Complex formulations,simple techniques:Can 3D printing technology be the Midas touch in pharmaceutical industry?
Shrawani Lamichhane,Santosh Bashyal,Taekwang Keum,Gyubin Noh,Jo Eun Seo,Rakesh Bastola,Jaewoong Choi,Dong Hwan Sohn,Sangkil Lee
College of Pharmacy,Keimyung University,Daegu 42601,Republic of Korea
Keywords:Three-dimensional printing Personalized medicine Fused deposition modeling Inkjet printing Complex formulations
ABSTRACT 3D printing is a method of rapid prototyping and manufacturing in which materials are deposited onto one another in layers to produce a three-dimensional object.Although 3D printing was developed in the 1980s and the technology has found widespread industrial applications for production from automotive parts to machine tools,its application in pharmaceutical area is still limited.However,the potential of 3D printing in the pharmaceutical industry is now being recognized.The ability of 3D printing to produce medications to exact specifications tailored to the needs of individual patients has indicated the possibility of developing personalized medicines.The technology allows dosage forms to be precisely printed in various shapes,sizes and textures that are difficult to produce using traditional techniques.However,there are various challenges associated with the proper application of 3D printing in the pharmaceutical sector which should be overcome to exploit the scope of this technology.In this review,an overview is provided on the various 3D printing technologies used in fabrication of complex dosage forms along with their feasibility and limitations.©2019 Shenyang Pharmaceutical University.Published by Elsevier B.V.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
The concept of three-dimensional (3D) printing dates to the 1980s when a Japanese doctor published a paper describing stereolithography technology[1].3D printing is also known as additive manufacturing since a digital design for a 3D structure is fabricated layer-by-layer from the bottom.Conventionally,3D printing was widely used for rapid prototyping molds and tooling, digital manufacturing, and personal fabrication.However, with the advancement of the technology, 3D printing has become a solid free form fabrication(FFF)technology.From products as simple as hearing aid to high-tech parts of military jets,the scope of 3D printing is growing rapidly[2].
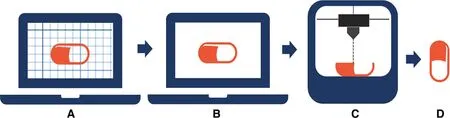
Fig.1-General process of 3D printing with reference to nozzle-based printing:design of the object using computer aided design software(CAD)and file saved in STL.(A)Format Slicing and printer setting using slicing software;(B)file saved in format supported by the printer(e.g.G-code);(C)printing of designed object using suitable 3D printing technology(e.g.nozzle-based technology);(D)finished printed object.
All 3D printing processes starts with a computer-aided design(CAD)model or a digital scan which is converted into the Standard Tessellation Language (STL) format and transferred to slicing software to cut the design into thin cross sections(Fig. 1). However, different technologies are available for the final printing process to deposit consecutive layers for fabricating the desired object. The thickness of each layer ranges from 0.001 to 0.1 inches [2]. 3D printing of a wide range of materials is possible,including thermoplastics,thermoplastic composites, pure metals, metal alloys, ceramics and various forms of food. However, the possibility of using cells, growth factors, and biological materials as the main materials have opened potential application in medical engineering.The ability to fabricate personalized organs greatly reduces the risk of graft rejection and the need for lifelong use of anti-rejection medicine [3]. The application of 3D printing in the medical sector also includes educational and training tools,preoperative planning,surgical instruments,and bio printing.Various studies have investigated practical applications in the medical sector and the scope of application continues to grow as the technology progresses. As such, 3D printing is very important in the medical and pharmaceutical sector. Additive manufacturing can provide various options from drug development or discovery to front line care. While the concept of additive manufacturing started in 1980s, it still is developing especially in the pharmaceutical sector.Recently,in 2015,The Food and Drug Administration (FDA) approved Spritam®(brand name of Levetiracetam) as a 3D-printed drug for the treatment of epilepsy which showed instantaneous disintegration of an active ingredient [4,5]. Various studies have investigated different formulations using various 3D printing technologies.
Personalized medicine is a move to away from the concept of ‘one-size fits-all’for the treatment and care of patients with a particular condition, to new approaches that better manage patients’health and that targets therapies to achieve the best therapeutic outcomes and proper management of the diseases [6]. With the advancements in pharmacogenetics, pharmacogenomics [7] and metabolomics, the need for personalized medicine is increasing. Furthermore, 3D printing might provide one of the techniques for personalized medicine and on-demand manufacturing[8,9].It also reduces the cost of purchase and the size of operation space required compared to conventional manufacturing processes, while it may have limitations relating to accuracy, and the size of models. Cost and risk associated with use of various kinds of excipients can also be reduced as printing is possible with least number of excipients [10,11]. Also, 3D printing comes with flexibility in designing the formulations of various shapes, sizes and compositions which can be used to optimize the dose and the kinetics of drug release. All these are possible with simple changes in designs using a software compared to time consuming conventional manufacturing processes. Apart from enabling the development of patient specific medicine, 3D technology can also be used to produce release-tailored medicine [12]. Fig. 2 summarizes the advantages of 3D printing in the pharmaceutical sector.
Various technologies such as fused deposition modeling(FDM), selective laser sintering (SLS), stereolithography (SLA)and inkjet or binder jet printing(IJP),have been studied for the development of different formulations [5] and have been described in this review.However,most of the studies have been carried out using FDM and inkjet printing,which are considered the simplest and most feasible methods for the formulation of dosage forms using 3D technology.Hence,in this review, special attention has been paid on the principles and studies done using FDM and ink-based printing technology as well as the challenges faced by 3D printing in the pharmaceutical sector.
2. Additive manufacturing technologies
2.1. Laser based writing systems
2.1.1. Stereolithography
The first commercial application of stereolithography as a solid free-form fabrication technique date to the late 1980s[13,14].This method was patented by Charles Hull,co-founder of 3D Systems, Inc., in 1986. The process of printing involves a uniquely designed 3D printing machine called a stereolithograph apparatus(SLA),which converts liquid plastic into solid 3D objects [15]. SLA was found to be mostly used in preparations of personalized scaffolds and drug-loaded scaffolds. In terms of accuracy and resolution, stereolithography is superior to all other solid free-form fabrication (FFF) techniques with an accuracy up to 20 μm [13]. Patient-specific models and functional parts, implantable devices, tissue engineering, and also cell-containing hydrogels are possible with this technology[13].

Fig.2-Advantages of 3D printing in pharmaceutical sector.

Fig.3-Different 3D printing technologies:(A)SLA bottom-up,(B)SLA top-down,(C)SLS,(D)IJP and(E)FDM.
Stereolithography is based on the polymerization of photopolymers (resins) cured by ultraviolet light [3,13,16]. Based on the same principle two approaches have been defined for STL technology:bottom-up approach and top-down approach,as shown in Fig.3.In the bottom-up approach UV light cures the surface or the very thin top layer of the photopolymer above the build platform. After the solidification of the first layer, the platform is moved down depending on the thickness of each cross-section so that another layer of photopolymer resin is exposed above the hardened polymer. As such,the second layer is formed and attached to the first layer.This process continues until the desired design is completed(Fig.3A).In contrast,in the top-down approach,the build platform is above,and the UV source is beneath the resin tank.The resin tank has a transparent window,which provides the UV laser a path to cure the resin.At the start,the build platform is lowered to the base of the resin tank leaving only the thin layer of resin to be cured. After the first layer is formed, the platform is elevated according to the thickness of each layer.Then, another layer of fresh resin is exposed below the first layer which is again cured by the UV laser, thus forming the second layer (Fig.3B).In both approaches,after the design is completed, the printed product should be washed with rubbing alcohol to remove the excess resin.Finally,post curing in a UV oven can be employed in order to strengthen the printed parts [1,13,14]. The choice of resin is very crucial in terms of the product’s physical properties (solidification rate in the presence of UV light) and should be FDA approved in case of pharmaceuticals.The limited number of resins available that are biocompatible,and biodegradable is considered the main limitation of this technique[13].The first resins developed for use in stereolithography were based on low-molecular weight polyacrylate or epoxy macromers that form glassy networks upon photo-initiated polymerization and cross linking. Several resins have been developed over the past two decades,and a wide range of mechanical properties of the networks can be obtained after curing. Various studies explored the scope of SLA technology in pharmaceutical sector.
For instance, Goyanes et al. described an innovative way of personalizing acne management using 3D scanning and 3D printing.Patients’noses were scanned with a commercial 3D scanner, and a medicated mask was formulated according to the morphology of the individual patient. A personalized anti-acne nose mask was made using SLA and FDM technology and compared.Commercially produced Flex EcoPLATM(FPLA)and polycaprolactone(PCL)filaments were loaded with salicylic acid for FDM, whereas for the SLA process, salicylic acid was dissolved in poly(ethylene glycol)diacrylate(PSGDA)and poly ethylene glycol PEG.SLA printing yielded 3D-printed devices (nose-shape) with higher resolution and higher drug loading(1.9%,w/w)than FDM,with no drug degradation.SLA printing was the most appropriate 3D printing technology to manufacture anti-acne devices with salicylic acid [17]. Martinez et al. used SLA printing to prepare ibuprofen-loaded hydrogels of cross-linked polyethylene glycol diacrylate. Hydrogels containing up to 30% (w/w) water, and 10% (w/w)ibuprofen, were prepared. The dissolution profiles showed that drug release rates were dependent on water content,with higher water content hydrogels releasing the drug faster[18]. Wang et al. also used SLA to prepare oral-modified release dosage form with acetaminophen and 4-aminosalicylic acid (4-ASA) as the model drugs. In their work polyethylene glycol diacrylate (PEGDA) was used as a monomer and diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide was used as a photo-initiator. Tablets were successfully printed and formulations with different properties were fabricated by adding polyethylene glycol 300(PEG 300)to the printing solution.The loading of paracetamol and 4-ASA in the printed tablets were 5.69% and 5.40%, respectively. In conclusion, SLA 3D printing technology allows the manufacture of drug-loaded tablets with specific extended-release profiles [16]. SLA technology is known to produce extreme details and have very thin layers of cross-section which is one of the major advantages of this technology. In the case of medicine and pharmaceutics,as discussed earlier, the availability of a suitable resin plays a very big part in its future use.However,with proper understanding of the process and advancement of the resins SLA technology,an alternative method for the formulation of various dosage forms can be proposed.
2.1.2. Selective laser sintering(SLS)
SLS,a laser-based technology,as the name suggests works by causing fusion in the powder bed by a controlled scanning laser beam (Fig. 3C) [19]. During the printing process, the laser is directed to draw a specific pattern onto the surface of the powder bed. Once the first layer is completed, a roller distributes a new layer of powder on top of the previous one.The object is built layer-by-layer, which is then recovered from underneath the powder bed. SLS has mostly been used in the formation of scaffolds in bioengineering with proper biocompatible materials. There are very few formulations studied using SLS as 3D printing technology.In 2017,immediate release and modified release formulations of paracetamol as a model drug and Kollicoat®IR or Eudragit®L100-55 as polymers were printed using SLS technology [20]. SLS uses lasers to harden the polymers by increasing the temperature of the polymer above melting temperature. However, there should be interaction between the laser beam and the powder particles for the process to occur. In this case, initial study did not manage to print the paracetamol formulations as the polymers used was not able to absorb the laser-light. So,Candurin®gold sheen, pharmaceutical excipient for coating of tablets, was used in various concentrations to print out the formulations. Candurin®gold sheen was able to absorb the laser light included in the sintering process. This study proved the application of SLS technology in formulation of pharmaceutical dosage forms. But the choice of polymer seems to be very important as the material should be able to absorb the laser-light of given wavelength. Recently, in 2018, orally disintegrating tablets (ODTs) with paracetamol as a model drug and hydroxypropylmethylcellulose (HPMC)and kollidon®VA 64 as polymers along with Candurin®gold sheen was successfully formulated using SLS technology[21].SLS has advantages of high-resolution printing and printing of medicine without the need of the solvent. However, highenergy lasers may cause degradation of drugs and polymers used.With proper study of this technology for the formulation of various dosage forms in the future and the availability of polymers able to absorb laser-light may provide an alternative technique for printing medicines using 3D technology.
2.2. Ink-based printing technology:inkjet printing
IJP is a collective term for various printing technologies based on formation and placement of digitally controlled droplets onto a substrate. IJP can be broadly categorized into continuous inkjet printing (CIJ) and drop-on-demand (DoD) inkjet printing [22].Further,DoD can be differentiated into thermal inkjet or piezoelectric inkjet,based on the type of printhead,and drop-on-drop or drop-on-solid, based on the substrate onto which the printhead shoots the formulated droplets(Fig. 4). If printhead shoots droplets onto other droplets to produce a solid material, it is known as drop-on-drop inkjet,whereas if it shoots onto a solid material,it is known as dropon-solid inkjet,which is also known as TheriformTMprocess.Since the drop-on-drop method is quite difficult to apply for pharmaceuticals, most studies in this field have employed drop-on-solid method[23].
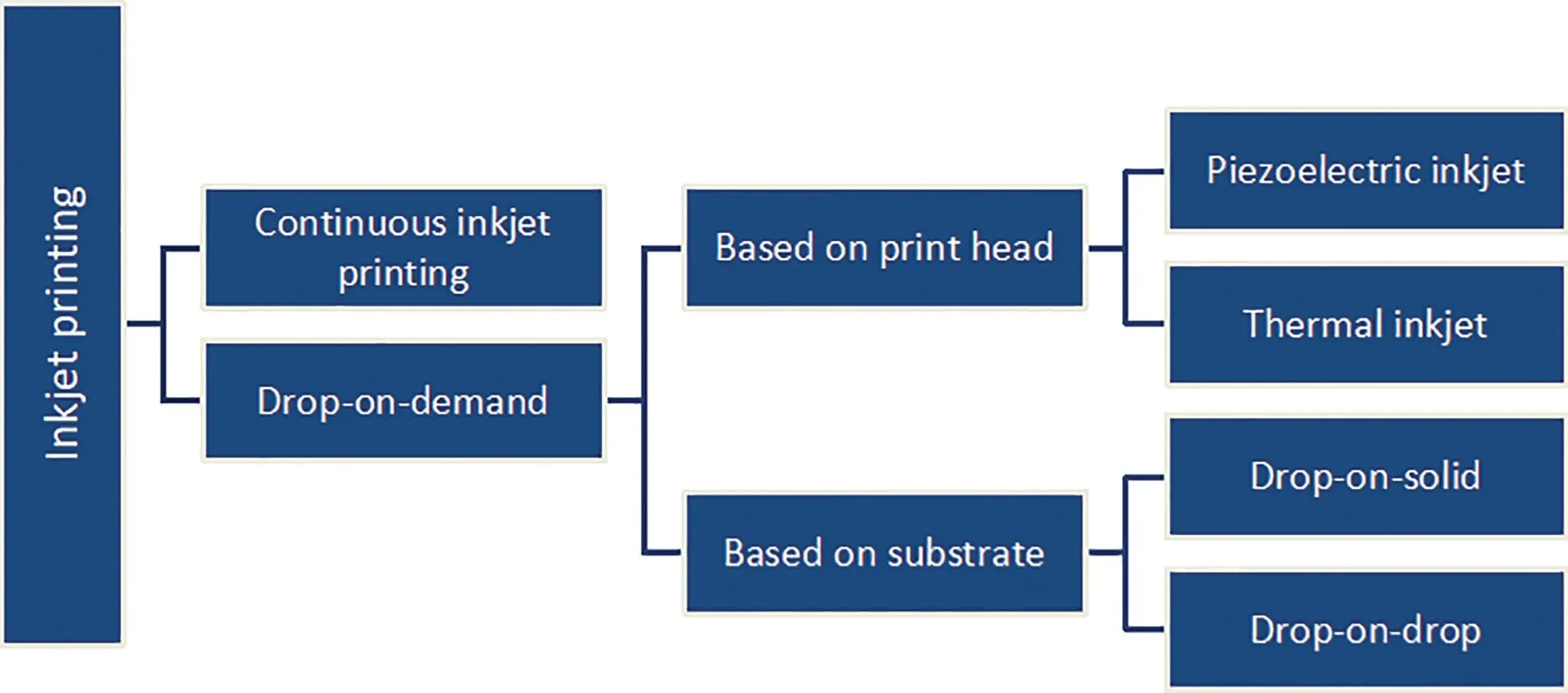
Fig.4-Different types of inkjet printing.
As described earlier,IJP is versatile and employs of various technologies. However, the basic printing principle remains same for all types of IJP.It consists of a powder bed and a liquid binder(Fig.3D).At the start of the process,a thin layer of powder is spread over the built platform with the help of a roller.Then the printhead releases precisely controlled droplets of the liquid binder onto the powder bed which causes fusion among the powder particles.After each layer is formed,a new layer of powder is spread across the built platform to form the new layer. The ejection of liquid droplets is governed by different mechanisms:piezoelectric,electrostatic and thermal.
Piezoelectricity,also known as pressure electricity,is a phenomenon where electricity is generated by the application of stress.Conversely,if electric force is applied,piezoelectric sensitive materials change their shape. Hence, in piezoelectric and electrostatic DoD,the application of current electricity or static electricity causes rapid changes in the shape of piezoelectric crystals or mechanical displacement adjacent to the liquid chamber respectively.This causes changes in the shape of liquid which generates pressure and forces ink out through the nozzle [24]. In thermal DoD, the print head is heated by heating elements,and small air bubbles are formed that produce pressure pulses to eject ink droplets out of the nozzle.
Inkjet printing is the first ever used solid FFF in pharmaceutics [25].A controlled drug delivery device was fabricated on a desktop version of 3D printing machine that was developed at the Massachusetts Institute of Technology in 1996.In that study,controlled-release drug delivery device was developed using Poly-ε-caprolactone (PCL) as the top and bottom layers and polyethylene oxide (PEO) as the middle layer. After that, various complex release profile dosage forms with complicated internal geometries, multiple layers with multiple excipients and actives were demonstrated in various studies [26-29]. Apart from the conventional formulations,oral films and orally dispersible formulations were some of the major fields of studies in pharmaceutics using IJP as FFF[30-32] (Table 1).Early studies included deposition of API (active pharmaceutical ingredient)onto the films made by potato starch using a simple desktop inkjet printer [32].An aqueous solution of salbutamol was successfully deposited onto the film.The study also demonstrated the importance of viscosity of the solution since greater viscosities (>2 mm2/s) made the ejection of the ink form cartridge difficult. Furthermore,with lower viscosities(<1 mm2/s),liquid flow was influenced by gravity which blocked the ink jetting action.
Fast disintegrating dosage formulations have been available since the late 1990s and are constantly in demand for rapid action and patient compliance due to ease of administration among pediatrics and geriatrics and in cases of dysphasia and incompliant psychiatric patients. To keep up with the demand of novel drug delivery systems and advancement of 3D printing, a rapid dissolving solid dosage form was patented with levetiracetam as a model drug which was later approved by FDA and was available in the market from early 2016.It was formulated using Aprecia’s proprietary ZipDose®Technology based on drop-on-solid technology.This achievement can be considered a major milestone in the application of 3D printing in pharmaceutics. Various rapid dissolving formulations have been developed following the FDA approval of Spritam®[30,31,33]. Inkjet printing is one the high-resolution 3D printing techniques as it can create droplets of binder fluid in micrometer sizes.This helps in accurate formulation of small particles and dosage form which were not possible by other 3D technologies.In comparison to other 3D printing technology,inkjet printing can revolutionize the secondary manufacturing by being able to print out particulates for injectables, inhalations or liquid based dosing,rapid dissolving formulations and thin films for oral or buccal delivery systems. Furthermore, inkjet printing has also been used to prepare drug delivery devices and packaging materials as well. In a way, inkjet printing can be used from preparing pharmaceuticals to final stages of dispensing as well. However, inkjet printing also comes with several challenges that needs to be addressed in order to employ the technology efficiently in the manufacturing of pharmaceutical products.
As discussed above, viscosity and surface tension of an ink in crucial for printing using inkjet printer. The properties of API and excipients also the combination ratio of API with excipient determines the final properties of the binder.Hence,extensive study might be necessary to optimize the flow of ink with droplets of desired size and flowability.Also,in dropon-solid technique, ink/binder fluid should be able to create nucleation process in the powder bed upon contact which depends on binder-powder wettability,droplet penetration into the powder voids and spreading behavior of the binder[22,23].
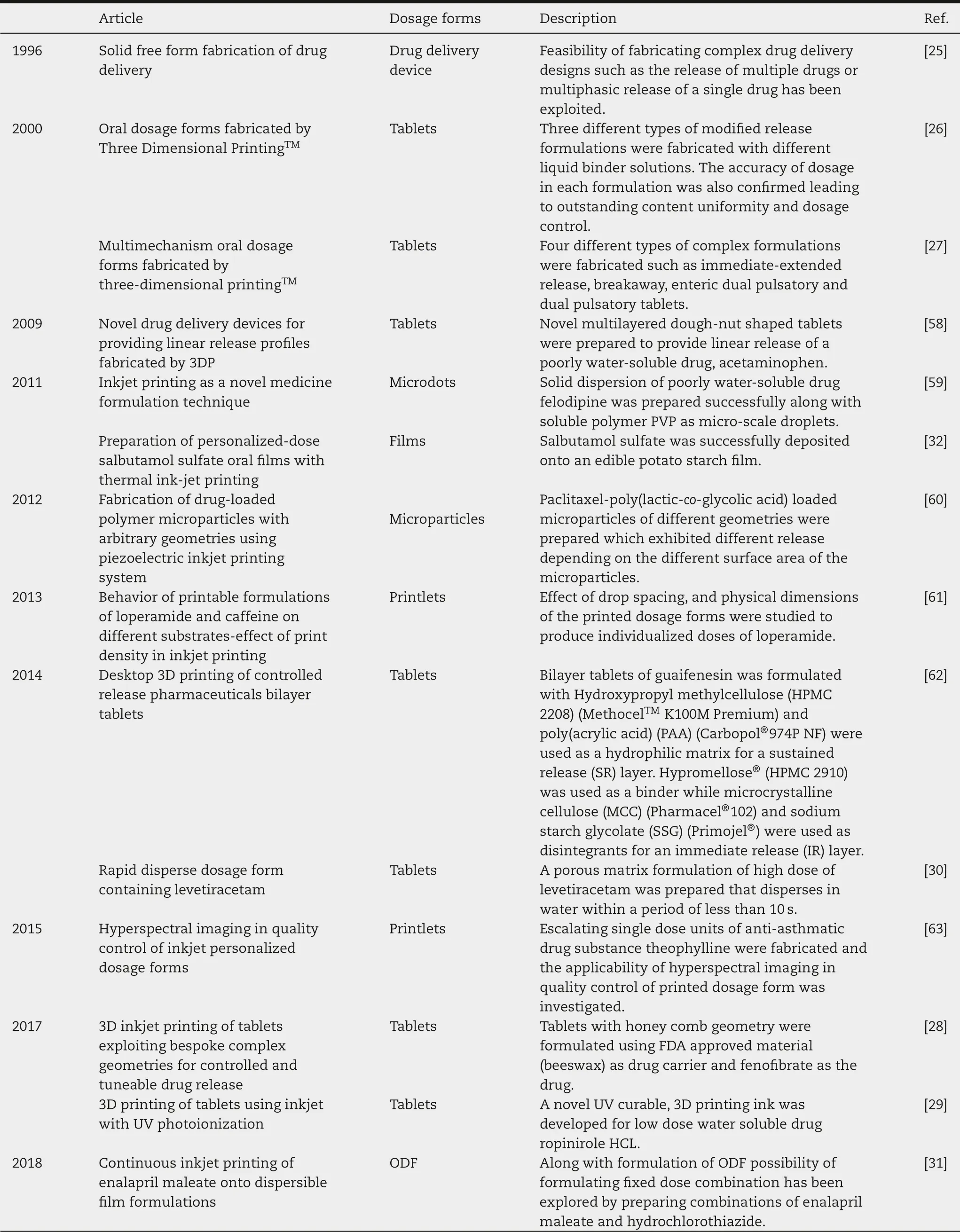
Table 1-Various significant studies done using ink-based printing technology in chronological order.
2.3. Material extrusion or nozzle-based deposition system:fused deposition modeling
Material extrusion is a process in which a filament of the desired material is prepared,and the melted material is pushed through an appropriate nozzle to build the desired product.In this technology, spools of filaments are present in rollers,which pass through a nozzle heated up to a temperature high enough to melt the filament.The melted filament is deposited on the build platform according to the design created using the software.The melted material is deposited layer-by-layer and fused together because the layers are in the molten state as the build platform moves downwards until the finished product is obtained.
In FDM technology, the nozzle moves horizontally, and the build platform moves vertically downwards as the process continues. After each layer, the build platform moves down,and another layer is deposited on top of previous layer(Fig.3E).The XY resolution of FDM is good but the Z resolution is not very good,and hence,the thickness is not uniform.Extra finishing process therefore may be required if a smooth surface is desired.A nozzle or small tip used in FDM technology typically ranges 50-100 μm[11].
The major polymers used in FDM are polylactic acid or polylactide (PLA), polyvinyl alcohol (PVA) and acrylonitrile butadiene styrene (ABS) [5,17], of which PLA and PVA are also used in the formulation of pharmaceutical dosage forms[17,34-36]. Polymers used should be thermally stable, nonvolatile and non-aerosolizing [8]. As a result, there are two major challenges faced by the FDM technology in the pharmaceutical sector:availability of appropriate filament and the thermal degradation of API due to extrusion and printing temperature.
Despite the challenges presented by FDM technology,most of the studies on 3D printing have employed the same technology.Various prototypes of dosage forms have been investigated in order to understand the feasibility of the process,the first of which is fabricating fluorescein-loaded PVA filaments (Fig.5A) for controlled release formulation.The possibility of loading drugs onto PVA filaments was demonstrated.Since an aqueous solution tends to dissolve the PVA filament,an alcoholic solution was used to incorporate drug into the filament.However,complete loading of the drug was not achieved. Drug release occurred by erosion and largely depended on the infill percentage of the drug. Complete drug release took 20 h for a 90%infill tablet[11].Various controlled release formulations have also been prepared by only using PVA mixed with desired APIs such as aminosalicylates(4-ASA and 5-ASA) [34] and prednisolone [37] as tablets and paracetamol or caffeine [36] and budesonide [35] as caplets.However,in few formulations developed as modified and extended release tablets excipients such as mannitol, triethyl citrate, and hydroxypropylcellulose have been used [38,39].Goyanes et al. formulated two aminosalicylate isomers used in the treatment of inflammatory bowel disease (IBD), 5-aminosalicylic acid(5-ASA,mesalazine)and 4-aminosalicylic acid (4-ASA), using FDM technology. Commercially PVA filaments were loaded with the drugs in an ethanolic drug solution similar to the fluorescein-controlled release formulation described above. Drug loading was relatively less in both filaments.A final drug-loading of 0.06% (w/w) and 0.25% (w/w)was achieved for the 5-ASA and 4-ASA filaments,respectively.Such less drug was found because of the significant thermal degradation (50%) of APIs especially 4-ASA, limiting its applicability to printing temperatures lower than the degradation point[34].Thus,thermolabile drugs might not be suitable for FDM; even though the heating time in nozzle is for a moment it can lead to significant degradation of the thermolabile drug [34]. This is the major drawback of FDM technology in the pharmaceutical field.However,to overcome this problem immediate release tablets of theophylline and dipyridamole as the model drugs were prepared at a relatively low temperature(90-100°C).The use of hydrophilic polymer polyvinyl pyrrolidone (PVP) to decrease the printing temperature has been successfully shown with more than 85% of drug release within 30 min [40]. In addition, to widen the range of FDM and to increase its applicability in formulation dosage forms, A. Melocchi et al. designed various filaments using a twin-screw extruder. Filaments based on insoluble (ethylcellulose, Eudragit®RL), promptly soluble (polyethylene oxide, Kollicoat®IR), enteric soluble (Eudragit®L, hydroxypropyl methylcellulose acetate succinate)and swellable/erodible(hydrophilic cellulose derivatives, polyvinyl alcohol, Soluplus®)polymers were successfully produced [41]. The possibility of designing a modified release formulation with appropriate API was demonstrated successfully.We already discussed the possibility of designing filaments with different polymers exhibiting various release profiles [41]. However, the initial step of FDM which is filament production is a crucial step that needs to be studied.Proper quality control parameters must also be established to secure FDM technology as one of the manufacturing technologies.Various studies have investigated the feasibility of combining the hot melt extrusion method with 3D printing technology [35,42,43]. With the aim of manufacturing filaments with good mechanical and rheological properties, as well as tablets with desired drug dissolution and release properties J. Zhang et al. fabricated controlled release tablets utilizing hot melt extrusion (HME) technology. When 3D-printed tablets,directly compressed mill-extruded tablets,and tablets prepared from a physical mixture were evaluated for drug release 3D-printed tablets were found to have better controlled release compared to the other two tablets. In this study, a three-point bending test was introduced as one of the quality control parameters of produced filaments in order to estimate the breaking stress and breaking distance as a representation of filament stiffness and brittleness, respectively.Although high temperature was used,no API or excipient degradation occurred during HME(140-160°C)and 3D printing(200°C)process.Acetaminophen was used as a model drug and the polymers used were HPC LF,HPMC E5,Eudragit®L100,Soluplus®,and EC N14[42].These studies show that even with FDM there is a possibility of expanding the scope of 3D printing together with a wide range of excipients and APIs(Table 2).

Fig.5-Examples of applications of 3D printing in pharmaceutical sector.(A)Images of PVA filament(left)and fluorescein-loaded filament(right)under UV light(Reproduced with permission from[11].Copyright 2014 Elsevier B.V.);(B)design and printed tablets of different geometrical shapes using FDM technology(Reproduced with permission from[43].Copyright 2015 Elsevier B.V.);(C)multicompartment capsular device printed using FDM technology(Reproduced with permission from[48].Copyright 2015 Elsevier B.V.);(D)dual-nozzle FDM(Reproduced with permission from[50].Copyright 2018 Elsevier B.V.);(E)channeled tablets(front and side view with channel sizes)(Reproduced with permission from[57].Copyright 2017 Elsevier B.V.).
Various designs of FDM printers are available with a wide range of versatility and precision. In particular, the availability of FDM 3D printers with multiple nozzles is a major breakthrough. As a result, an enteric coated tablet could be fabricated using a dual-nozzle single-step FDM 3D printing process. The gastric-resistant tablets were engineered by employing a range of shell-core designs using PVP and methacrylic acid copolymer for the core and shell structures respectively;such polymers have been also broadly exploited in supporting other advance techniques. Theophylline was used initially as a model drug for its thermal stability and feasibility of studying the release study [44]. As such, poly pills,layered tablets,breakable tablets,and coated tablets can be prepared in a single-step process. However, a combined formulation of chemically incompatible APIs has always been the challenge faced by many pharmaceutical technologists.One of the solutions to this problem is a bilayer tablet with different formulation blends to modify the release and to separate the APIs.Recently,Genina et al.presented an alternative method of formulating the combination of anti-tuberculosis drugs rifampicin and isoniazide by physically separating the APIs in a unique dual compartment dosage unit designed with CAD and fabricated using 3D printing based on FDM[45]. To exploit yet another advantage of using 3D printing technology in pharmaceutics,Goyanes et al.designed various shaped (cube,pyramid,cylinder,sphere,and torus) paracetamol tablets(Fig.5B)and studied the effect of geometry on the drug release. Dissolution profile of differently shaped tablets with constant surface area/volume ratio were not similar,contributing to the fact that geometry plays a major role in drug release profile and erosion plays a major role in defining the release kinetics[40].In addition,by utilizing the advantage of printing hollow and porous tablets,various gastro-retentive floating formulations were also developed [46,47]. Of which,floating hollow tablets of domperidone [43] were prepared using FDM technology. Domperidone-HPC filaments were prepared using HME and cylindrical hollow tablets were designed.Optimized formulation of density 0.77 g/cm3was able to float for 10 h in vitro and 8 h in vivo. In vivo studies of FDMprinted tablets were carried out for the first time using New Zealand rabbits’stomach. Tablets were labeled with BaSO4and the pharmacokinetic parameters were studied compared to commercial products (Table 3). The oral bioavailability of domperidone was significantly increased owing to the prolonged residence of domperidone from in the stomach.

Table 2-Various significant studies done using FDM technology in chronological order.

Table 2(continued)

Table 3 - Pharmacokinetic parameters of domperidoneprinted tablets compared to commercial tablet[46].
Apart from fabricating drugs as modified release systems,capsular devices have also been designed. A. Melocchi et al. manufactured swellable/erodible capsular device using hyproxypropyl cellulose(HPC)filaments(Fig.5C)[48].Furthermore, A. Maroni et al. designed a multi-compartment capsular device using HPC (hydroxypropyl methyl cellulose),KIR(kollicoat®IR), HPMCAS (hydroxypropyl methyl cellulose acetate succinate) and PEG (polyethylene glycol) 400 and 8000[49].To this date,capsules are prepared by injection molding with numerous steps until the finished product is obtained.3D technology might provide a single-step alternative process for the designing and production of capsule devices of different size and materials.
Recently to help develop personalized medicine and to deliver a poorly soluble drug in a liquid form,a fully automated additive manufacturing process for liquid capsule with the capability to control the dispensed dose has been introduced.In this study,a dual FDM 3D printer was modified to include a syringe-based liquid dispenser (Fig. 5D). This was used to fabricate a capsule shell through FDM 3D printing and instantaneously dispense either a suspension or a solution formulation of the model drug[50].As another recent advancement in FDM technology, the FDM printability of active ingredients ibuprofen-loaded sustained release polymer EC was investigated by introducing indices of melt rheology and mechanical property.Furthermore,sustained release tablets with predesigned scaffold structures were prepared by the FDM process[12].Although various studies investigated tablets and capsular devices as dosage forms,other dosage forms,such as oral films,were not studied until recently.Fast dissolving films(FDF) of paracetamol were formulated using PVA filaments and PEO as the polymer. Although the disintegration time was slightly higher than that for commercial FDFs,this study proves the applicability of FDM in the formulation of FDFs[51].Formulations by FDM provides good mechanical strength and high resolution, but the used thermoplastic material should be suitable for extrusion and infill percentage of drug should be optimized to obtain the desired release profile.FDM has been most commonly discussed as the best technology for medicine printing and it can also formulate very complex geometrical dosage forms, which was not feasible with conventional manufacturing process.FDM technology surely opens a new revelation in personalized medicine.
3. Challenges and future of 3D printing in pharmaceutical sector
We already discussed about the possibilities and technological challenges presented by different 3D printing technologies, during the formulation of various dosage forms, to this day. However, for 3D printing to thrive well in pharmaceutical sector,various other issues need to be addressed.Some of the issues like quality control of printed dosage forms, legal and regulatory matters,cost-effectiveness,availability of materials and equipment needed to produce medicine of better quality,if solved in future,would ascertain the success of the 3D printing in this area(Fig.6).
3.1. Concerns regarding quality control and regulatory issues
The first and foremost dispute regarding the application of 3D printing technology might be the concerns regarding quality control.Even though various studies have proven the feasibility of production of different dosage forms,regulatory requirements could be an obstacle to be cleared. FDA has already accepted the use of 3D printing in production of medical devices with about 200 FDA-approved 3D-printed devices available that can be tailored to fit a patient’s anatomy.A work shop entitled “Additive Manufacturing of Medical Devices:An Interactive Discussion on the Technical Considerations of 3D Printing”was held by FDA to discuss about optimal characterization and assessment methods for the final finished devices,as well as optimal process validation and acceptance methods for these devices. As a result, FDA developed guidance for industry and food and drug administration staffs which was broadly organized into two topic areas:Design and Manufacturing Considerations and Device Testing Considerations [52]. Also, FDA has recently approved the 3D printed solid dosage form for the treatment of epilepsy. However,3D printing is quite different from the conventional manufacturing processes, and hence, the quality control process should also be different. As such, concerns regarding critical parameters affecting the printability of various materials into drug products,critical process parameters for various printing technologies, assessment of the performance of 3D printed drug products,proper in-vitro release study procedure, sterilization issues,critical characteristics of intermediate products such as filaments,inkjets,and photopolymers,etc.still exists.To address these concerns the Office of Testing and Research(OTR) in Center for Drug Evaluation and Research (CDER)’s Office of Pharmaceutical Quality is conducting research to further understand the application of this technology to drug products [6]. Proper analysis techniques and parameters,from the design of the dosage forms to the finished products, should be developed. Owing to the numerous benefits of additive manufacturing in the pharmaceutical sector in terms of precision medicine and reduction of conventional manufacturing constraints, development of proper quality control for 3D-printed products seems like the next step.

Fig.6-Past,present and future of 3D printing in pharmaceutical sector.
3.2. A tool for personalized medicine
We have already discussed about the possibilities of personalized therapy with 3D printing in relation to the flexibility in design and formulation of medicine according to the patient’s need.The availability of limited strength of medicines in the market,specifically those with narrow therapeutic index and high potency, poses greater difficulty in personalizing the medicine to patient’s therapeutic response. In such cases,a simple modification in the 3D design can optimize the required dose.Also,the fixed-dose combination formulations,easily possible by this technique, can help achieve better therapeutic outcome by the synergistic effect and reducing the incidence of adverse effects[53,54].However,the practical application of 3D printing technology in personalized medication is a big challenge.The practice of every new technology and invention comes with a big price.3D printing being a very new technology in the pharmaceutical sector, its only practical application so far is the development of Spritam (FDA approved) for epileptic seizure, which was prepared using Zipdose®technology,a patented-technology developed by the Aprecia pharmaceuticals. Due to this reason, the cost of the medicine at this moment is higher than that of the other commercialized brands of levetiracetam prepared via traditional manufacturing processes. So, the cost effectiveness of the technology also depends largely on the expiration of patents.Also, the concept of developing 3D printing technology to personalize medicine in hospital and community pharmacy is very futuristic. Although, the technology has been proven to be applicable to develop various dosage forms, the actual setup of the technology needs proper legal and technological support.No doubt,this technology gives the view of the future,where digital clinical data can be sent directly to 3D printer to print out the medicine designed to meet the patient’s individual therapeutic requirements(Fig.7).However,the quality control of medicine before dispensing also plays a vital role in the determination of whether the technology can sustain in the future or not. To aid to this problem, recently process analytical technologies (PAT) such as near infrared (NIR) and Raman spectroscopy have been introduced to analyze the drug distribution and solid-state characteristics within the printed formulations. These non-destructive method for the characterization of printed medicine were comparable to conventional methods and were found to provide a feasible alternative to conventional destructive methods [55]. These findings backed up with more researches on the regulatory and financial constraints related to the establishment of 3D printing technology for personalized medicine will determine the future of 3D printing technology in pharmacotherapy.
3.3. Availability of materials
Another issue might be the availability of appropriate materials for the manufacture of desired dosage form that should be compatible with 3D printing technology. The accessibility of biodegradable, biocompatible and physically and chemically stable materials has been the major concern of 3D printing in medicine. For example, expansion of the presently available photo-curable resins for SLA,availability of non-toxic solvents to increase the binding capacity for binder-jet printing,accessibility of laser curable APIs and excipients for SLS and convenience of using thermally stable polymers other than thermoplastic polymers for FDM[56]would be one of the measures to enhance the scope of 3D printing in pharmaceutical industry.
3.4. Resolution of 3D printers
While 3D technology can produce great details in rapid prototyping and solid FFF,its resolution for printing medicine is still low.For smooth finishing and precise design of dosage forms,high-resolution equipments should be developed. Industrial 3D printers can now reach extremely small build layers such as 16-μm layer thickness for SLA (Polyjet, Stratasys), 178-μm layer thickness for FDM(Fortus 900mc,Stratasys),80-μm layer thickness for SLS(sPro 230HS,3D Systems)and 75-μm resolution for SLA (3D Systems) [14]. Among various technologies,SLA has the highest resolution till date,but these equipments are not optimized for biomaterials and pharmaceutical polymers. Hence, there still is the possibility of developing these technologies to include materials needed for medical application and pharmaceutics.
3.5. Scale-up manufacturing

Fig.7-Personalized medicine using 3D printing.
Although on-demand manufacturing and personalized formulation is possible, large-scale production is still challenging. Injection modeling has always been the most practical approach for large-scale manufacturing.However,with addictive manufacturing various shapes, sizes and dosage forms will be possible in a single run without much change in the machine parts and material preparation process. Although large-scale production of dosage forms using additive manufacturing technique might still be tricky, the ability to print customized dosage forms according to patients’needs can revolutionize the concept of drug delivery[23].
4. Conclusion
As such, 3D printing can be one of the major contrivances to realize the concept of personalized medicine at a realistic level.Apart from the possibility of producing personalized scaffolds and organs,the likelihood of preparing dosage forms with precise release profile and dosing strength is a major milestone in the scope of 3D printing.Large scale production of pharmaceuticals might be a long way from now, but personalized medicine is possible soon.
Additive manufacturing also has the capacity to reduce the plethora of intricate manufacturing processes.Complex structure and formulation can be prepared in a single run,and thus,the overall cost and time for 3D printing is lower than that of conventional manufacturing processes. Cost effectiveness may be recognized owing to the low-cost production of items.Large-scale manufacturing is still cheaper by traditional manufacturing processes. Mass production of oral dosage form is rapidly increasing, and the technology is also progressing.Thus,the question of how additive manufacturing can actually benefit the process in terms of space required,time consumption, and economy must be answered. However, smallscale production runs,and on-demand manufacturing is quite feasible for now. 3D printing in the pharmaceutical sector is an interdisciplinary technology that consists of designing,material engineering, pharmaceutics, etc. All divisions come together to play a significant role in the final development processes.
The simplicity with which 3D printing technologies can develop complex formulations in a cost-and time-effective way can provide a solution to difficulties and impossibilities faced by conventional manufacturing processes. Not only that, 3D printing technology can be beneficial from early drug development to personalized medicine for individualized therapy.Along these lines 3D printing can be the“one solution for all”or the Midas touch in pharmaceutics. 3D printing technology needs to be adopted by pharmaceutical sector and should be able to explore the marvels that can be brought by the technology.
Acknowledgment
This research was supported by Keimyung University Research Grant of 2017.
Conflict of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
 Asian Journal of Pharmacentical Sciences2019年5期
Asian Journal of Pharmacentical Sciences2019年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases:Focus on recent advances
- Design,mechanism,delivery and therapeutics of canonical and Dicer-substrate siRNA
- Improving the protective effects of aFGF for peripheral nerve injury repair using sulfated chitooligosaccharides
- Exploring the relationship of hyaluronic acid molecular weight and active targeting efficiency for designing hyaluronic acid-modified nanoparticles
- Redox-sensitive micelles for targeted intracellular delivery and combination chemotherapy of paclitaxel and all-trans-retinoid acid
- Modulating intestinal mucus barrier for nanoparticles penetration by surfactants
