和异柠檬酸脱氢酶(isocitrate dehydrogenase,IDH)。SDH和FH被认为是肿瘤抑制剂,这2种酶的突变会导致肉瘤、肾癌或者其他类型的癌症[48-50]。研究表明,在胶质瘤[51]和急性粒细胞白血病[52]及其他癌症[53-54]中存在
IDH突变。这些突变导致IDH生成一种名叫(R)-2-羟基戊二酸的新代谢物,其在体外可以改变细胞的生存[55],不过机制仍不清楚。许多研究表明,2-羟基戊二酸在细胞中可以庇护IDH突变,并且可以抑制甲基化酶,导致甲基化DNA和干细胞的保留[55-56]。
TCA循环中特异性小分子是迄今为止治疗肿瘤最伟大的发现之一。小分子抑制剂成功锁定突变的SDH和FH的能力是有限的,因为这是一种失去了功能的突变。然而,已经表明,在临床前和临床中一些新化合物可以成功地抑制突变IDH的功能活性。在临床前研究中,突变IDH的小分子抑制剂会严重减少2-羟基戊二酸的产生并使肿瘤细胞向一个更正常的表型分化[57-58]。
5 肿瘤的合成代谢
无论是良性肿瘤还是恶性肿瘤,都必须不断增殖,不断产生新的物质和能量,这就促使肿瘤中脂质、蛋白质和核酸的合成途径不断加快。磷酸戊糖途径可以产生5-磷酸核糖和NADPH。NADPH是脂质和核酸合成所必需的,也是重要的抗氧化剂。6-磷酸葡萄糖脱氢酶(glucose-6-phosphate dehy⁃drogenase,G6PD)是磷酸戊糖途径的限速酶,它的异常过表达会改变小鼠NIH-3T3成纤维细胞[59]。磷酸甘油酸酯变位酶1(phosphoglycerate mutase 1,PGAM1)活性或基因的抑制剂可以减缓肿瘤生长,是由于G6PD抑制3-磷酸甘油酸酯的作用[60]。即便如此,在某些地方,对G6PD活性有影响的基因的缺失很普遍[61]。然而,这些遗传基因缺陷似乎并没有增加这些人群的各种肿瘤发展的风险[62]。丙酮酸激酶M2亚型(pyruvate kinase M2 isoform,PKM2)在磷酸戊糖途径中促进糖分解,激活后可以抑制肿瘤生长[63]。3-磷酸甘油酸酯经多步反应转变为丝氨酸,磷酸甘油酸脱氢酶(phosphoglycerate dehydrogenase,PHGDH)是第一步反应的催化剂,它在人类乳腺癌和黑色素瘤中的量增加,这些肿瘤细胞对PHGDH的消耗特别敏感,说明PHGDH有助于某些肿瘤的发展[64]。PHGDH是新型抗癌药物开发的潜在靶点。
mTOR可以感知氨基酸的缺失,进而抑制蛋白质的翻译,激活细胞自噬作用,从而抑制肿瘤细胞增殖[65]。除了谷氨酰胺非必需氨基酸对肿瘤细胞而言是营养缺失的[66-67]。抑制mTOR是抑制肿瘤细胞增殖的一种方式[68]。核苷酸合成的代谢通路在很早以前就被锁定为抗肿瘤药物研发的靶点。因此,抑制叶酸代谢、胸腺嘧啶核苷的合成、脱氧核苷酸的合成和核酸伸长等是人类肿瘤的标准化疗方案的一部分[69]。
针对肿瘤代谢中肿瘤治疗的代谢靶点研发了很多药物或方法,整理如表1。
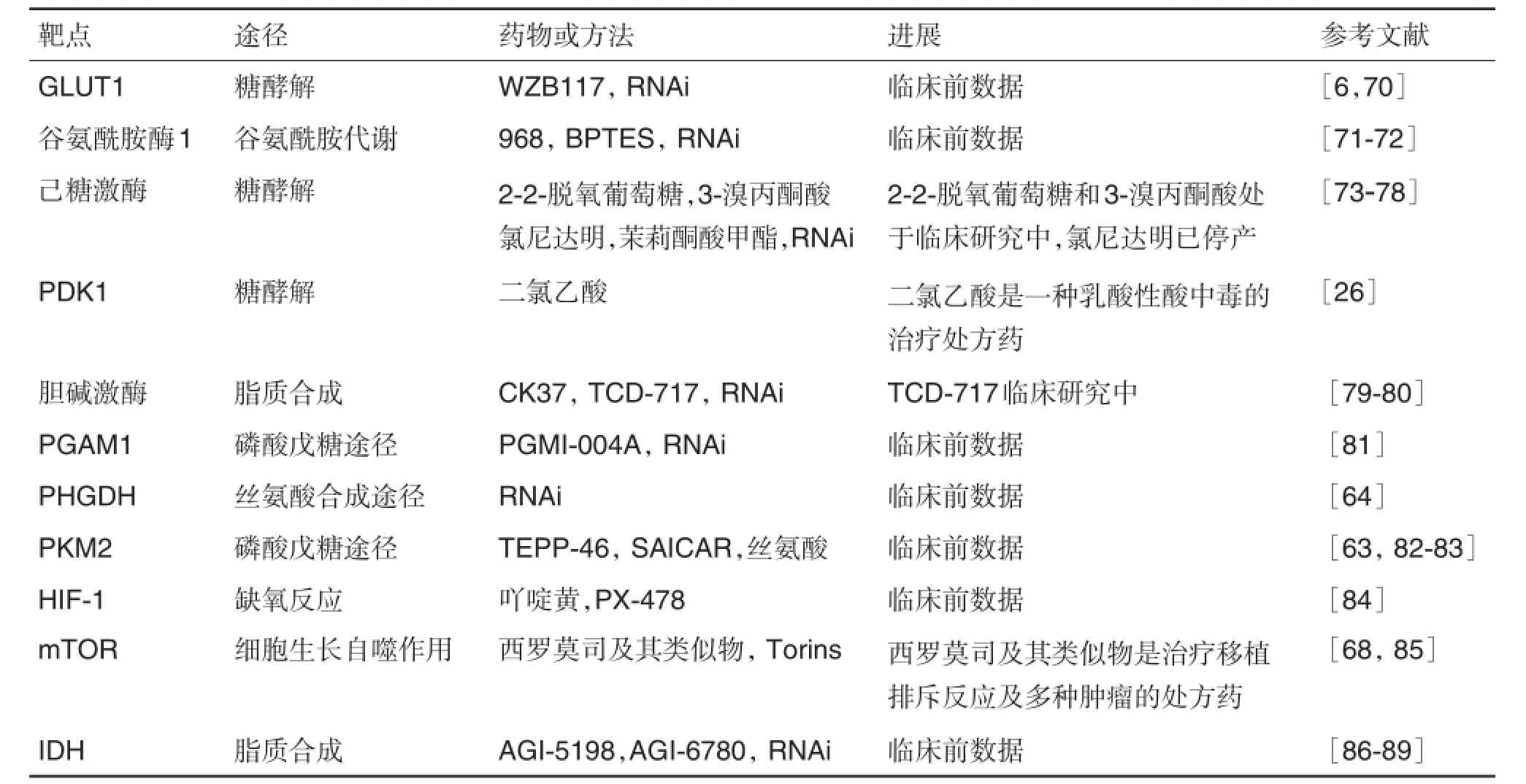
表1 用于癌症治疗的有前景的代谢靶点
6 与肿瘤代谢靶点相关的信号通路
一些不同的致癌因素引起的信号通路与相关的肿瘤代谢变化有一定的联系。致癌基因Ras和BRAF突变与GLUT1增加有关,这使得肿瘤细胞能够在微环境中生存[90]。GLUT1增加不仅与致癌基因的表型相关,而且可能成为特定治疗干预中的对恶性肿瘤耐药的早期标志物[91]。当肿瘤细胞的葡萄糖摄取下降时,它对磷脂酰肌醇3激酶(phos⁃phoinositide 3-kinase,PI3K)和mTOR产生双重抑制作用。PI3K在恶性肿瘤中被过度激活,它的表达可以激活受体酪氨酸激酶(receptor tyrosine kinases,RTK),反之也可激活Akt1和mTOR复合物1[93]。Akt1按以下方式促进有氧糖酵解:①通过促进GLUT1在质膜内合成[92];②通过稳定己糖激酶2与线粒体酶间的关系,从而增加酶的活性[93];③通过激活PFKFB3[94]。另外,一些RTK例如ERBB2,在大量的乳腺癌中过度表达,并通过Akt1的依赖性和非依赖性途径刺激糖酵解,ERBB2尤其促进Akt1非依赖性途径和热休克因子1介导的LDHa基因的激活[95]。研究发现,突变的SDH和FH与家族性癌症和散发性癌症有关,是琥珀酸和延胡索酸的积累所致,说明代谢产物可能直接导致肿瘤的发生[96]。最近的研究证明了这一点,在FH缺陷的小鼠中肾囊肿的形成不需要HIF-1,但却涉及半胱氨酸残基的酶改性,它破坏了Kelch样ECH相关蛋白1(Keap1)抑制NF-E2相关因子2(Nrf2)调控的抗氧化反应的能力[97]。
7 靶向肿瘤代谢的挑战
肿瘤代谢中有许多潜在的治疗靶点,但将抑制代谢途径作为临床治疗肿瘤的一种手段仍然存在重大的挑战。首先是只针对肿瘤细胞有效治疗,而对正常细胞没有抑制作用,尤其是免疫系统中快速增殖的细胞,因为它的代谢途径类似于肿瘤细胞。如T细胞和B细胞依赖于有氧糖酵解[98-99]和谷氨酰胺代谢[100-101]来维持免疫作用。免疫细胞代谢被抑制会减弱它们抵抗癌细胞的能力,且使患者更易感染。其次是许多肿瘤细胞的代谢具有灵活性。这种代谢的灵活性限制了单一靶向治疗的有效性。
8 结语
细胞代谢是一切生命体的基础,肿瘤细胞为适应细胞生长和转移进行自身代谢改变。肿瘤细胞代谢会进行自我调整,但是肿瘤细胞代谢会形成哪些新的途径?例如,降低与糖酵解有关酶的活性,或者控制食物中能量的供给,使肿瘤细胞长期处于一个能量匮乏的微环境中。有氧糖酵解的描述已经证实了肿瘤细胞代谢改变的靶向治疗潜力。在过去10年里,已经明确了通过改变细胞能量代谢途径及如何运用这些改变设计新的治疗方案来抵抗疾病。最近研究表明,许多潜在的途径和靶点会成为肿瘤治疗的有价值的目标。每一种肿瘤都有自己特有的代谢途径,大多数肿瘤的代谢特点表现为Warburg效应。大部分研究都是侧重在糖酵解和谷氨酰胺代谢过程中关键酶和限速酶的靶向治疗。TCA循环中关键酶的研究相对比较少。相信今后将发现更加有潜力的治疗靶点。现仍然存在许多关于靶向肿瘤代谢的问题,包括代谢抑制剂的非靶向性和免疫细胞的抑制,但或许将代谢抑制剂联合应用于临床更有效。
[1]Warburg O.On the origin of cancer cells[J]. Science,1956,123(3191):309-314.
[2]Jones RG,Thompson CB.Tumor suppressors and cell metabolism:a recipe for cancer growth[J].Genes Dev,2009,23(5):537-548.
[3]Flavahan WA,Wu Q,Hitomi M,Rahim N,Kim Y,Sloan AE,et al.Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake[J].Nat Neurosci,2013,16(10):1373-1382.
[4]Smith TA.Mammalian hexokinases and their abnormal expression in cancer[J].Br J Biomed Sci,2000,57(2):170-178.
[5]Liu T,Kishton RJ,Macintyre AN,Gerriets VA,Xiang H,Liu X,et al.Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabo⁃lism and resistance to apoptosis[J].Cell Death Dis,2014,5:e1470.
[6]Liu Y,Cao Y,Zhang W,Bergmeier S,Qian Y,Akbar H,et al.A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis,induces cell-cycle arrest,and inhibits cancer cell growth in vitro and in vivo[J].Mol Cancer Ther,2012,11(8):1672-1682.
[7]Murata H,Hruz PW,Mueckler M.The mecha⁃nism of insulin resistance caused by HIV proteaseinhibitor therapy[J].J Biol Chem,2000,275(27):20251-20254.
[8]McBrayer SK,Cheng JC,Singhal S,Krett NL,Rosen ST,Shanmugam M.Multiple myeloma exhibits novel dependence on GLUT4,GLUT8,and GLUT11:implications for glucose transport⁃er-directed therapy[J].Blood,2012,119(20):4686-4697.
[9]Klepper J,Voit T.Facilitated glucose transporter protein type 1(GLUT1)deficiency syndrome:impaired glucose transport into brain-a review[J].Eur J Pediatr,2002,161(6):295-304.
[10]Patra KC,Wang Q,Bhaskar PT,Miller L,Wang Z,Wheaton W,et al.Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer[J].Cancer Cell,2013,24(2):213-228.
[11]Gershon TR,Crowther AJ,Tikunov A,Garcia I,Annis R,Yuan H,et al.Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neuro⁃genesis and pathogenesis of medulloblastoma[J].Cancer Metab,2013,1(1):2.
[12]Ciavardelli D,Rossi C,Barcaroli D,Volpe S,Consalvo A,Zucchelli M,et al.Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment[J].Cell Death Dis,2014,5:e1336.
[13]Kole HK,Resnick RJ,Van Doren M,Racker E. Regulation of 6-phosphofructo-1-kinase activity in ras-transformed rat-1 fibroblasts[J].Arch Bio⁃chem Biophys,1991,286(2):586-590.
[14]Bobarykina AY,Minchenko DO,Opentanova IL,Moenner M,Caro J,Esumi H,et al.Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expres⁃sion in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers[J].Acta Biochim Pol,2006,53(4):789-799.
[15]Lin BY,Li X,Zhang HT.Potential therapeutic target of energy metabolism for cancer[J].Chem Life(生命的化学),2015,35(1):45-50.
[16]Zhang YL,Fang NZ,YOU JC,Zhou QH. Advances in the relationship between tumor cell metabolism and tumor metastasis[J].Chin J Lung Cancer(中国肺癌杂志),2014,17(11):812-818.
[17]Van Schaftingen E,Hue L,Hers HG.Fructose 2,6-bisphosphate,the probably structure of the glucose-and glucagon-sensitive stimulator of phos⁃phofructokinase[J].Biochem J,1980,192(3):897-901.
[18]Telang S,Yalcin A,Clem AL,Bucala R,Lane AN,Eaton JW,et al.Ras transformation requires meta⁃bolic control by 6-phosphofructo-2-kinase[J].Onco⁃gene,2006,25(55):7225-7234.
[19]Clem B,Telang S,Clem A,Yalcin A,Meier J,Simmons A,et al.Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glyco⁃lytic flux and tumor growth[J].Mol Cancer Ther,2008,7(1):110-120.
[20]Clem BF,O′Neal J,Tapolsky G,Clem AL,Imbert-Fernandez Y,Kerr DA 2nd,et al.Targeting 6-phosphofructo-2-kinase(PFKFB3)as a therapeu⁃tic strategy against cancer[J].Mol Cancer Ther,2013,12(8):1461-1470.
[21]Jha MK,Suk K.Pyruvate dehydrogenase kinase as a potential therapeutic target for malignant gliomas[J].Brain Tumor Res Treat,2013,1(2):57-63.
[22]Fan J,Kang HB,Shan C,Elf S,Lin R,Xie J,et al.Tyr-301 phosphorylation inhibits pyruvate dehydrogenase by blocking substrate binding and promotes the Warburg effect[J].J Biol Chem,2014,289(38):26533-26541.
[23]Hur H,Xuan Y,Kim YB,Lee G,Shim W,Yun J,et al.Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeu⁃tic target[J].Int J Oncol,2013,42(1):44-54.
[24]Bonnet S,Archer SL,Allalunis-Turner J,Haromy A,Beaulieu C,Thompson R,et al.A mitochondria-K+channel axis is suppressed in cancer and its normal⁃ization promotes apoptosis and inhibits cancer growth[J].Cancer Cell,2007,11(1):37-51.
[25]Sutendra G,Dromparis P,Kinnaird A,Stenson TH,Haromy A,Parker JM,et al.Mitochondrial activation by inhibition of PDKⅡsuppresses HIF1a signaling and angiogenesis in cancer[J]. Oncogene,2013,32(13):1638-1650.
[26]Stacpoole PW,Nagaraja NV,Hutson AD.Efficacy of dichloroacetate as a lactate-lowering drug[J].J Clin Pharmacol,2003,43(7):683-691.
[27]Shen YC,Ou DL,Hsu C,Lin KL,Chang CY,Lin CY,et al.Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma[J].Br J Cancer,2013,108(1):72-81.
[28]Koukourakis MI,Giatromanolaki A,Winter S,Leek R,Sivridis E,Harris AL.Lactate dehydroge⁃nase 5 expression in squamous cell head and neck cancer relates to prognosis following radicalor postoperative radiotherapy[J].Oncology,2009;77(5):285-292.
[29]Koukourakis MI,Giatromanolaki A,Simopoulos C,Polychronidis A,Sivridis E.Lactate dehydro⁃genase 5(LDH5)relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer[J].Clin Exp Metastasis,2005,22(1):25-30.
[30]Le A,Cooper CR,Gouw AM,Dinavahi R,Maitra A,Deck LM,et al.Inhibition of lactate dehydroge⁃nase A induces oxidative stress and inhibits tumor progression[J].Proc Natl Acad Sci USA,2010,107(5):2037-2042.
[31]Fantin VR,St-Pierre J,Leder P.Attenuation of LDH-A expression uncovers a link between glycolysis,mitochondrial physiology,and tumor⁃maintenance[J].Cancer Cell,2006,9(6):425-434.
[32]Baggstrom MQ,Qi Y,Koczywas M,Argiris A,Johnson EA,Millward MJ,et al.A phaseⅡstudy of AT-101(gossypol)in chemotherapy-sensi⁃tive recurrent extensive-stage small cell lung cancer[J].J Thorac Oncol,2011,6(10):1757-1760.
[33]Manerba M,Vettraino M,Fiume L,Di Stefano G,Sartini A,Giacomini E,et al.Galloflavin(CAS 568-80-9):a novel inhibitor of lactate dehydroge⁃nase[J].Chem Med Chem,2012,7(2):311-317.
[34]Gao P,Tchernyshyov I,Chang TC,Lee YS,Kita K,Ochi T,et al.c-Myc Suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism[J].Nature,2009,458(7239):762-765.
[35]Son J,Lyssiotis CA,Ying H,Wang X,Hua S,Ligorio M,et al.Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway[J].Nature,2013,496(7443):101-105.
[36]Cory JG,Cory AH.Critical roles of glutamine as nitrogen donors in purine and pyrimidine nucleo⁃tide synthesis:asparaginase treatment in child⁃hood acute lymphoblastic leukemia[J].In Vivo,2006,20(5):587-589.
[37]Hensley CT,Wasti AT,DeBerardinis RJ.Gluta⁃mine and cancer:cell biology,physiology,and clinical opportunities[J].J Clin Invest,2013,123(9):3678-3684.
[38]Jung SY,Jeon HK,Choi JS,Kim YJ.Reduced expression of FASN through SREBP-1 down-regu⁃lation is responsible for hypoxic cell death in HepG2 cells[J].J Cell Biochem,2012, 113(12):3730-3739.
[39]Liao Y.Akt signaling pathway in the regulation of glucose metabolism in cancer cells[J].Electron J Metab Nutr Cancer(肿瘤代谢与营养电子杂志),2014,1(3):61-69.
[40]Liu QL,Huang QY.Characteristics of energy me⁃tabolism of tumor cells and its significance[J]. Chem Life(生命的化学),2015,35(3):387-391.
[41]Son J,Lyssiotis CA,Ying H,Wang X,Hua S,Ligorio M,et al.Glutamine supports pancreatic cancer growth through a KRAS-regulated meta⁃bolic pathway[J].Nature,2013,496(7443):101-105.
[42]Kaira K,Sunose Y,Ohshima Y,Ishioka NS,Arakawa K,Ogawa T,et al.Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer[J].BMC Cancer,2013,13:482.
[43]Wang Q,Tiffen J,Bailey CG,Lehman ML,Ritchie W,Fazli L,et al.Targeting amino acid transport in metastatic castration-resistant pros⁃tate cancer:effects on cell cycle,cell growth,and tumor development[J].J Natl Cancer Inst,2013,105(19):1463-1473.
[44]Nicklin P,Bergman P,Zhang B,Triantafellow E,Wang H,Nyfeler B,et al.Bidirectional transport of amino acids regulates mTOR and autophagy[J].Cell,2009,136(3):521-534.
[45]Hassanein M,Hoeksema MD,Shiota M,Qian J,Harris BK,Chen H,et al.SLC1A5 mediates glu⁃tamine transport required for lung cancer cell growth and survival[J].Clin Cancer Res,2013,19(3):560-570.
[46]Emadi A,Jun SA,Tsukamoto T,Fathi AT,Minden MD,Dang CV.Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations[J].Exp Hematol,2014,42(4):247-251.
[47]Qing G,Li B,Vu A,Skuli N,Walton ZE,Liu X,et al.ATF4 regulates MYC-mediated neuroblasto⁃ma cell death upon glutamine deprivation[J]. Cancer Cell,2012,22(5):631-644.
[48]Pollard PJ,Wortham NC,Tomlinson IP.The TCA cycle and tumorigenesis:the examples of fu⁃marate hydratase and succinate dehydrogenase[J].Ann Med,2003,35(8):632-639.
[49]Gottlieb E,Tomlinson IP.Mitochondrial tumour suppressors:a genetic and biochemical update[J].Nat Rev Cancer,2005,5(11):857-866.
[50]Raimundo N,Baysal BE,Shadel GS.Revisiting the TCA cycle:signaling to tumor formation[J]. Trends Mol Med,2011,17(11):641-649.
[51]Balss J,Meyer J,Mueller W,Korshunov A,Hartmann C,von Deimling A.Analysis of the IDH1 codon 132 mutation in brain tumors[J].Acta Neuropathol,2008,116(6):597-602.
[52]Rakheja D,Konoplev S,Medeiros LJ,Chen W. IDH mutations in acute myeloid leukemia[J]. Hum Pathol,2012,43(10):1541-1551.
[53]Terunuma A,Putluri N,Mishra P,Mathé EA,Dorsey TH,Yi M,et al.MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis[J].J Clin Invest,2014,124(1):398-412.
[54]Borger DR,Tanabe KK,Fan KC,Lopez HU,Fantin VR,Straley KS,et al.Frequent mutation of isocitrate dehydrogenase(IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping[J].Oncologist,2012,17(1):72-79.
[55]Lu C,Ward PS,Kapoor GS,Rohle D,Turcan S,Abdel-Wahab O,et al.IDH Mutation impairs histone demethylation and results in a block to cell differentiation[J].Nature,2012,483(7390):474-478.
[56]Chowdhury R,Yeoh KK,Tian YM,Hillringhaus L,Bagg EA,Rose NR,et al.The oncometabolite 2-hydroxyglutarate inhibits histone lysine demeth⁃ylases[J].EMBO Rep,2011,12(5):463-469.
[57]Rohle D,Popovici-Muller J,Palaskas N,Turcan S,Grommes C,Campos C,et al.An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells[J].Science,2013,340(6132):626-630.
[58]Wang F,Travins J,DeLaBarre B,Penard-Lacro⁃nique V,Schalm S,Hansen E,et al.Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation[J].Science,2013,340(6132):622-626.
[59]Kuo W,Lin J,Tang TK.Human glucose-6-phos⁃phate dehydrogenase(G6PD)gene transforms NIH 3T3 cells and induces tumors in nude mice[J].Int J Cancer,2000,85(6):857-864.
[60]Hitosugi T,Zhou L,Elf S,Fan J,Kang HB,Seo JH,et al.Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth[J].Cancer Cell,2012,22(5):585-600.
[61]Howes RE,Battle KE,Satyagraha AW,Baird JK,Hay SI.G6PD deficiency:global distribution,genetic variants and primaquine therapy[J].Adv Parasitol,2013,81:133-201.
[62]Pisano M,Cocco P,Cherchi R,Onnis R,Cherchi P.Glucose-6-phosphate dehydrogenase deficiency and lung cancer:a hospital based case-control study[J].Tumori,1991,77(1):12-15.
[63]Anastasiou D,Yu Y,Israelsen WJ,Jiang JK,Boxer MB,Hong BS,et al.Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis[J].Nat Chem Biol,2012,8(10):839-847.
[64]Possemato R,Marks KM,Shaul YD,Pacold ME,Kim D,Birsoy K,et al.Functional genomics reveal that the serine synthesis pathway is essential in breast cancer[J].Nature,2011,476(7360):346-350.
[65]Ye J,Mancuso A,Tong X,Ward PS,Fan J,Rabinowitz JD,et al.Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation[J].Proc Natl Acad Sci USA,2012,109(18):6904-6909.
[66]Bach SJ,Swaine D.The effect of arginase on the retardation of tumour growth[J].Br J Cancer,1965,19:379-386.
[67]Jain M,Nilsson R,Sharma S,Madhusudhan N,Kitami T,Souza AL,et al.Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation[J].Science,2012,336(6084):1040-1044.
[68]Sabatini DM.mTOR and cancer:insights into a complex relationship[J].Nat Rev Cancer,2006,6(9):729-734.
[69]Chabner BA,Roberts TG Jr.Timeline:Chemotherapy and the war on cancer[J].Nat Rev Cancer,2005,5(1):65-72.
[70]Gautier EL,Westerterp M,Bhagwat N,Cremers S,Shih A,Abdel-Wahab O,et al.HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders[J]. J Exp Med,2013,210(2):339-353.
[71]Wang JB,Erickson JW,Fuji R,Ramachandran S,Gao P,Dinavahi R,et al.Targeting mitochondrial glutaminase activity inhibits oncogenic transfor⁃mation[J].Cancer Cell,2010,18(3):207-219.
[72]Seltzer MJ,Bennett BD,Joshi AD,Gao P,Thomas AG,Ferraris DV,e t al.Inhibition of gluta⁃minase preferentially slows growth of glioma cells with mutant IDH1[J].Cancer Res,2010,70(22):8981-8987.
[73]Dwarakanath BS,Singh D,Banerji AK,Sarin R,Venkataramana NK,Jalali R,et al.Clinical studies for improving radiotherapy with 2-deoxy-D-glucose:present status and future prospects[J].J Cancer Res Ther,2009,5(Suppl 1):S21-S26.
[74]Wolf A,Agnihotri S,Micallef J,Mukherjee J,Sabha N,Cairns R,et al.Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme[J].J Exp Med,2011,208(2):313-326.
[75]Goldin N,Arzoine L,Heyfets A,Israelson A,Zaslavsky Z,Bravman T,et al.Methyl jasmonate binds to and detaches mitochondria-bound hexo⁃kinase[J].Oncogene,2008,27(34):4636-4643.
[76]Jae HJ,Chung JW,Park HS,Lee MJ,Lee KC,Kim HC,et al.The antitumor effect and hepato⁃toxicity of a hexokinaseⅡinhibitor 3-bromopyru⁃vate:in vivo investigation of intraarterial adminis⁃tration in a rabbit VX2 hepatoma model[J].Kore⁃an J Radiol,2009,10(6):596-603.
[77]Hamanaka RB,Chandel NS.Targeting glucose metabolism for cancer therapy[J].J Exp Med,2012,209(2):211-215.
[78]Vander Heiden MG.Targeting cancer metabo⁃lism:a therapeutic window opens[J].Nat Rev Drug Discov,2011,10(9):671-684.
[79]Clem BF,Clem AL,Yalcin A,Goswami U,Arumugam S,Telang S,et al.A novel small mole⁃cule antagonist of choline kinase-α that simultane⁃ously suppresses MAPK and PI3K/AKT signaling[J].Oncogene,2011,30(30):3370-3380.
[80]Yalcin A,Clem B,Makoni S,Clem A,Nelson K,Thornburg J,et al.Selective inhibition of choline kinase simultaneously attenuates MAPK and PI3K/AKT signaling[J].Oncogene,2010,29(1):139-249.
[81]Hitosugi T,Zhou L,Elf S,Fan J,Kang HB,Seo JH,et al.Phosphoglycerate mutase 1 coordi⁃nates glycolysis and biosynthesis to promote tumor growth[J].Cancer Cell,2012,22(5):585-600.
[82]Chaneton B,Hillmann P,Zheng L,Martin AC,Maddocks OD,Chokkathukalam A,et al.Serine is a natural ligand and allosteric activator of pyru⁃vate kinase M2[J].Nature,2012,491(7424):458-462.
[83]Keller KE,Tan IS,Lee YS.SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions[J].Science,2012,338(6110):1069-1072.
[84]Wilson WR,Hay MP.Targeting hypoxia in cancer therapy[J].Nat Rev Cancer,2011,11(6):393-410.
[85]Benjamin D,Colombi M,Moroni C,Hall MN. Rapamycin passes the torch:a new generation of mTOR inhibitors[J].Nat Rev Drug Discov,2011,10(11):868-880.
[86]Dang L,White DW,Gross S,Bennett BD,Bittinger MA,Driggers EM,et al.Cancer-associ⁃ated IDH1 mutations produce 2-hydroxyglutarate[J].Nature,2009,462(7274):739-744.
[87]Ward PS,Patel J,Wise DR,Abdel-Wahab O,Bennett BD,Coller HA,et al.The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alphaketoglutarate to 2-hydroxyglutarate[J].Cancer Cell,2010,17(3):225-234.
[88]Wang F,Travins J,DeLaBarre B,Penard-Lacro⁃nique V,Schalm S,Hansen E,et al.Targeted inhibition of mutant IDH2 in leukemia cells induc⁃es cellular differentiation[J].Science,2013,340(6132):622-626.
[89]Rohle D,Popovici-Muller J,Palaskas N,Turcan S,Grommes C,Campos C,et al.An inhibitor of mutant IDH1 delays growth and promotes differ⁃entiation of glioma cells[J].Science,2013,340(6132):626-630.
[90]Ying H,Kimmelman AC,Lyssiotis CA,Hua S,Chu GC,Fletcher-Sananikone E,et al.Oncogen⁃ic Kras maintains pancreatic tumors through regu⁃lation of anabolic glucose metabolism[J].Cell,2012,149(3):656-670.
[91]Engelman JA.Targeting PI3K signalling in cancer:opportunities,challenges and limitations[J].Nat Rev Cancer,2009,9(8):550-562.
[92]Barthel A,Okino ST,Liao J,Nakatani K,Li J,Whitlock JP Jr,et al.Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1[J].J Biol Chem,1999,274(29):20281-20286.
[93]Majewski N,Nogueira V,Bhaskar P,Coy PE,Skeen JE,Gottlob K,et al.Hexokinase-mito⁃chondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak[J].Mol Cell,2004,16(5):819-830.
[94]Deprez J,Vertommen D,Alessi DR,Hue L,Rider MH.Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades[J].J Biol Chem,1997,272(28):17269-17275.
[95]Zhao YH,Zhou M,Liu H,Ding Y,Khong HT,Yu D,et al.Upregulation of lactate dehydroge⁃nase A by ERBB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth[J].Oncogene,2009,28(42):3689-3701.
[96]Gottlieb E,Tomlinson IP.Mitochondrial tumour suppressors:a genetic and biochemical update[J].Nat Rev Cancer,2005,5(11):857-866.
[97]Adam J,Hatipoglu E,O′Flaherty L,Ternette N,Sahgal N,Lockstone H,et al.Renal cyst forma⁃tion in FH1-deficient mice is independent of the HIF/Phd pathway:roles for fumarate in KEAP1 succination and Nrf2 signaling[J].Cancer Cell,2011,20(4):524-537.
[98]Macintyre AN,Gerriets VA,Nichols AG,Michalek RD,Rudolph MC,Deoliveira D,et al. The glucose transporter Glut1 is selectively essen⁃tial for CD4 T cell activation and effector function[J].Cell Metab,2014,20(1):61-72.
[99]Caro-Maldonado A,Wang R,Nichols AG,Kuraoka M,Milasta S,Sun LD,et al.Metabolic reprogramming is required for antibody produc⁃tion that is suppressed in anergic but exaggerat⁃ed in chronically BAFF-exposed B cells[J].J Immu⁃nol,2014,192(8):3626-3636.
[100]Le A,Lane AN,Hamaker M,Bose S,Gouw A,Barbi J,et al.Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells[J].Cell Metab,2012,15(1):110-121.
[101]Nakaya M,Xiao Y,Zhou X,Chang JH,Chang M,Cheng X,et al.Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTOR C1 kinase activation[J].Immunity,2014,40(5):692-705.
Research progress in metabolic targets for cancer therapy
WEN Shi-yuan,JIANG Miao-miao
(Tianjin State Key Laboratory of Modern Chinese Medicine,Tianjin University of Traditional Chinese Medicine,Tianjin 300193,China)
Cancer cells can change metabolic pathways,including glycolysis and glutamine metabolism,and produce the raw materials needed for rapid proliferation and survival.Therefore,research on metabolic pathways of cancer cells might help find the targets of cancer therapy.In this review,we outlined the metabolic features of aerobic glycolysis,glutamine metabolism and(tricarboxylic acid) TCA cycle in cancer.We also described metabolic targets for cancer therapy and therapeutic agents for the corresponding targets in these metabolic pathways,and finally discussed some of the challenges related to tumor metabolism as a therapeutic target in cancer therapy.
tumor metabolism;aerobic glycolysis;glutamine
JIANG Miao-miao,E-mail:miaomiaojiang@126.com,Tel:15822829059
2016-07-05接受日期:2017-03-17)
(本文编辑:齐春会)
国家自然科学基金(81573547)
温时媛,硕士研究生,主要从事中药化学研究,E-mail:13072001326@163.com
姜苗苗,E-mail:miaomiaojiang@126.com,Tel:15822829059
R730.5
:A
:1000-3002-(2017)03-0269-10
10.3867/j.issn.1000-3002.2017.03.011
Foundation item:The project supported by National Natural Science Foundation of China(81573547)

