欧洲睡眠中心认证标准
INTRODUCTION
A clinical centre for the diagnosis and treatment of a wide variety of sleep disorders is called a Sleep Medicine Centre (SMC) in this document.The accreditation of SMCs is essential to ensure and improve patient care.The voluntary accreditation of sleep laboratories in Germany has been carried out by the Deutsche Gesellschaft fur Schlafforschung und Schlafmedizin (DGSM),by means of a questionnaire and site inspection,since 1989.The initiation of this procedure was based on a consensus publication (Penzel et al.,2000),and subsequent papers dealing with specific aspects of the accreditation process (Fischer et al.,1999; Gugger,1998; Niewerth and Wiater,2000; Penzel et al.,1993,1994,1998; Peter et al.,1995; Wiater and Niewerth,2000).Other European countries have been successful as well in developing specific accreditation procedures for their SMCs (Bassetti,2000; Gugger,1998).At a meeting of delegates of the European National Sleep Societies (ENSS) in Mallorca in 2004,under the auspices of the European Sleep Research Society (ESRS),it was decided that an accreditation process,based on the DGSM guidelines,but taking into account similar experiences in other European countries,should be developed to form the basis of a Europewide voluntary accreditation procedure.A preliminary version was drafted after obtaining input from different ENSS.This version was subsequently reviewed by representatives of the ENSS at the business meeting of the ESRS in Bayrischzell,Germany (April 2005).The revised draft manuscript was then circulated to all ENSS for comments,and subsequently reworked,taking into account the recommendations that were received.The final document was reviewed and approved by the Executive Board of the ESRS.
The status of accredited SMCs does not preclude the existence of other clinical facilities with more limited capabilities in the diagnosis of specific sleep disorders.However,such facilities are not eligible for accreditation as an SMC according to the present guidelines.
The accreditation process examines and certifies the staff and facilities of the laboratory that constitute the SMC.The process quality and outcome quality of the results are checked and ensured by other measures,which will be subject to the discussion and eventual publication of a separate guideline.
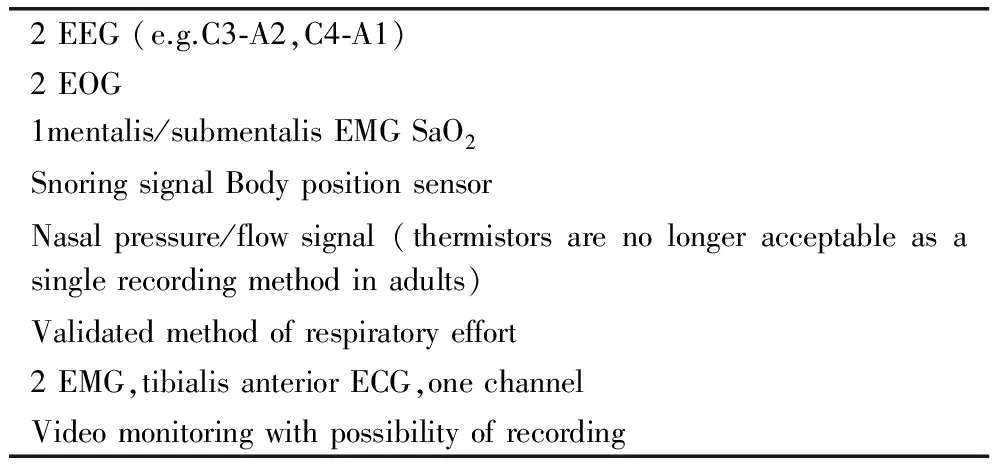
Table 1 Minimum montage for standard sleep medicine polysomnography
Accreditation of an SMC is achieved by a two-step process: firstly a questionnaire is completed,and then a site inspection visit is conducted by a panel of independent specialists experienced in sleep medicine.
These guidelines set out the requirements of the accreditation procedure and the assessment criteria used.The local National Sleep Society (NSS) is entitled to set an administrative fee,charged for the costs with respect to the application process and the site inspection visit.
An accredited SMC must have the diagnostic measures available to diagnose common sleep disorders (see Table 1 in the Accreditation Questionnaire),in accordance with ICSD-2 (Sateia,2005).However,it should also be noted that accredited SMCs are not required to be able to manage the entire spectrum of sleep disorders.The SMC should be able to demonstrate appropriate referral routines.
It is envisaged that this document will be reviewed,and where necessary revised,4 years after publication.
STAFF,FACILITIES,EQUIPMENT,AND STRUCTURAL REQUIREMENTS FOR A SLEEP MEDICINE CENTRE
Management and medical staff
The head of the SMC should have a permanent position at the institution in order to ensure continuity of medical care for patients with sleep disorders.
An SMC must have a responsible physician (MD) who can demonstrate comprehensive knowledge of the diagnostic spectrum of sleep disorders.
To be eligible for accreditation by the NSS,the applicant must be a member of the NSS and have,or will obtain,a National Board Certification of Sleep Medicine,if available.1
The head of the SMC is responsible for the continual quality assurance in the sleep laboratory.It is not felt appropriate that the same person acts as the head of more than one SMC at any one time.
Medical emergency care must be guaranteed.In case of emergency,a physician must be available to attend the SMC,at clinically appropriate,short notice.Medical care must be ensured at all times: an attending physician in the clinic is considered sufficient.
Overall,the staff policy of the hospital should ensure that the sleep laboratory is an independent entity; e.g.those nurses and technologists performing night duty in the sleep laboratory must not have any further responsibilities,such as night duty in another ward.
Sleep laboratories that are part of a ward need to be able to demonstrate that they have a staff dedicated to doing the recording routines.
Technical staff
The operation of an SMC with both day and night examinations and a sleep outpatient clinic with ambulatory diagnostics requires an adequate staff.
Nurses and technologists must have sufficient knowledge of the diagnostic and therapeutic procedures,the polysomnographic measuring methods,the procedures performed during the day,as well as ambulatory measuring procedures.
Polysomnography (PSG) technologists for nocturnal and diurnal recordings are required to ensure the proper,artefactfree functioning of the recording devices,to detect problems and resolve them.This makes their presence during the entire recording process an absolute necessity.They are also required to continuously monitor the patient′s vital signs and take appropriate measures in case of emergency.It is recommended that one member of the night staff is responsible for no more than four patients.
The technical and nursing staff of the SMC must also be knowledgeable about the diagnosis of sleep disorders in accordance with ICSD-2,and to this end they should obtain professional certification in the field of Somnology,if available (Vorstand der DGSM,1999).
Administrative staff
The SMC should have a permanent secretarial staff,who can adequately answer and refer the patients′ calls as well as organize appointments and keep patients′ records.
Facilities
The following criteria must be met for the approval of a polysomnographic bed:
Only those beds that are located in single bedrooms and in which the polysomnograph allows recording of all relevant biosignals will be approved as polysomnographic beds.
The bedrooms must be equipped to allow professional diagnosis and therapy in the field of sleep medicine to be carried out and to enable both nocturnal examinations and assessment of daytime sleepiness.

Table 2 Non-exhaustive list of supplementary signals to be recorded,depending on the clinical specialization of the SMC
The recording and examination rooms should be of an adequate size that complies with local specifications.2The rooms must be sound and light attenuated and equipped with temperature and ventilation controls.To enable daytime assessments,such as the multiple sleep latency test (MSLT),the rooms must be adequately darkened.
Adequate sanitary facilities must be available near the bedrooms.
Each bedroom should be equipped with a suitable video monitoring system.
A two-way communication system must be installed which allows the patient and night duty technician to communicate with each other,and to enable biosignal calibration.
A separate room,which is also sufficiently large and which ensures undisturbed working conditions,must be available for the monitoring equipment and the technical/nursing staff.Polysomnographic recording next to the patient′s bed is not acceptable.
Recording techniques and criteria
Polysomnography
Polysomnography is a diagnostic technique comprising the simultaneous recording of neurophysiological,cardiorespiratory and other biosignals during an entire nocturnal sleep period.The suggested minimum PSG recording montage to be used in an SMC is listed in Table 1.Supplementary signals to be recorded depending on the clinical specialization of the SMC are listed in Table 2.
Polysomnographs can either be recorded on paper or digitally.However,if digital recordings are performed,then a printout on paper must be possible.The digital recording device must allow the viewing of previous epochs during the recording.In digital recording the monitor screens must have a sufficiently high resolution to allow accurate assessment and evaluation of all recorded biosignals.3
As mentioned above,the ongoing recording should be monitored by trained technical or nursing staff.
Concisedescriptionofothertechniquesandtests
·Polygraphy: a diagnostic technique comprising the simultaneous recording,usually not attended,of certain cardiorespiratory and other biosignals but not EEG during an entire nocturnal sleep period.
·MSLT: a neurophysiological test comprising EEG,EMG and EOG,for a duration of 20 min,in four or five sessions with 2-h intervals.The primary outcome is the evaluation of the tendency to fall asleep (Carskadon et al.,1986).
·Maintenance of Wakefulness Test (MWT): a neurophysiological test comprising EEG,EMG and EOG,for a duration of 40 min,in four or five sessions with 2-h interval.The primary outcome is the evaluation of the capacity to stay awake (Mitler et al.,1982).
·A description of techniques and measurements not listed here can be found in the literature (Chervin,2005).
Patient reports,documentation and archive facilities
Sleep disorders must be classified in accordance with ICSD-2 (Sateia,2005).The protocol of the PSG (descriptive-statistical sleep characteristics,hypnogram and other all-night trends) and findings of the accompanying examinations must be documented in the patient′s medical record.This also applies to the documentation of the titration of nocturnal ventilation therapy.
A comprehensive report should be prepared within a reasonable time delay4.A brief discharge report is also recommended.
The comprehensive report has to comprise the patient′s sleep history as well as a description and interpretation of relevant sleep parameters (sleep stages,sleep latency,etc.).The inclusion of all-night graphical trends of relevant signals,e.g.SaO2,hypnogram,etc.,is desirable.The report should be based on human scoring of the signals that constitute the PSG by professionals trained in sleep medicine.
A filing system needs to be maintained so that polysomnographic records and patient-related findings can be easily accessed.The entire patient record,including the raw data,is to be archived for a period that is in accordance with the relevant statutory instructions of the SMCs′ host country5.
GUIDELINES FOR THE COMPLETION AND EVALUATION OF THE ACCREDITATION QUESTIONNAIRE
The accreditation questionnaire is not included in this manuscript.It is made available through the official website of the ESRS.
Staff
NameofSMC
The name of the SMC and the clinic in which the SMC is located is given here.If available,the address is to be supplemented with the e-mail address and internet homepage.
SMCmanagement
The name of the head of the SMC is given here.This person should be a member of the NSS and,if applicable,should have a Board Certification in Sleep Medicine.The head of the SMC should have a permanent position at the institution.
Responsible physician of the SMC.
This part is to be filled out if different from above.
Consultingservices
Proof should be provided that a comprehensive sleep medical diagnosis can be established.
In accordance with the nature of sleep disorders,consulting services from other medical and non-medical specialties are required and must be specified.
Details of outpatient sleep clinic activities should also be given under this heading.It is strongly recommend that the SMC offers an outpatient clinic for prescreening,follow-up and therapy.This approach may be useful to reduce inpatient admissions.
Staff
A full list of the staff members working in the SMC should be given along with a description of their knowledge of and experience in sleep medicine.An indication of the percentage of total job time spent working in the sleep laboratory is to be given.From the composition of the staff,it should be clear that the continuity of medical services is guaranteed by the SMC.
Advancedtraining
Sleep medicine is undergoing rapid changes due to the continuous increase in scientific knowledge.For this reason,regular sleep-related training activities should be conducted for the laboratory′s own staff and/or the possibility of attending external training should be ensured.The provision of sleepmedical educational activities for other hospitals or physicians is welcomed,but optional.
Patient and sleep-medical services
The sleep-medical services offered provide information on the interdisciplinarity of the SMC.In particular,the diagnostic measures should enable the laboratory to diagnose common sleep disorders in accordance with ICSD-2.
The diagnostic and therapeutic process should be economical and correspond to national and international standards,respectively.The patient′s length of stay in the laboratory should be oriented to the diagnostic and therapeutic standards.
DiagnosticprofileoftheSMC
The numerical data,including ICSD-2 code and outpatient/ inpatient status,provide information on the focus of the laboratory,differential diagnosis,and its size,as shown by the number of patients.The data given here should provide statistics as exact as possible.The current waiting periods for outpatient and inpatient diagnosis are to be given.
TherapeuticprofileoftheSMC
The numerical data (including outpatient/inpatient status) provide information on the therapeutic main foci of the laboratory.Referral policies for sleep disorders that are not routinely treated must be described.
Equipment and rooms
PSGrecorders
A description of the PSG recording equipment and montages used in the laboratory is to be given here.
Additionaldevices
Under this heading the polygraphs used for inpatients should be mentioned.Polysomnographic recorders that are operated in rooms without continuous monitoring should also be mentioned here.
Routinemeasurements
A description of the routine nocturnal measurements performed in the laboratory of the SMC is to be included here.
Otherequipment
Existing independent systems for follow-up diagnoses and controls should be listed here.This heading is to provide space for devices that are not only used in a sleep medical context,e.g.
·Actigraphy
·Long-term EEG
·Long-term ECG
·Ambulatory blood pressure monitoring or continuous monitoring
·Ambulatory pH measurement
·Pulse oximeters,which can be used independently of the SMC
·Other.
Facilities
A description of the bedrooms,recording rooms,sanitary facilities,etc.should be given here.
Diagnostic tests
Additional diagnostic tests that are routinely performed at the SMC should be listed here,e.g.
·Physical examination
·Clinical investigations (e.g.laboratory analyses,radiology,pulmonary function tests,etc.)
·MSLT
·MWT
·Sleep diary
·Sleep questionnaires [e.g.Pittsburg Sleep Quality Index (PSQI),Epworth Sleepiness Scale (ESS),Stanford Sleepiness Scale (SSS),etc.]
·Psychological and personality questionnaires (e.g.MMPI,etc.)
·Neuropsychological examinations (vigilance,psychometric and cognitive tests)
·Other.
Medical documentation and archive
A description of the reporting procedure and relevant documentation should be given here along with a description of the archive system used.
Invoicing
The invoicing procedure for sleep-medical services can be briefly listed here.During the site visit,an exchange of experiences on this topic can take place.
Miscellaneous
Additional comments and information thought relevant should be given here.
SCENARIO FOR THE SITE VISIT
Once the SMC has submitted a completed questionnaire a site inspection visit is arranged.The exact time and modalities of the visit must be communicated at least 4 weeks in advance.The Accreditation Committee of the NSS will assign independent experts to constitute an Inspection Panel.6
These individuals should have expertise in quality management and sleep medicine.They will each be given a copy of the site questionnaire which will form the basis of the initial discussion.The site visit is a three-part procedure.The Inspection Panel should represent different but appropriate specialties in order to assess the work of the site to be visited.First,there is an initial discussion with the staff of the SMC.This is then followed by a demonstration of skilfulness in the required techniques,as well as a tour and assessment of the facilities.The final stage is a discussion of the findings of the Inspection Panel with the management of the SMC.The Accreditation Committee of the NSS is entitled to charge a fee to the SMC for administration costs and the reimbursement of the travel expenses made by the members of the Inspection Panel.
Initial discussion
The head of the SMC and the technical staff will participate in this discussion.If the SMC is interdisciplinary,the staff members of the respective disciplines should also be present.Whether predominantly children or adults are diagnosed and treated must have been clarified before the site visit,because a paediatrician must be part of the Inspection Panel if the former is the case.
The discussion will be comprehensive and will usually last at least an hour,frequently longer.It should take place in a calm atmosphere and be uninterrupted.The diagnostic and therapeutic procedures of the SMC will be reviewed.
Demonstration of technical skills
At the beginning of the initial discussion,the laboratory staff will be informed that a complete ‘hook-up’ of electrodes and sensors is to be carried out on a test subject,followed by a test polysomnographic recording.At the beginning of the demonstration the technical staff will be allowed to proceed without interference so that they perform the procedure in their usual manner.Following the initial discussion,the quality of hookup and recording will be evaluated by the Inspection Panel.
Subsequently,polysomnographic recordings performed in the sleep laboratory will be assessed for good quality.When showing these records,the SMC staff will be asked to demonstrate their practical knowledge in analysis and evaluation of such data.
Finally,an examination of randomly selected patient records will be carried out.This should demonstrate that clinical investigations have been performed in an appropriate medical context,ultimately leading to the diagnosis of the sleep disorder for which the patients consulted the SMC.
An inspection of all the rooms listed in the questionnaire will be conducted to see whether they fulfil the relevant criteria.
Final discussion
The Inspection Panel will retreat on site to identify critical points and prepare these items for the final discussion.Subsequently,the final discussion will take place with the head and/or the responsible physician of the SMC.The Inspection Panel will inform them on the result of the site visit.
The Inspection Panel can only make recommendations.The decision on the accreditation is the competence of the Accreditation Committee of the NSS.
Four major outcomes can be differentiated in the Accreditation Recommendation.
·Recommendation to accredit the SMC in its present form with no restrictions.
·Recommendation to accredit the SMC after correction of slight deficiencies.The SMC must notify the Accreditation Committee in writing of the correction of the deficiencies.Another site visit is not required.
·Recommendation that deficiencies qualified to be ‘ubstantial’ first be corrected and that the decision on a possible accreditation is made dependent on a new site visit.
·No accreditation.
The oral statement of the Inspection Panel must absolutely agree with the contents of the written report.In cases of considerable uncertainty,the Inspection Panel should not make any definitive decision on site,but first consult the Accreditation Committee of the NSS.In this case the result of the final discussion remains open.
OUTLINE OF THE ACCREDITATION REPORT
The report must be written by a member of the Inspection Panel on stationary of the NSS and signed by each of the visiting experts.
The items in the report correspond to the structure of the Accreditation Questionnaire.
Initial essential information
This information includes the date of the visit,composition of the Inspection Panel,address,telephone and fax of the SMC,and,if applicable,also the e-mail and website addresses.The names of the head and/or responsible physician of the SMC are given.
General information
This section contains the description of the clinic/department in which the SMC is based and how the SMC is integrated into the structure,as well as its origin and development.This section also includes the consulting services provided with names of the relevant physicians and allotment of time to the SMC,transfer information for the sleep outpatient clinic.Staff and fraction of its working time spent in SMC are described.
Sleep laboratory routine
This section gives a description of diagnostic and therapeutic procedures.Diagnostic and therapeutic profiles must be also be mentioned in detail.
Equipment and facilities
Here a description is given of the following items:
·Diagnostic tests
·Documentation
·Demonstration of placement of all measuring devices
·Demonstration of recording
·Review of past nocturnal recordings
·Review of files and physicians′ reports
·Remuneration of expenses.
Assessment and recommendations
This section contains the following points:
·General summary of the site visit
·Recommendations listed point by point
·Final summary of the recommendation with statement:
·Accreditation immediately
·Accreditation after fulfilment of improvements without revisit
·Accreditation after fulfilment of improvements with revisit
·No accreditation
·Signatures of the three experts
After being signed,the report is to be sent to the Accreditation Committee of the NSS and a letter will be sent from there to the respective SMC.A copy of the report must be filed in the archives of the NSS.
RESPONDING TO AN ACCREDITATION REPORT
The Accreditation Committee will send a copy of the site visit report to the SMC within a reasonable time span.It is recommended that the Accreditation Committee of the NSS and the SMC agree upon the span for the delivery of the report,before the site visit is undertaken.7The letter that accompanies the report will again state in which of the four categories the SMC was placed during the site visit.If improvements are required the SMC must document the alterations that have been carried out.If this is to be checked without a renewed site visit,documentary evidence (e.g.photographs of the structural alterations) is to be submitted.If the improvements refer to signals (e.g.oesophageal pressure,tibialis EMG),copies of epochs of the measuring curves of three to five patients are to be submitted.
If it is recommended that further training in another SMC of excellence is required for members of SMC staff,then details of such training should be given.
In summary,the SMC is to write a statement on all points of the recommendations.This statement will be forwarded to the Inspection Panel experts with the included documents and receipts.They can then directly declare their agreement with accreditation or require the submission of further documents.If necessary,they can directly contact the SMC to clarify details of the improvements.If the SMC has fulfilled all the recommendations,accreditation will be granted after agreement of the experts.If the subsequently submitted documents do not satisfy the experts,another site visit can be taken into consideration.Depending on the subsequently submitted documents,the number of experts can be reduced for a renewed site visit.All experts must agree to this procedure.
If the SMC disagrees with an unfavourable outcome,after passing the whole procedure,appeal may be acknowledged to the Board of the NSS,that subsequently decides on appropriate actions.
RE-EVALUATION OF LABORATORIES
Re-evaluation of SMCs is required every 2 years.This is achieved by means of a further questionnaire based on the original one.In this process,the general information of the SMC and its equipment will be confirmed.Inquiries will be made about Board Certification in Sleep Medicine,if available,of people in leading positions.
NOTIFICATION OF CHANGES OF THE PHYSICIAN IN CHARGE OF THE SMC AND CHANGES IN CAPACITY OR CHANGES IN LOCATION
The SMCs′ accreditation is limited to the laboratory facilities and their heads.The Accreditation Committee of the NSS must be informed of any changes within 2 weeks after the change has occurred.
In cases involving a change of the head and/or the responsible physician,a renewed site visit is required only if the newly appointed officer does not possess a Board Certification of Sleep Medicine (if available).
In cases involving changes in capacity (i.e.expansion) of the SMC,the head of the respective SMC must submit a detailed report on the extent and type of expansion with regard to technical capabilities,facilities and staff.If this report contains indications that the criteria are no longer fulfilled,the Accreditation Committee of the NSS may arrange for a renewed site visit.
If the SMC moves into new facilities in another building (address change),even if part of the staff and equipment are retained,a new site visit is required.This new site visit can be performed by one appointed expert,who will make an assessment of the facilities and prepare a brief report on them.If the capacity has not changed,it is not necessary to fill out a new questionnaire.
AUTHORSHIP
The European guidelines for the accreditation of Sleep Medicine Centres were written by the members of the Steering Committee of the European Sleep Society: Pevernagie D.(Belgian Association for the Study of Sleep),Stanley N.(British Sleep Society),Berg S.(Svensk Forening for Somnforskning och Somnmedicin),Krieger J.(Societe Francaise de Recherche et Medecine du Sommeil),Fischer J.(Deutsche Gesellschaft fur Schlafforschung und Schlafmedizin).
ACKNOWLEDGEMENTS
The authors are indebted to delegates and members of the ENSS who reviewed the text and made helpful comments: Popovic R.M.,Hogl B.(Austrian Sleep Research Association),Hack M.(British Sleep Society),Dogas Z.,Hodoba D.(Croatian Sleep Research Association),Sonka K.(Czech Sleep Research and Sleep Medicine Society),Jennum P.(Danish Sleep Research Association),Kerkhof G.(Dutch Society for Sleep and Wake Research),Saaresranta T.,Hasan J.,Harma M.(Finnish Sleep Research Society),Vecchierini M.-F.(French Society of Sleep Research),Mayer G.,Penzel T.(DGSM),Soldatos C.,Papavassiliou A.(Hellenic Sleep Research Association),Halasz P.(Sleep Research Section of the Hungarian EEG Society),Gislason T.(Icelandic Sleep Research Society),Ferini-Strambi L.(Italian Association of Sleep Medicine),Vecvagars A.(Latvian Sleep Society),Liesiene V.(Lithuanian Sleep Society),Szelenberger W.,Jacitowicz J.(Polish Sleep Research Society),Paiva T.(Portuguese Sleep Association),Cuartero P.,Canellas Dols F.,Bove Ribe A.(Iberic Sleep Research Society),Bloch K.E.,Hess C.,Tobler I.(Swiss Society of Sleep Research,Sleep Medicine and Chronobiology),Kaynak H.(Turkish Sleep Research Society).
Finally,the authors want to thank the Board of the ESRS,especially Pollmacher T.,Bassetti C.and Cirignotta F.,for reviewing the drafts and for their valuable suggestions.
Bassetti,C.Akkreditierte Schlafzentren in der Schweiz.Schweiz.
Arzteztg.,2000,81: 886-888.
Carskadon,M.A.,Dement,W.C.,Mitler,M.M.,Roth,T.,Westbrook,P.R.and Keenan,S.Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness.Sleep,1986,9: 519-524.
Chervin,R.D.Use of clinical tools and tests.In: M.H.Kryger,T.Roth and W.C.Dement (Eds) Principles and Practice of Sleep Medicine,4th edn.Elsevier,Philadelphia,PA,USA,2005: 602-614.
Fischer,J.,Raschke,F.and Kutschmann,M.Die Checkliste qualitatsrelevanter Prozessmerkmale fur das Peer-Review-Verfahren der Deutschen Gesellschaft fur Schlafforschung und Schlafmedizin (DGSM) zur Sicherung der Prozessqualitat im akkreditierten Schlaflabor.Somnologie,1999,3: 335-346.
Gugger,M.Einleitende Bemerkungen zu den Richtlinien zur Zertifizierung von Zentren fur Schlafmedizin zur Durchfuhrung von Polysomnographien.Schweiz.Arztezetg.,1998,79: 2604-2614.Mitler,M.M,Gujavarty,K.S.and Browman,C.P.Maintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolence.Electroencephalogr.Clin.Neurophysiol.,1982,53: 658-661.
Niewerth,H.J.and Wiater,A.Polysomnographische Untersuchungen fur Sauglinge und Kinder±Anleitung fur die Laborarbeit.Somnologie,2000,4: 43-52.
Penzel,T.,Hajak,G.,Hoffmann,R.M.,Lund,R.,Podszus,T.,Pollmacher,T.,Schafer,T.,Schulz,H.,Sonnenschein,W.and Spieweg,I.Empfehlungen zur Durchfuhrung und Auswertungen polygraphischer Ableitungen im diagnostischen Schlaflabor.Ztschr.EEG EMG,1993,24: 65-70.
Penzel,T.,Berger,M.,Clarenbach,P.and Peter,J.H.Zur Qualitats kontrolle von Schlaflabors in der Bundesrepublik Deutschland.Wien.Med.Wschr.(Sonderheft),1994: 120-124.
Penzel,T.,Brandenburg,U.,Fischer,J.,Jobert,M.,Kurella,B.,Mayer,G.,Niewerth,H.J.,Peter,J.H.,Pollmacher,T.,Schafer,T.,Steinberg,R.,Trowitzsch,E.,Warmuth,R.,Weefi,H.G.,Wolk,C.and Zulley,J.Empfehlungen zur computergestutzten Aufzeichnung und Auswertung von Polygraphien.Somnologie,1998,2: 42-48.
Penzel,T.,Hein,H.,Rasche,K.,Weess,H.G.,Fischer,J.,Hajak,G.,Mayer,G.,Wiater,A.and Zulley,J.Leitfaden fur die Akkreditie-rung von schlafmedizinischen Zentren der DGSM.Somnologie,2000,4: 181-187.
Peter,J.H.,Kohler,D.,Knab,B.,Mayer,G.,Penzel,T.,Raschke,F.and Zulley,J.(Eds) Weifibuch Schlafmedizin.Roderer Verlag,Regensburg,1995.
Sateia,M.J.(Ed.),The International Classification of Sleep Disorders,2nd edn.American Academy of Sleep Medicine,Westchester,IL,USA,2005.
Vorstand der DGSM.Qualifikationsnachweis Somnologie fur techni-sche und pflegerische Mitarbeiter in den Schlafmedizinischen Zent-ren der DGSM.Somnologie,1999,3: 283-286.
Wiater,A.and Niewerth,H.J.Polysomnographic standards for infants and children.Somnologie,2000,4: 39-42.
1The description of the postgraduate curriculum and certification of sleep specialists is subject of a separate publication.
2A minimum bedroom surface of 12 m2 is recommended.
3A resolution of 1600 x 1200 pixels is considered sufficient to display EEG in 30 s epochs.
4A maximum of 4 weeks is recommended.
5A minimum period of 10 years is recommended.
6A panel of three experts is recommended (see Introduction).These experts are selected by the NSS,but not necessarily from the NSS.When conflict of interest may exist,it is advised to appoint at least one foreign expert,who may be recommended by the ESRS.
7It is recommended that this delay should not exceed 8 weeks.
Correspondence′.Dirk Pevernagie,MD,PhD,Sleep Disorders Centre,Ghent University Hospital,De Pintelaan 185,9000 Gent,Belgium.Tel.: +32-9-240 26 11; fax: +32-9-240 2341; e-mail: dirk.pevernagie@ ugent.be

