钕负载二氧化钛-炭杂化气凝胶的制备及其光催化性能
邵 霞, 潘 峰, 郑 励, 张 睿, 张文雅
(1.上海应用技术大学,材料科学与工程学院,上海201418;2. 上海应用技术大学,化学与环境工程学院,上海201418)
1 Introduction
Due to the potential application in air purification and water treatment, photo-catalytic oxidation techniques have attracted much attention in recent years[1,2]. Photo-catalysts can effectively convert solar energy into chemical energy,which can be used for decomposing toxic organic and inorganic pollutants to purify water and air[3]. Among various photo-catalysts, TiO2has been regarded as one of the most promising one owing to its stability to light irradiation, non-toxicity, low cost and strong oxidizing power[4,5,6]. However, the wide band gap of TiO2(3.2 eV) and high rate of electron-hole recombination limit its efficiency only up to UV light region[7]. Since the UV light only accounts for less than 5% of the solar spectrum[8], development of active photo-catalytic materials under solar light is a subject of extensive current research in this field.
It is known that TiO2has three crystallographic phases in nature: brookite, anatase and rutile[9]. Anatase TiO2shows the best photo-catalytic activity owing to its valence band characteristics, conduction and crystal structure[10]. In recent years, the main study of anatase TiO2has focused on the improvement of its photo-catalytic performance and optical absorption by cation and/or anion doping[11]. Particularly, lanthanide ions possess the special 4f electron configurations,which can significantly enhance the photo-catalytic activity of TiO2[12].
For example, TiO2nanoparticles modified by Yb and Er could be used to degrade phenol in aqueous solution under visible light irradiation[13]. Obregon et al.[14]reported that the erbium-doped TiO2, synthesized by the hydrothermal method, showed a high photocatalytic activity in phenol and methylene blue degradation in aqueous phase as well as toluene degradation in the gas phase. In particular, doping Nd has been proved to be an efficient route to alter the photo-activity of TiO2for selected reactions[15]. Du et al.[16]synthesized some porous Nd-doped TiO2monoliths using polystyrene spheres as a template through a facile sol-gel method, and the result showed that Nd doping could suppress the h+/e recombination rate by extending the photo-produced-carrier lifetime. In addition[17], its porous structure can provide a large surface area, leading to an enhanced adsorption and fast transfer of pollutants.
In this paper, the Nd-doped TiO2-C hybrid aerogels were prepared by a combined sol-gel and impregnation methods. The effects of different amounts of Nd on the structure morphology and the photo-catalytic properties of the samples were investigated , with the aim of guiding the development of synthesis of photo-catalysts and improving their photo-catalytic activities.
2 Experimental
2.1 Materials
All the chemicals used in this paper were of analytical grade. Titanium tetrachloride (TiCl4), resorcinol, formaldehyde and absolute ethyl alcohol were supplied by Sinopharm Chemical Reagent Co Ltd. The salt precursor of neodymium nitrate hydrate (Nd(NO3)3·6H2O) and methylene blue were from Shanghai Zhanyun Chemical Reagent Co., Ltd.
2.2 Catalyst preparation
The Nd-doped TiO2-Cphoto-catalysts with different contents of neodymium (Nd) were prepared by the combined sol-gel and impregnation methods with the following procedure[18]. The right amount of formaldehyde was dissolved into the mixed solution of 7.185 g resorcinol and 0.007 g anhydrous sodium carbonate and stirred for 1.5 h to obtain a sol. Then the sol was dispensed into vial, sealed, first stood at room temperature, then placed in a bath for gelation and aging at 70 ℃ for 7 d. Finally, the wet gels were soaked in absolute ethanol for 5 d to replace the water and chloride ion in the wet gels.
The obtained wet gels were subjected to supercritical drying in a high pressure autoclave at 240 ℃ and 6 MPa for 1 h to get dried RF aerogels using n-hexane as the drying medium with a heating rate of 2 ℃/min. Then the RF aerogels were carbonized in a vertical furnace at 800 ℃ for 3 h with a heating rate of 2 ℃/min under a high purity nitrogen flow to get carbon aerogels .
Ti and Nd were doped into the carbon aerogels by an impregnation method[19]. A moderate amount of anhydrous ethanol was dropped into 2.375 g titanium tetrachloride at a constant stirring speed. Then, 0, 0.02, 0.04, 0.06, 0.08 and 0.10 g of neodymium nitrate were added to above solution and dissolved to obtain a series of light yellow transparent solutions. Each solution was poured into a certain amount of the carbon aerogels and held for 24 h. Finally, the impregnated carbon aerogels were carbonized at 500 ℃ for 3 h in a nitrogen-protected carbonization furnace to obtain the Nd-doped TiO2-C hybrid aerogels. The obtained six samples were named as TiO2-C-Nd%-1, TiO2-C-Nd%-2, TiO2-C-Nd% -3, TiO2-C-Nd%-4, TiO2-C-Nd%-5 and TiO2-C-Nd%-6 for the amount of neodymium nitrate, 0, 0.02, 0.04, 0.06, 0.08 and 0.10 g, respectively.
2.3 Characterization
X-ray diffraction (XRD) patterns of the as-prepared samples were recorded with an Rigaku D/max-Brm X-ray diffractometer using CuKαradiation. Nitrogen adsorption was performed on a V-Sorb 2800TP. The specific surface areas were calculated by the Brunauer-Emmet-Teller (BET) method. The morphology of samples was evaluated by field emission scanning electron microscopy (SEM, Hitachi S-3400N, Japan) with an accelerating voltage of 15 kV and a current of 50 mA. The chemical composition of the samples was determined by an energy dispersive X-ray spectrometer (EDS) attached to the SEM. Transmission electron microscopy (TEM) images were taken over a Tecnai G2 F30 transmission electron microscope. The FTIR analysis of the samples was carried out in the region ~4 000 - 400 cm-1on a FTIR Shimadzu UV-3600 instrument. KBr was used as a mulling agent for preparing the samples. XPS spectra were recorded on an ESCALAB250Xi X-ray photo-electron spectrometer (Thermo Fisher Scientific, USA) using AlKa radiation (1 486.6 eV) and operating at 150 W.
2.4 Adsorption properties and photo-degradation tests
The photo-catalytic activities of all samples were evaluated by degradation of methylene blue (MB) under UV-light irradiation. A 500 W mercury lamp was used as the light source, which was hung in the center of the photo-reactor and kept at about 12 cm from 8 glass tubes distributed around the light source. A general photo-catalytic reaction was carried out as following. 30 mg different catalysts were suspended in MB dye solutions (C0= 20 mg/L, 50 mL). The suspensions were stirred in the dark for 60 min to ensure an establishment of adsorption-desorption equilibrium of MB dye. The samples were collected at 20 minutes intervals, and the absorbance change of MB solutions were measured by using an UV-vis spectrometer at 660 nm (λmax). The photo-catalytic activities of the samples were evaluated by the degradation ratio (w) of the samples, according to the following equation.
(1)
Wherewwas the degradation ratio, andA0was the initial absorbance of MB at 20 mg/L, andAtwas the absorbance of MB after irradiation for “t” minutes.
3 Results and discussion
Fig. 1 shows the XRD patterns of the Nd-doped TiO2-C hybrid aerogels. The diffraction peaks at 2θof 25.2°, 37.5°, 47.8°, 54.3° and 62.5° correspond to (101), (004), (200), (105) and (204)
of anatase TiO2, respectively[20]. There is no rutile or brookite type TiO2. No peaks of carbon are observed in the XRD patterns, which indicates that the carbon in the samples is present in an amorphous form[21]. Xu et al.[22]indicated that rare earth metal salts could be converted into rare earth metal oxides in the process of calcining, therefore, it could be speculated that Nd3+is dispersed on the surface of TiO2particles in the form of Nd2O3. Moreover, no Nd peaks are found in the XRD patterns, which can be accounted for by the following two reasons. For one reason, the dosage of Nd is too small to be detected with the XRD instrument. For another reason, Nd3+enters into the lattice of TiO2, and forms Ti-O-Nd bond in the process of doping[23]. Moreover, the crystal structure of TiO2does not change from anatase to rutile when the carbonization temperature is as high as 500 ℃. It may be due to the strong cross-linking between TiO2and polymer molecules during the preparation of the hybrid aerogels[24]. Carbon and TiO2in the samples are highly hybridized, which hinders the structural transformation of the crystal.
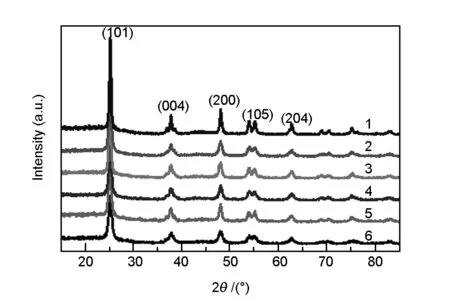
Fig. 1 XRD patterns of the samples
Based on the XRD data of the (101) full width at half maximum values, the average crystallite sizes of the synthesized photo-catalysts are calculated by the Debye-Scherrer equation and the results are shown in the Table 1. It reveals that the crystallite size decreases from 3.93 to 2.04 nm with the increasing of Nd content, which indicates that the Nd doping can inhibit the growth of TiO2crystal particles.

Table 1 Crystallite sizes of the samples.
The microstructure and morphology of the prepared samples were observed by the scanning electron microscopy. Fig. 2 shows the SEM images of the Nd-doped TiO2-C hybrid aerogels. Along with the incorporation of rare earth elements Nd, it can be seen that all the prepared samples are irregular granules, and the surface of the samples is not smooth, but the micro-morphology of the sample TiO2-C-Nd%-4 has the best pore structure, which is conducive to increase the surface area and performance.

Fig. 2 SEM images of the samples
In order to further understand the structure of the samples, the prepared Nd-doped TiO2-C aerogels were also characterized by TEM. As shown in Fig. 3, the Nd-doped TiO2-C aerogel samples exhibit highly uniform nano crystalline structures with observed particle sizes in 5-10 nm range,and the sample of TiO2-C-Nd%-4 has the most uniform particle size distribution.
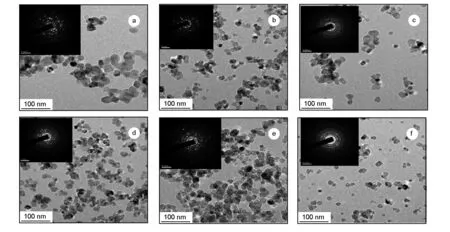
Fig. 3 TEM images of the samples
In order to investigate if the element Nd has been doped into the samples, EDS analysis is carried out to identify the elemental composition of TiO2-C-Nd%-6. According to the EDS result, there are Ti, O, C and Nd elements, from which Nd is certainly the dopant neodymium nitrate. There is no nitrogen impurity, which indicates that the doped nanoparticles are free of nitrate impurities that come from dopant precursors[25]. In the EDS elemental composition (the inset of Fig. 4), It can be found that the atomic content of Nd in the sample is 0.81%. Therefore, the samples prepared by this method have Nd doped into the TiO2-C aerogels indeed.

Fig. 4 EDS spectrum of the TiO2-C-Nd%-6 sample.
UV-visible diffuse reflectance spectra of the samples before calcination are shown in Fig. 5. As can be seen from this figure, the descending absorption intensities of the samples in the ultraviolet band are following: TiO2-C-Nd%-4 > TiO2-C-Nd%-3 > TiO2-C-Nd%-1> TiO2-C-Nd%-6> TiO2-C-Nd%-5> TiO2-C-Nd%-2, indicating that there is an appropriate amount of neodymium that can effectively improve the absorption of the samples. The phenomenon of TiO2-C-Nd%-1 > TiO2-C-Nd%-6 > TiO2-C-Nd%-5 > TiO2-C-Nd%-2 shows that too much or too little Nd content may reduce absorption.
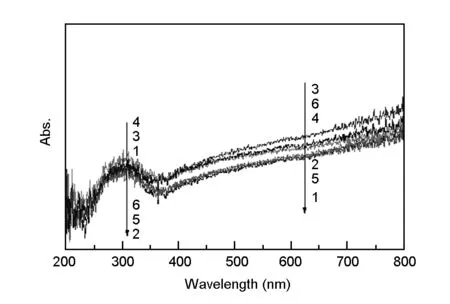
Fig. 5 UV-visible diffuse reflectance spectra of the samples before calcination.
To evaluate the surface state of the samples, the samples of TiO2-C-Nd%-1 and TiO2-C-Nd%-3 were characterized by XPS. The high resolution XPS of C 1s in TiO2-C-Nd%-3 is shown in Fig.6(b). The binding energies of C1s are about 284.7, 285.9 and 288.1 eV, which have a bit of deviation as compared with TiO2-C-Nd%-1 (284.2, 285.1 and 288.3 eV). This indicates that the doping amount of the Nd ions affects the internal structure of the crystals.
In the XPS spectrum of TiO2-C-Nd%-3 (Fig.6(b)), the Ti 2p 3/2 and Ti 2p 1/2 peaks are located at binding energies of 459.4 and 465.2 eV, respectively, which is consistent with the value of Ti4+in the TiO2lattice[26]. O 1sspectrum in Fig.6(b) shows the main peak at 531.2 eV due to the metallic oxides Ti-O bond, which is consistent with binding energy of O2-in the TiO2lattice[26]. The peak appearing at 532.8 eV can be attributed to adsorbed OH-on the surface of TiO2[27].
Table 2 shows the pore structure parameters of Nd-TiO2-C aerogels with different Nd doping contents. It can be seen that the specific surface area of the prepared samples varies with the increase of the Nd doping amount while the other conditions are kept the same. The descending order of specific surface area is following: TiO2-C-Nd%-4 > TiO2-C-Nd%-5 > TiO2-C-Nd%-6>TiO2-C-Nd%-1 > TiO2-C-Nd%-3>TiO2-C-Nd%-2.
The N2adsorption-desorption isotherms and the Barrett-Joyner-Halenda (BJH) pore size distributions of the synthetic samples are given in Fig. 7 a and b, respectively. From this figure it can be seen that the isotherms are of type IV curve with a H1 hysteresis loop, which implies that the representative samples exhibit a mesoporous structure with a high degree of pore size uniformity[28]. The nitrogen adsorption-desorption isothermal curves show well-defined adsorption steps. At low relative pressure (p/p0< 0.4), the isotherms exhibit a high adsorption, which indicates the powders are mesoporous. At a middle relative pressure (p/p0= 0.4-0.8), the curves exhibit a H1 hysteresis loop, which can further confirm the powders are mesoporous photocatalysts[29].
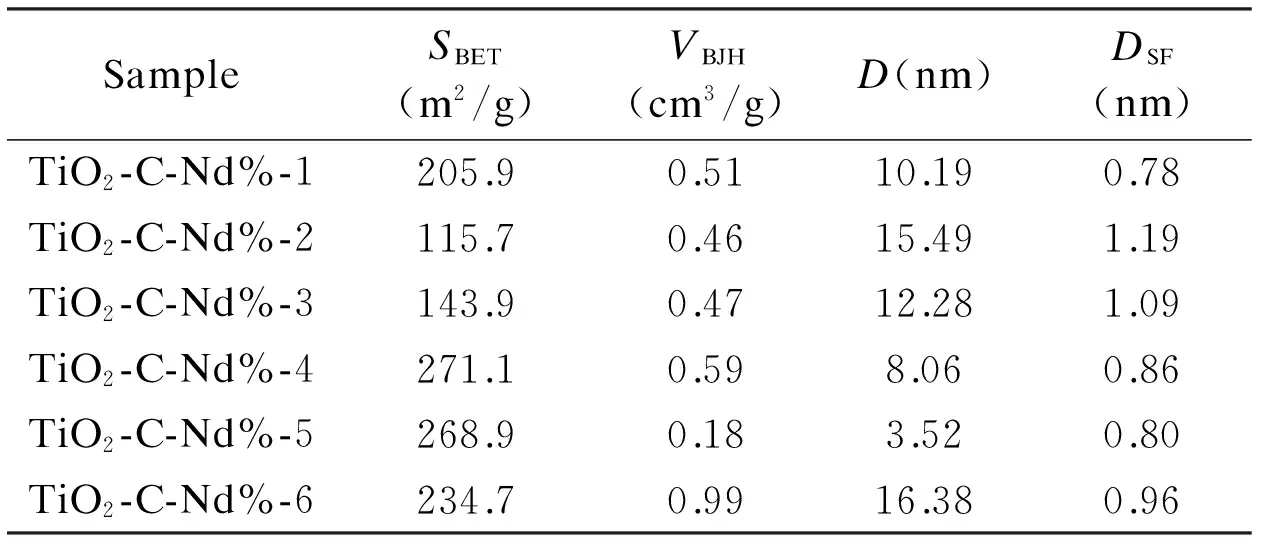
Table 2 Pore structure parameters of the samples.
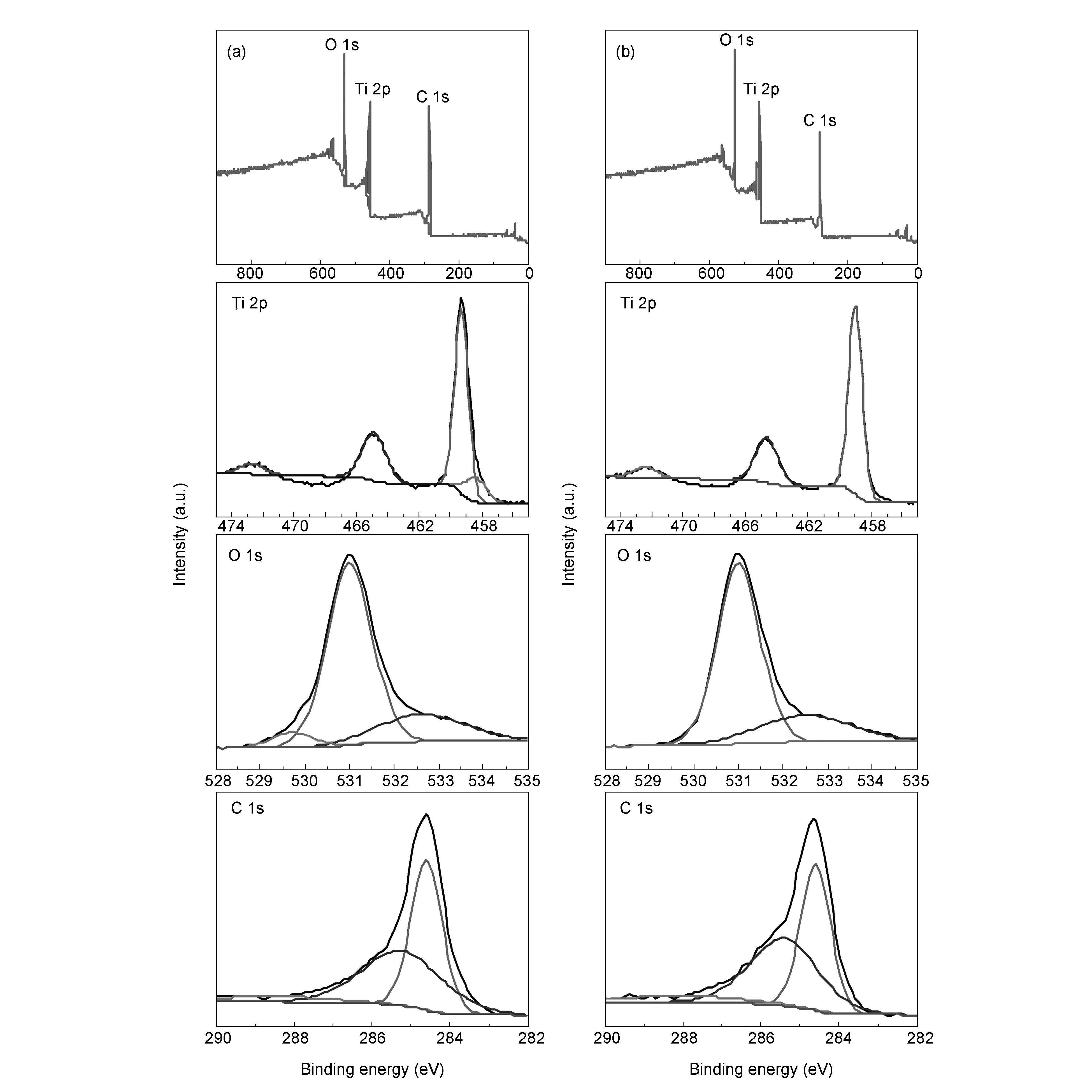
Fig. 6 XPS spectra of the survey, Ti 2p, C 1s, and O 1s:

Fig. 7 Porous properties of the samples:
The specific surface areas of the TiO2-C-Nd%-4 and TiO2-C-Nd%-5 are 271.1 and 268.9 m2/g, respectively, which is much larger than that of TiO2-C-Nd%-1 (205.9 m2/g). A high specific surface area can improve the adsorption ability of a photo-catalyst, because the photo-catalytic activity strongly depends on the adsorption capacity of organic substance and the interfacial charge transfer can also be improved[30]. From this it can be deduced that the doping of Nd can improve the specific surface area of the nanoparticles, and further improve its photo-catalytic performance. However, the amount of dopant has a most suitable value, when the amount of Nd is too much, the excess of Nd may block the pore holes, resulting in a decrease in specific surface area.
It can be seen from Fig. 7b that the samples prepared by the experimental scheme have pore size distribution in the micropore and mesopore region. The pore size distribution further confirm that the pore size distributions in the samples vary with the doping amount of Nd, and the amount of Nd doping has the most suitable value.
Fig. 8 shows the FT-IR spectrum of the TiO2-C-Nd%-3 particles. Absorption at 450-800 cm-1is assigned to the stretching vibrations of Ti-O-Ti, Ti-O, and O-Ti-O bonds[31]. The absorption band at about 1 647 cm-1is attributed to the bending vibration of H-O—H bonds, which is assigned to the chemisorption water[32]. Compared with the FT-IR spectra of the TiO2-C-Nd%-3 adsorbed with MB before and after light irradiation and MB, it is found that TiO2-C-Nd%-3 particles have the strong stretching vibration of O-H absorption peak at 3 388 cm-1and the characteristic absorption peak of CO2at 2 389 cm-1. Obviously, Nd doped TiO2-C aerogel particles have a high ability of absorbing water, which results in more hydroxyls on their surface. Hydroxyls can form hydrogen bonds with water, and therefore the surface property of the samples is significantly enhanced.
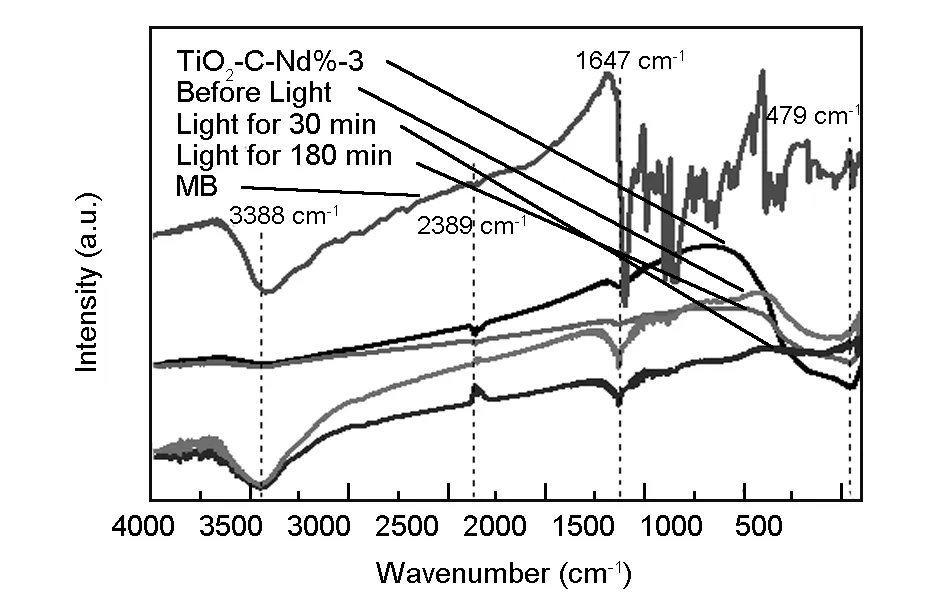
Fig. 8 FT-IR spectra of the TiO2-C-Nd%-3 in MB solution.
The photo-catalytic activities of the prepared samples were determined by the degradation of MB in solutions. The concentration of the MB solution is 20 mg/L, and 30 mg of catalysts is introduced per 50 mL MB solution. As shown in Fig. 9a, the adsorption curve of MB solution is linear within 60 min before irradiation, indicating that the degradation rate of the sample is very slow during that time. After 20 min of lighton, the adsorption rate is gradually increasing. The changing trend of degradation ratio after the degradation reaction carries out for 80 min is as follow: TiO2-C-Nd%-4>TiO2-C-Nd%-3>TiO2-C-Nd%-5>TiO2-C-Nd%-2>TiO2-C-Nd%-1> TiO2-C-Nd%-6, indicating that the proper amount of Nd doping can improve the performance of the catalysts to a certain extent. Fig. 9b shows the degradation ratios after a light irradiation for 60 min. It is found that TiO2-C-Nd%-4 has the highest degradation ratio, which is ascribed to its largest specific surface area.
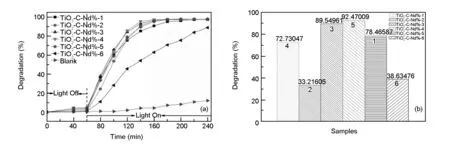
Fig. 9 Photocatalytic activities
Fig. 10 shows the absorbance curves of ultraviolet photo-catalysis of methylene blue over 40 mg sample at different times. It can be seen from the figure that the absorbance of methylene blue at 660 nm is in the range of 2.8 to 2.9 within 60 min of irradiation, but after UV irradiation for 160 min, the absorbances of the samples decrease sharply, and after light on for 240 min, the absorbances of the samples are almost close to zero, which indicates that the MB is decomposed completely. From Fig. 10b, it can be seen that the sample TiO2-C-Nd%-4 has the best performance, and the absorbance is substantially reduced to zero after 160 min of light exposure. And there are no other miscellaneous peaks appearing in these spectral data, indicating that no other substances except CO2and H2O are formed during the photocatalytic process.
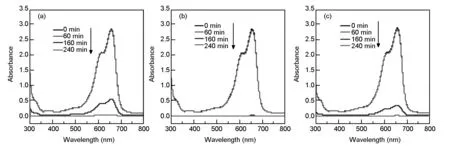
Fig. 10 The Absorbance curves of ultraviolet photocatalysis of methylene blue over 40 mg samples at different times.
4 Conclusions
Nd-doped TiO2-C hybrid aerogels were prepared by a combined sol-gel and impregnation methods. The samples were characterized by SEM, TEM, EDS, FTIR, XRD, nitrogen adsorption and XPS. The photo-catalytic activities of the samples were tested by using MB in solutions as a target substance under UV light irradiation. The main conclusions were as following: (1) Carbon aerogels were first prepared, and the TiO2-C hybrid aerogels with different Nd doping contents were prepared by impregnation. (2) The prepared samples are irregular granular, and the particle size of the samples reduces with the increase of the doping amount of Nd, which indicates that the doping of neodymium inhibits the growth of the particles, and also hinders the crystal type transform from anatase to rutile. At the same time the doped Nd3+is present as a Ti-O-Nd bond in the titanium dioxide lattice. (3) The samples show good photo-catalytic activities for MB degradation in solutions under ultraviolet light. By investigating the degradation of MB in solutions with samples of different ratios of Nd doping, it is found that a moderate amount of Nd3+content could improve the photo-catalytic activity. When the ratio of neodymium nitrate to titanium dioxide as a percentage is 3%-4%, the prepared sample has the best performance. After light irradiation for 180 min, the degradation ratio of MB reaches almost 100%.
[1] James L G, John D S, Clemens B, et al. Highly efficient formation of visible light tunable TiO2-xNxphotocatalysts and their transformation at the nanoscale[J]. ChemInform, 2006, 35: 1230-1240.
[2] Baia L, Diamandescu L, Barbu-Tudoran L, et al. Efficient dual functionality of highly porous nanocomposites based on TiO2and noble metal particles[J]. Journal of Alloys & Compounds, 2011, 509: 2672-2678.
[3] Sharma M, Jain T, Singh S, et al. Photocatalytic degradation of organic dyes under UV-Visible light using capped ZnS nanoparticles[J]. Solar Energy, 2012, 86: 626-633.
[4] Martha M, Wilson F. Remediation of pesticide contaminated soil using TiO2mediated by solar light[J]. Catalysis Today, 2002, 76: 201-207.
[5] Ramacharyulu P, Prasad G, Ganesan K, et al. Photocatalytic decontamination of sulfur mustard using titania nanomaterials[J]. Journal of Molecular Catalysis A Chemical, 2012, s353-354: 132-137.
[6] Sahoo C, Gupta A K. Characterization and photocatalytic performance evaluation of various metal ion-doped microstructured TiO2under UV and visible light[J]. Journal of Environmental Science and Health, Part A, 2015, 50: 659-668.
[7] Wahi R K, William W Y, Liu Y P , et al. Photodegradation of congo red catalyzed by nanosized TiO2[J]. Journal of Molecular Catalysis A Chemical, 2005, 242: 48-56.
[8] Anuja B, Mrinal R P, Anjali A, et al. Surface modified Nd doped TiO2nanoparticles as photocatalysts in UV and solar light irradiation[J]. Solar Energy, 2013, 91: 111-119.
[9] Singh D P, Ali N. Synthesis of TiO2and CuO nanotubes and nanowires[J]. Science of Advanced Materials, 2010, 2: 295-335.
[10] Periyat P, Suresh C P, Declan E, et al. Improved high-temperature stability and sun-light-driven photocatalytic activity of sulfur-doped anatase TiO2[J]. J.Phys.Chem.C, 2008, 112: 7644-7652.
[11] Yang X, Ma F, Li K, et al. Mixed phase titania nanocomposite codoped with metallic silver and vanadium oxide: new efficient photocatalyst for dye degradation[J]. Journal of Hazardous Materials, 2010, 175: 429-38.
[12] Hoffmann M R, Choi W Y, Bahnemann. Environmental applications of semiconductor photocatalysis[J]. Chemical Reviews, 1995, 95: 69-96.
[13] Grabowska E, Reszczyńska J, Zaleska A. Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review[J]. Water Research, 2012, 46: 5453-71.
[14] Obregon S, Kubacka A, Fernandez-Garcia M, et al. High-performance Er3+-TiO2system: Dual up-conversion and electronic role of the lanthanide[J]. Journal of Catalysis, 2013, 299: 298-306.
[15] de la Cruz Romero D, Torres Torres G, Arévalo J C. et al. Synthesis and characterization of TiO2doping with rare earths by sol-gel method: photocatalytic activity for phenol degradation[J]. Journal of Sol-Gel Science and Technology, 2010, 56: 219-226.
[16] Du J M, Chen H J , Yang H , et al. A facile sol-gel method for synthesis of porous Nd-doped TiO2monolith with enhanced photocatalytic activity under UV-Vis irradiation[J]. Nanotechnology & Precision Engineering, 2013, 182: 87-94.
[17] Zhang Q W, Wang J S, Tang Q, et al. Preparation of nitrogen-doped titania with high visible light induced photocatalytic activity by mechanochemical reaction of titania and hexamethylenetetramine[J]. Journal of Materials Chemistry, 2003, 13: 2996-3001.
[18] Shao X, Lu W C, Zhang R. et al. Enhanced photocatalytic activity of TiO2-C hybrid aerogels for methylene blue degradation[J]. Scientific Reports, 2013, 3: 3018.
[19] Carolina S M, Javier R D, Carlos J L, et al. Low concentration Fe-doped alumina catalysts using sol-gel and impregnation methods: the synthesis, characterization and catalytic performance during the combustion of trichloroethylene[J]. Materials 2014, 7: 2062-2086.
[20] Pavasupree, Sorapong, et al. Synthesis of titanate, TiO2(B), and anatase TiO2nanofibers from natural rutile sand[J]. Journal of Solid State Chemistry, 2005, 24: 3110-3116.
[21] Bart R, Karl C G, Sandeep P, et al. Enhanced efficiency and stability of perovskite solar cells through Nd-doping of mesostructured TiO2[J]. Advanced Energy Materials, 2016, 6 : 1501868.
[22] Xu A W, Gao Y, Liu H Q, The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2nanoparticles[J]. Journal of Catalysis, 2002, 207: 151-157.
[23] Meng Z H, Wan L H, Zhang L J, et al. One-step fabrication of Ce-N-codoped TiO2nano-particle and its enhanced visible light photocatalytic performance and mechanism[J]. Journal of Industrial & Engineering Chemistry, 2014, 20: 4102-4107.
[24] Zhang X H, Luo L T, Duana Z H, Preparation and application of Ce-doped mesoporous TiO2oxide[J]. Reaction Kinetics, Mechanisms and Catalysis, 2005, 87: 43-50.
[25] Biswajit C, Bikash B, Amarjyoti C, Ce-Nd codoping effect on the structural and optical properties of TiO2nanoparticles[J]. Materials Science & Engineering B, 2013, 178: 239-247.
[26] Erdem B, Hunsicker R A, Simmons G W, et al. XPS and FTIR surface characterization of TiO2particles used in polymer encapsulation[J]. Langmuir, 2001. 17: 2664-2669.
[27] Wu Y M, Zhang J L, Xiao L,et al. Properties of carbon and iron modified TiO2photocatalyst synthesized at low temperature and photodegradation of acid orange 7 under visible light[J]. Applied Surface Science, 2010. 256: 4260-4268.
[28] Liu J K, An T C, Li G Y, et al. Preparation and characterization of highly active mesoporous TiO2photocatalysts by hydrothermal synthesis under weak acid conditions[J]. Microporous & Mesoporous Materials, 2009, 124: 197-203.
[29] Shi H X, Zhang T Y, An T C, et al. Enhancement of photocatalytic activity of nano-scale TiO2particles co-doped by rare earth elements and heteropolyacids[J]. Journal of Colloid & Interface Science, 2012, 380: 121-127.
[30] Pan L, Zou J J, Zhang X, et al. Water-mediated promotion of dye sensitization of TiO2under visible light[J]. Journal of the American Chemical Society, 2011, 133: 10000.
[31] Mills A, An overview of the methylene blue ISO test for assessing the activities of photocatalytic films[J]. Applied Catalysis B Environmental, 2012, 128: 144-149.
[32] Du J, Gu X, Wu Q, et al. Hydrophilic and photocatalytic activities of Nd-doped titanium dioxide thin films[J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 2601-2607.
——材料科学与工程