Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation
Barry Burstein,Division of Pulmonary and Critical Care Medicine,Mayo Clinic,Rochester,MN 55905,United States
Patrick M Wieruszewski,Yan-Jun Zhao,Department of Pharmacy,Mayo Clinic,Rochester,MN 55905,United States
Nathan Smischney,Department of Anesthesia,Mayo Clinic,Rochester,MN 55905,United States
Abstract
Key words:Extracorporeal membrane oxygenation; Anticoagulants; Antithrombins;Bivalirudin; Argatroban; Heparin
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is increasingly used in patients with refractory respiratory or cardiogenic shock[1].Patients can be supported in either a veno-venous (VV) or veno-arterial (VA) configuration.Systemic anticoagulation is necessary and crucial due to continuous contact between the patient's blood and the foreign surfaces of all components of the ECMO circuit.This interaction triggers the coagulation cascade and can lead to pump or oxygenator thrombi,or fibrin stranding within the inflow cannula resulting in potentially devastating thromboembolic events[2].The ideal anticoagulant has rapid onset and offset,is easily titratable based on available monitoring parameters,is reversible,and is not affected by organ dysfunction commonly seen in ECMO patients[3].Intravenous unfractionated heparin(UFH) is the standard anticoagulant in most centers due to its availability,rapid onset of action,reversibility,cost profile,and familiarity among practitioners[4].
Complications related to UFH are common:bleeding,non-immune and immune heparin-induced thrombocytopenia (HIT),and heparin-resistance have all been described[5,6].HIT is of particular concern,with mortality as high as 20%-30%[7].The incidence of HIT in patients treated with UFH is approximately 2.6%,although this does not reflect the incidence in ECMO patients[8].HIT is often suspected in ECMO patients; however available data are inconsistent,suggesting that the incidence ranges from less than 0.36% to 17%[9-12].HIT reverses the anticoagulant effect of heparin and leads to massive platelet activation and thrombosis,which can be catastrophic[13].All forms of heparin must be immediately discontinued once the diagnosis of HIT is suspected.In ECMO patients,the heparin-coated elements of the circuit must also be exchanged.UFH can also lead to acquired antithrombin deficiency which may result in heparin resistance and suboptimal anticoagulation[2].This is of particular concern in patients receiving ECMO secondary to the inability to liberate from cardiopulmonary bypass due to high intraoperative heparin loads.Alternative anticoagulation strategies have been proposed.In non-ECMO patients with suspected or confirmed HIT,direct thrombin inhibitors (DTIs) are the primary alternative[13].This article will review DTI anticoagulation strategies in adult ECMO patients,as well as available safety and outcome data.Heparin alternatives in pediatric ECMO patients have previously been reviewed[14].
PHARMACOLOGY OF DIRECT THROMBIN INHIBITORS
Thrombin is a serine protease that plays a crucial role in the coagulation cascade and the generation and stabilization of clot.Upon activation,thrombin facilitates the formation of insoluble fibrin from soluble fibrinogen[15,16].Thrombin contains three binding sites that are essential to its coagulant-and thereby anticoagulant-effects,including the catalytic site,exosite-1,and exosite-2.
The DTIs obtain their name from their direct binding to thrombin to exert anticoagulant effects.This is in contrast to UFH and low molecular weight heparins,which are indirect thrombin inhibitors.These indirect inhibitors bind to antithrombin,a hepatically-synthesized glycoprotein,forming a heparin-antithrombin complex which subsequently binds to exosite-2 on thrombin and blocks the catalytic site[16,17].Because of this binding,indirect thrombin inhibitors only exert effects on circulating thrombin,as the catalytic site of fibrin-bound thrombin is occupied in preformed clot[17].DTIs,on the other hand,bind directly to either the catalytic site or both the catalytic site and exosite-1 on thrombin,depending on the valency of the agent,in the absence of antithrombin[15,17].Because of this,a major pharmacologic advantage over indirect thrombin inhibitors is the ability of DTIs to bind both circulating and fibrinbound thrombin.DTIs for clinical use vary based on their valency,modality of binding (reversiblevs.irreversible),and pharmacokinetic profile (Table1).
ADVANTAGES OF DIRECT THROMBIN INHIBITORS
Anticoagulation is an essential element of ECMO support.While UFH remains the standard at most ECMO centers,DTIs are emerging as a reasonable and safe alternative due to several advantages when compared to UFH.The advantages of DTIs over UFH are:(1) Direct binding of both circulating and clot-bound thrombin,which results in increased efficacy relative to UFH[17]; (2) Anticoagulant effect that is independent of antithrombin,allowing for more consistent and predictable effect without concern for antithrombin depletion[15,17]; (3) Avoidance of HIT,as thrombocytopenia is common among ECMO patients and the diagnosis of HIT in this setting is challenged by the multitude of factors that can precipitate a drop in platelet count.Healthcare teams must maintain a low threshold of concern when HIT is suspected.DTIs appear to be at least as safe as UFH with no evidence of increased bleeding or thrombosis,and evidence suggests that patients who receive DTIs are more often in therapeutic targeted range of anticoagulation.While DTIs are an appropriate choice in the setting of suspected or confirmed HIT,they may be a reasonable option as first-line anticoagulation for better efficacy in maintaining ECMO circuit patency and to avoid concerns for HIT altogether.Although DTIs do not have target-specific antidote,their half-life is very short and the anticoagulant effect tapers off rapidly despite the widely prevalent end organ dysfunction in ECMO patients.ECMO patients rarely require complete reversal of anticoagulation given the high risk of thrombosis.
AVAILABLE AGENTS
Bivalirudin
Bivalirudin is a synthetic,bivalent DTI,which binds directly to both the catalytic site and exosite-1 on thrombin in a reversible fashion[16](Table1).Bivalirudin dissociates from the catalytic site following proteolytic cleavage,reconstituting thrombin's ability to facilitate fibrin formation[18].This may be problematic during states of blood stasis as will be discussed.It is administered by intravenous infusion,has an onset of action within minutes,and has a half-life of 25 min in patients with normal renal function[18].Bivalirudin has a low volume of distribution and is therefore widely distributed in plasma and has negligible protein binding.
Bivalirudin is available as an adjunct to anticoagulation therapy during percutaneous coronary interventions,as well as the primary anticoagulant during coronary artery bypass grafting in patients with HIT[19].Use in ECMO,with or without HIT,is off-label.The available literature for use in adults supported by ECMO is derived from retrospective studies,a single case control trial,and multiple case reports[20-26](Table2).Two studies compared bivalirudin to a matched control group who received UFH[20,25].Four studies described patients with suspected or confirmed HIT due to previous UFH use[21,23,24,26]; the remaining studies used bivalirudin as the initial anticoagulation strategy[20,22,24,25].Both VV and VA configurations are described.There is one published protocol describing initial bivalirudin dosing and subsequent dose adjustments[22].
In most reported studies of bivalirudin,initial bolus doses were administered followed by a weight-based infusion.The described dosing is heterogeneous; bolus dosing ranges from 0.04 mg/kg to 2.5 mg/kg.In reports without bolus dosing there was no sign of increased thromboembolic risk during the time until therapeutic anticoagulation was achieved[21,24-26].The maintenance infusion was adjusted based on monitoring parameters,and doses ranged from 0.025 mg/kg/h to 2.5 mg/kg/h.In studies where average infusion rate is described,the dose ranges from 0.05 mg/kg/h to 0.26 mg/kg/h to maintain therapeutic targets[21,23,26].When compared to patients receiving UFH infusions,patients treated with bivalirudin are more often in therapeutic range[20,24].
优质黑米、小米清洗→温水浸泡12 h→加大枣、枸杞等蒸煮30 min→搅拌散冷→加糖化酶→搅拌散冷加水、调糖、调酸等→加酿酒曲、酯化红曲→前发酵→加蔗糖、蜂蜜等调糖→后发酵→压榨、煎酒、除菌→半成品酒
Renal dysfunction:Bivalirudin is metabolized in the plasma by proteolytic cleavageand has a prolonged half-life in the setting of renal dysfunction[27].Renal dysfunction is therefore an important consideration when initiating and adjusting bivalirudin infusions.Initial dosing,as well as adjustments in the maintenance infusion rate,may vary,and over-anticoagulation is an important concern in these patients.Limited studies with established protocols describe lower initial bolus doses in patients with renal dysfunction[25],while others use the same bolus dose as patients without renal dysfunction while adjusting subsequent maintenance infusion rates[22].Ranucciet al[25]administered half-dose initially in patients with renal dysfunction,followed by the regular dose adjustment protocol.The protocol by Netleyet al[22]stratified patients based on creatinine clearance above 30 mL/min,between 10-29 mL/min,and less than 10 mL/min or requiring intermittent hemodialysis.The initial bolus dose was standardized for all patients regardless of creatinine clearance,and subsequent dose adjustments were limited as the severity of renal dysfunction progressed.
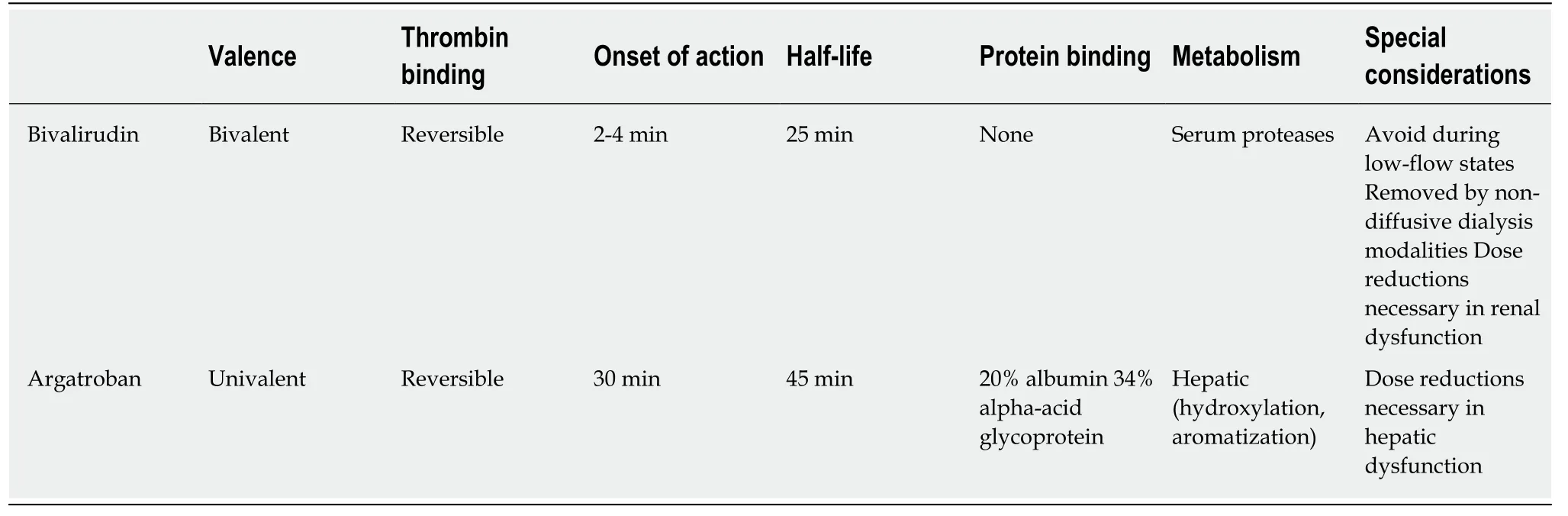
Table1 Pharmacology of direct thrombin inhibitors
Continuous renal replacement therapy (CRRT) utilizing convective (hemofiltration)or combination convective/diffusive (hemodiafiltration) modes has been found to modestly remove bivalirudin,but purely diffusive modalities are not expected to be major determinants of clearance due to bivalirudin's larger molecular size (1980 Da)[28].There are multiple reports of successful anticoagulation with bivalirudin while supporting patients with CRRT[20,22,24].Walkeret al[26]described moderately reduced dose requirements in patients on CRRT,although the doses were higher than those with renal dysfunction who were not receiving CRRT.While it would be expected that patients with renal dysfunction require less bivalirudin to maintain a similar anticoagulation profile,retrospective data suggest that these patients may require higher doses[24].The reasons for this finding are unclear.Given the tendency to reduce initial and/or maintenance bivalirudin doses in patients with renal dysfunction,careful attention should be paid to avoid under-anticoagulation which may result in thrombotic events.The currently available reports do not signal an increased risk of thrombosis in patients with renal dysfunction,although the number of studied patients is limited.
Hepatic dysfunction:There is no bivalirudin dose adjustment necessary in patients with hepatic dysfunction,although there are no specific reports of outcomes in patients with hepatic disease.
Monitoring:Bivalirudin prolongs the activated clotting time (ACT),activated partial thromboplastic time (aPTT),thrombin time,prothrombin time,and international normalized ratio[19].Ecarin clotting time and chromogenic anti-factor IIa assay are the most reliable methods for DTI monitoring; however,they are not readily available in clinical laboratories.The aPTT testing is the most commonly used and well validated assay to monitor DTIs.The manufacturer recommends monitoring with ACT in certain conditions,namely HIT,with an ACT target greater than 225 s.No specific recommendation from the manufacturer is available in ECMO patients as this is an off-label indication.Most reviewed studies,including available protocols,used aPTT as a monitoring parameter.Netleyet al[22]used aPTT alone with adjustable target based on physician preference; 40-60 s,50-70 s,or 60-80 s were all described,with adjustments in bivalirudin doses based on the difference between the measured aPTT and the target aPTT.The aPTT was measured 2 h after the initial dose and then every 4 h afterwards.Ranucciet al[25]adjusted bivalirudin dosing based on ACT primarily(target 160-180 s),followed by aPTT (target 50-80 s) and then kaolin-activated thromboelastography (TEG,TEG 5000; Haemonetics Corp,Braintree,MA,UnitedStates)rtime (target 12-30 min).Parameters were checked every 4,12,and 8 h,respectively.In the retrospective study by Bereiet al[20],aPTT targets between 45-65 s(low intensity) and 60-80 s (high intensity) were chosen based on physician preference.Given the wide variability of monitoring modalities used and goal ranges reported in the literature,and the possibility of DTI resistance and unreliable aPTT monitoring at very high DTI doses,it is of utmost importance to use clinical indicators including ECMO circuit patency and potential thrombotic and bleeding complications as ultimate guidance for anticoagulation titration rather than solely relying on numbers of certain lab values.
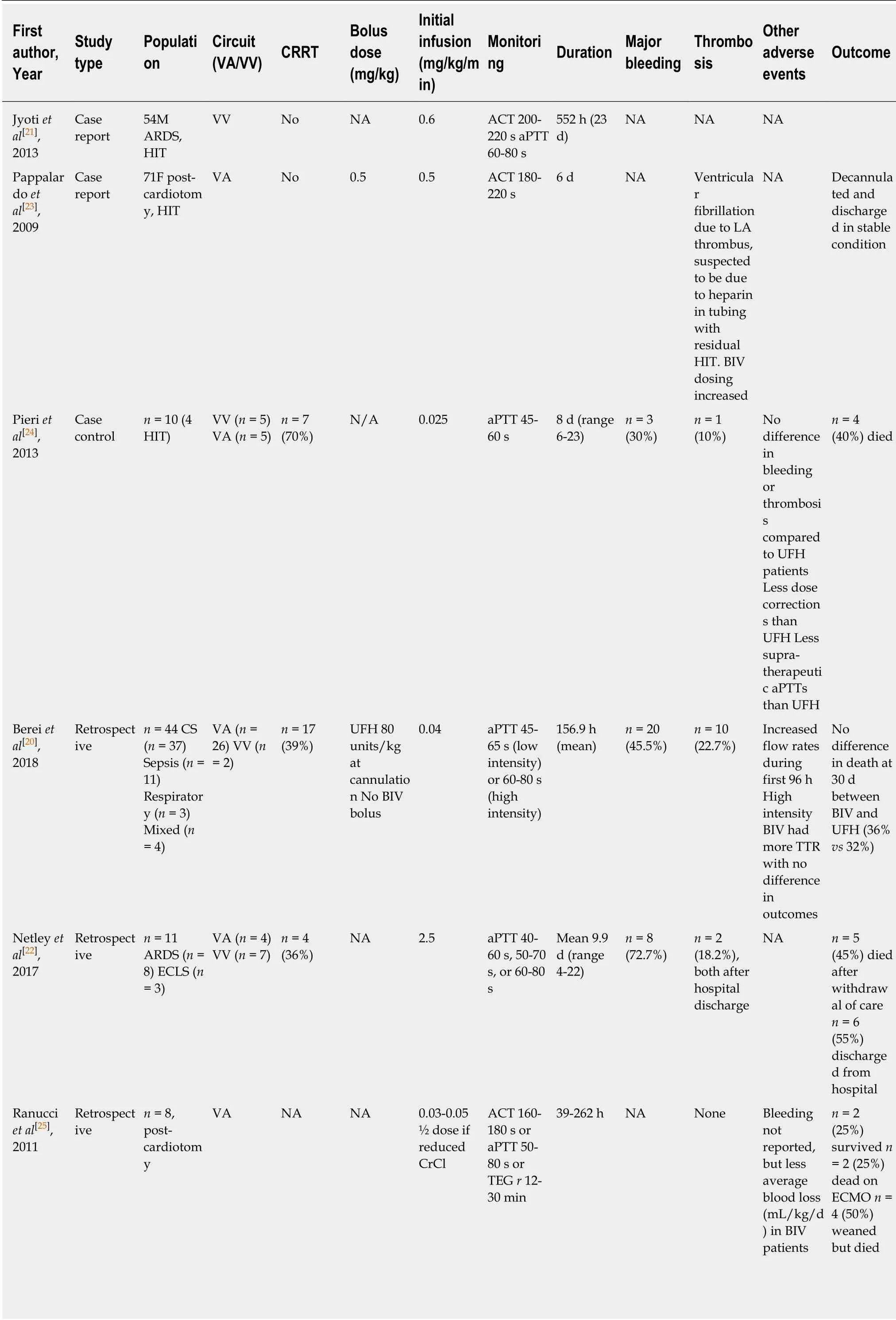
Table2 Summary of studies reporting on argatroban in adult patients supported with extracorporeal membrane oxygenation
ACT:Activated clotting time; aPTT:Activated partial thromboplastin time; ARDS:Acute respiratory distress syndrome; BIV:Bivalirudin; CRRT:Continuous renal replacement therapy; CS:Cardiogenic shock; ECLS:Extra-corporeal life support; HIT:Heparin-induced thrombocytopenia; NA:Not available; TEG:Thromboeslastography; UFH:Unfractionated heparin; VA:Veno-arterial; VV:Veno-venous.
Safety and outcomes:Bivalirudin appears to be a safe anticoagulation strategy for patients supported by ECMO,with no overall evidence of increased bleeding or thrombotic complications relative to UFH[20,24,25].One study demonstrated an increased incidence of bleeding events in patients treated with bivalirudin compared to those receiving UFH,without meeting statistical significance[20].Walkeret al[26]described 4 of 14 patients who required reduction in aPTT targets or complete suspension of bivalirudin due to bleeding,although this rate is similar to reported bleeding rates in UFH patients.Some reports suggest a lower risk of thrombosis and vascular complications,as well as decreased transfusion requirements relative to UFH[20,25],although cases requiring circuit exchange due to thrombosis have been reported[26].Reports of patients who required ECMO support up to 23 d suggest that outcomes with prolonged bivalirudin use are similar to UFH[21,24].
Argatroban
Argatroban is a synthetic,univalent DTI,and thereby binds directly to the catalytic site on thrombin in a reversible fashion[16].Compared to the other DTIs,it has a relatively small molecular size (527 Da).It is administered by intravenous infusion,has an onset of action within 30 minutes,and has a half-life of 45 min in patients with normal hepatic function (Table1).Of the DTIs,argatroban has the greatest serum protein binding,with 20% to albumin and 34% to alpha1-acid glycoprotein.Lidocainean antiarrhythmic used on occasion in cardiothoracic surgical patients-may decrease argatroban concentrations up to 20% due to its high affinity for alpha1-acid glycoprotein[29].
Dosing:Only one patient in the reviewed literature received the manufacturerrecommended initial dose of 2 µg/kg/min.The patient subsequently suffered major bleeding complications and required a rapid dose reduction[30].In all other reported cases,patients subsequently received doses at a rate approximately 10% the manufacturer recommended dose,with most initial doses ranging from 0.1-0.3µg/kg/min.This is consistent with literature from other critically ill populationswhich suggests that an initial dose of 0.2 µg/kg/min results in adequate dosing based on aPTT measurement,without excessive bleeding or thrombosis[39].
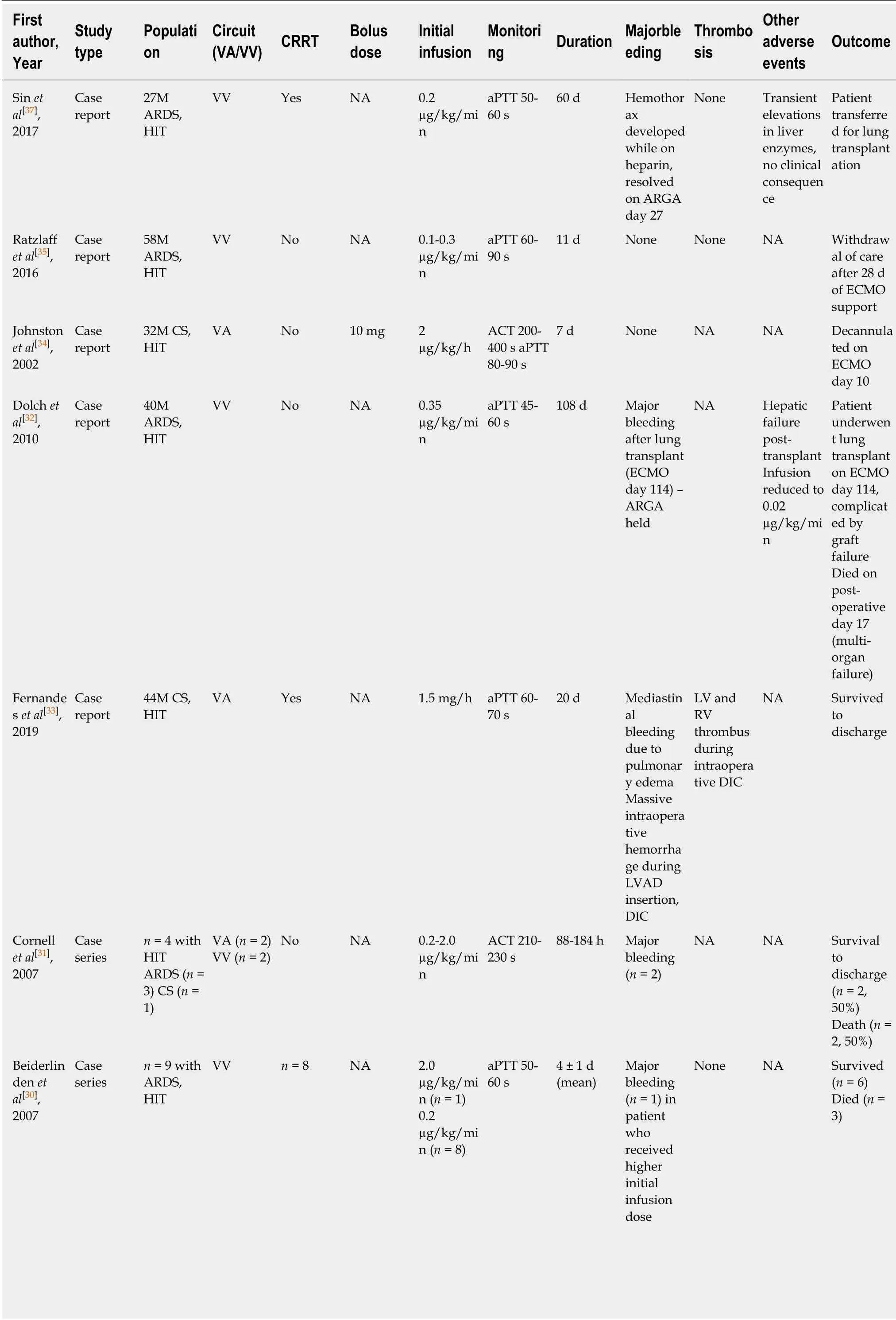
Table3 Summary of studies reporting on argatroban in adult patients supported with extracorporeal membrane oxygenation

ACT:Activated clotting time; aPTT:Activated partial thromboplastin time; ARDS:Acute respiratory distress syndrome; ARGA:Argatroban; CRRT:Continuous renal replacement therapy; CS:Cardiogenic shock; DIC:Disseminated intravascular coagulation; ECLS:Extra-corporeal life support; HIT:Heparin-induced thrombocytopenia; LVAD:Left ventricular assist device; NA:Not available; TEG:Thromboeslastography; UFH:Unfractionated heparin;VA:Veno-arterial; VV:Veno-venous.
Renal dysfunction:No dose adjustment is required for patients with renal dysfunction,with or without renal replacement therapy,which is a major advantage relative to other agents.There are several reports of successful clinical outcomes with argatroban in patients who require CRRT while supported by ECMO[30,33,37].
Hepatic dysfunction:Argatroban is metabolized hepatically by hydroxylation and aromatization,and therefore has a prolonged half-life of up to four times normal in the setting of liver dysfunction[40].While CYP3A4 and CYP3A5 provide a minor metabolic pathway,co-administration of inhibitors or inducers of these enzymes do not result in significant changes in argatroban concentrations[41].
Argatroban is not contraindicated in patients with hepatic dysfunction; however significant dose reductions may be required.There are few described cases of argatroban use in patients with liver dysfunction.One reported case of a patient with acute respiratory distress syndrome who underwent lung transplantation complicated by postoperative hepatic dysfunction describes a maintenance dose of 0.02µg/kg/min in order to achieve the target aPTT[32].The patient did not suffer from any adverse bleeding or thrombotic events related to the relatively low dose requirement.
Monitoring:The majority of reported cases and the single retrospective study used aPTT as the target for dose adjustment[30,32,33,35,37,38].Some cases reported use of ACT,either alone or in conjunction with aPTT,as the therapeutic monitoring parameter[31,34].In cases where aPTT was used,there was no standardized target; most reported a goal of 50-70 s.Of note,Menket al[38]found that bleeding events occurred when the maximum aPTT was above 50-60 s,and two thirds of bleeding events occurred when the maximum aPTT was above 75 s.Conversely,transient aPTT values below 50 s did not signal an increase in thromboembolic events.The authors of that study therefore recommended strict aPTT monitoring with a target of approximately 50 s.This is consistent with published guidelines which suggest an aPTT goal range of 1.5-2.5 times baseline[38].ACT monitoring,although less frequently used,varies substantially with reported targets between 200 and 400 s.As discussed previously,clinical end points remain the ultimate guidance for adequacy of anticoagulation in these critically ill and complex ECMO patient populations.
Safety and outcomes:There are many reported cases of successful clinical outcomes using argatroban in ECMO patients with suspected or confirmed HIT.In cases in which patients did not survive,none were reported to be directly associated with argatroban use.The reported duration of support is as long as 95 d.The rate of bleeding or thrombosis is low.In the retrospective study of 39 ECMO patients treated with argatroban,the rate of major bleeding or thrombosis was comparable to patients who received heparin[2,38].There are few reported major bleeding events with argatroban,many of which were related to surgical interventions.Bleeding at cannulation sites is reported,and blood transfusions were frequent,however the reported rate is similar to that seen with heparin[38].
Other agents
Desirudin and lepirudin are recombinant-DNA forms of the naturally occurring peptide,hirudin,present in the salivary glands of leeches.These recombinant hirudins are bivalent DTIs and thereby bind directly to both the catalytic and exosite-1 of thrombin in an irreversible fashion[42].Because they are composed of non-human proteins,anti-hirudin antibodies may be formed leading to potential immunologic reactions including anaphylaxis[42].The clinical use of recombinant hirudins during ECMO is limited by these factors and the more favorable pharmacokinetic profiles of the newer synthetic DTIs.Only lepirudin has been reported in adult patients requiring ECMO support.The single case report noted a successful outcome[43].Lepirudin was withdrawn from market in 2012 and is no longer in production.
Dabigatran etexilate is an oral,synthetic,peptide-like DTI[44].The onset of action is approximately 1 h and the half-life is 12 to 17 h.Renal excretion is the primary determinant of dabigatran removal,with up to 80% in the urine,and therefore the half-life is prolonged in the setting of renal dysfunction.Dabigatran is a substrate of p-glycoprotein and is therefore prone to a large number of clinically relevant drug interactions with p-glycoprotein inhibitors or inducers.Because of all of these reasons,there is no role for dabigatran in anticoagulation during ECMO.
LIMITATIONS AND SPECIAL USE CONSIDERATIONS
Bleeding
Bleeding is a common cause of morbidity and mortality among ECMO patients,regardless of anticoagulation strategy[2].There are no formal recommendations for anticoagulation management when bleeding is encountered while on ECMO.For patients receiving DTI therapy,holding DTIs temporarily or short term can be considered.As previously mentioned,there is no specific antidote for DTIs; however,the short half-life of available agents results in rapid offset of anticoagulant effect when the infusion is held or decreased.Once surgical bleeding has been excluded,treating teams can consider performing TEG to further assess the cause of bleeding and transfuse blood products as needed.In patients with hyperfibrinolysis,successful TEG-guided use of tranexamic acid has been described in patients on ECMO and DTIs[45].Once hemostasis has been achieved,DTI infusions can be restarted at a lower infusion rate,targeting a lower anticoagulation goal based on aPTT or ACT.In the protocol published by Netleyet al[22],significant bleeding triggered the reduction of the bivalirudin infusion rate,targeting the lower limit of the therapeutic range (i.e.,aPTT 40 s).Prothrombin complex concentrates may be used in life-threatening situations[2].As a general rule,ECMO flow should be increased whenever anticoagulation is reduced or suspended in order to minimize the risk of thrombus formation.
Prothrombin time interference
DTIs commonly prolong the prothrombin time and international normalized ratio in a dose-dependent manner,which may confound monitoring of warfarin in clinical practice.The derangement in these values may depend on the specific assay used[46].However,it typically does not interfere with ECMO anticoagulation as we primarily use aPTT/ACT.Factor Xa testing may be considered during DTI transitions to warfarin.
Low circuit flow states
While argatroban undergoes mostly hepatic metabolism,bivalirudin is primary metabolized by proteolytic enzymes which rapidly cleave the molecule.This results in the short half-life that has been described.However,instances where blood is stagnant may induce thrombosis due to rapid local cleavage of bivalirudin[47].Although ECMO is a continuous circuit without any “low-flow” chambers,in cases of cardiac dysfunction the cardiac chambers may act as a source of stagnation.This may particularly be realized in patients who are undergoing active ECMO “wean” trials where circuit flows are down-titrated to assess ability to decannulate.In addition,any setting wherein the native myocardium experiences low pulsatility and allows blood to pool in the ventricles is of very high concern.This may result in localized thrombosis despite adequate bivalirudin dosing.This can be particularly problematic in post-cardiotomy ECMO patients with prosthetic heart valves as valve thrombosis may lead to significant morbidity and mortality.It has been suggested that these circumstances may be avoided by minimizing intracardiac blood flow in cases of cardiogenic shock or avoid low-pulsatility states,and by using UFH as alternative anticoagulation in cases where intracardiac spontaneous echo contrast,or “smoke effect,” is noted,or low-flow states are suspected[47].During EMCO weaning and trialoff period,especially in setting of VA ECMO wean and if the duration of the trial-off is prolonged,preemptive heparin administration is essential to minimize thrombus formation.
Direct thrombin inhibitor resistance and high-dose response
Resistance to UFH has been reported both in ECMO and non-ECMO cases,with multiple potential explanations[48,49].Less is known regarding DTI resistance,although cases have been reported using both bivalirudin and argatroban[50-53].Very few described cases included patients requiring extracorporeal support.In cases of DTI resistance,increasing doses are required to achieve the target aPTT.This has been reported with initial dosing of DTI,although there are also delayed presentations with therapeutic levels initially followed by progressive unexplained dose increases to maintain therapeutic aPTT levels[26,50,53].The mechanism of DTI resistance is unclear but may be associated with elevated factor VIII and fibrinogen,which has been reported in some cases[52,53].Notably,these patients ultimately require very high doses of DTI to achieve aPTT targets,often well above the maximum recommended dose.It has been suggested that increases in DTI dosage may result in less aPTT prolongation at high doses than at low doses,which may explain why patients on high doses of DTI require greater than expected dose increases[51].Conversely,the increase in the international normalized ratio may be more pronounced at high DTI doses[51,53].Early recognition and rapid titration are essential,as there are multiple reports of clinical thrombosis due to subtherapeutic anticoagulation during delayed titration to target aPTT levels[53].This point highlights the importance of applying clinical end points(circuit patency,bleeding vs.thrombosis) as ultimate guidance for ECMO anticoagulation management.In cases of DTI resistance,alternative monitoring parameters have been proposed as aPTT may be unreliable.These include ACT,thrombin time,or direct drug level measurement[53,54].The therapeutic targets for these parameters,however,are variably defined.
CONCLUSION
Systemic anticoagulation with DTIs in ECMO patients is a feasible and safe alternative,with several advantages over UFH.The primary indication for DTIs is in cases of suspected or confirmed HIT,however reports suggest that DTIs may be effective as an initial anticoagulation strategy for all ECMO patients.Multiple dosing and monitoring protocols have been proposed for both bivalirudin and argatroban,and further prospective trials should determine the optimal pathway to safe,effective anticoagulation in this critically ill population.
 World Journal of Critical Care Medicine2019年6期
World Journal of Critical Care Medicine2019年6期
- World Journal of Critical Care Medicine的其它文章
- Fatal Legionella pneumophila serogroup 1 pleural empyema:A case report
