Improving the protective effects of aFGF for peripheral nerve injury repair using sulfated chitooligosaccharides
Ynmei Liu,Fenglin Yu,Beiei Zhng,Meng Zhou,Yu Bei,Yifn Zhng,Jinzhong Tng,Yn Yng,Yong Hung,,Qi Xing,,*,Yueping Zho,Qin Ling,Yng Liu
aInstitute of Biomedicine and Guangdong Provincial Key Laboratory of Bioengineering Medicine,Jinan University,Guangzhou 510632,China
bCollege of Pharmacy,Jinan University,Guangzhou 510632,China
cDepartment of Pharmacy,the First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,China
dDepartment of Stomatology,Jinan University Medical College,Guangzhou 510632,China
Keywords:Acidic fibroblast growth factor Neuroprotection Peripheral nerve injury RSC96 cells Sulfated chitooligosaccharides
ABSTRACT Injury to the peripheral nerves can result in temporary or life-long neuronal dysfunction and subsequent economic or social disability.Acidic fibroblast growth factor(aFGF)promotes the growth and survival of neurons and is a possible treatment for peripheral nerve injury.Yet,the actual therapeutic utility of aFGF is limited by its short half-life and instability in vivo.In the present study,we prepared sulfated chitooligosaccharides(SCOS),which have heparinlike properties,to improve the bioactivity of aFGF.We investigated the protective effects of SCOS with or without aFGF on RSC96 cells exposed to Na2S2O4 hypoxia/reoxygenation injury. Cell viability was measured by MTT assay and cytotoxicity induced by Na2S2O4 was assessed by lactate dehydrogenase (LDH) release into the culture medium. Pretreatment with aFGF and SCOS dramatically decreased LDH release after injury compared to pretreatment with aFGF or SCOS alone.We subsequently prepared an aFGF/SCOS thermo-sensitive hydrogel with poloxamer and examined its effects in vivo.Paw withdrawal thresholds and thermal withdrawal latencies were measured in rats with sciatic nerve injury.Local injection of the aFGF/SCOS hydrogels(aFGF:40,80 μg/kg)increased the efficiency of sciatic nerve repair compared to aFGF(80 μg/kg)hydrogel alone.Especially aFGF/SCOS thermo-sensitive hydrogel decreased paw withdrawal thresholds from 117.75±8.38(g,4 d)to 65.74±3.39(g,10 d),but aFGF alone group were 140.58 ± 27.54 (g,4 d) to 89.12 ± 5.60 (g,10 d) (aFGF dose was 80 μg/kg,P <0.05,n=8).The thermal withdrawal latencies decreased from 11.61±2.26(s,4 d) to 2.37 ±0.67 (s,10 d).However,aFGF alone group were from 17.69 ± 1.47 (s,4 d) to 4.65 ± 1.73 (s, 10 d) (P <0.05, n=8). Furthermore, the aFGF/SCOS hydrogels also exhibited good biocompatibility in mice. In summary, SCOS improved the protective effects of aFGF in RSC96 cells injured with Na2S2O4 and increased the efficiency of nerve repair and recovery of function in rats with sciatic nerve injury.These findings pave an avenue for the development of novel prophylactic and therapeutic strategies for peripheral nerve injury.©2018 Shenyang Pharmaceutical University.Published by Elsevier B.V.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Peripheral nerve injuries are mainly caused by trauma, birth injury,bone dysplasia,elevated levels of lead in the blood,or alcoholism,and are associated with some degree of disruption or complete disturbance of sensory and/or functional abilities in the areas innervated by the affected nerve[1].Since peripheral nerve system acts as a bridge between the central nervous system and the periphery including the internal organs,glands, skeletal muscle, and skin, peripheral nerve injuries may create disability[2].Recently,the proportion of peripheral nerve injuries among trauma patients increased to approximately 2.8% and more than 50% of these patients failed to recover normal motor and sensory functions following treatment [3,4]. The fact that the majority of patients with these types of injuries constitute working population is significant socioeconomic problem nowadays[1].
The sciatic nerve is the thickest peripheral nerve in the human body and extends from the lumbosacral spinal cord to the foot.Given its length,the sciatic nerve is particularly vulnerable to injury[5].Sciatic nerve injury is thought to contribute to sciatica,coccygodynia,pififormis syndrome,and muscle atrophy [6]. At present, there are several surgical techniques available for the treatment of sciatic nerve injury, including direct repair, nerve autografting, tubulization technique, and cell-based treatment.Several growth factors and biomaterials such as hydrogels,films,and aligned fibers have been used to facilitate sciatic nerve repair in preclinical studies [7]; however, additional research is required to determine the safety of these approaches.
Acidic fibroblast growth factor(aFGF or FGF-1)is one of 23 members of the FGF family and is a universal FGF that activates all FGF receptors[8].Previous studies have demonstrated that aFGF promotes the growth and survival of neurons and plays a critical role in the development and function of the nervous system including effects on neural stem cell proliferation and differentiation. Accordingly, aFGF promotes repair and functional recovery after peripheral nerve injury[9].Moreover,aFGF was recently demonstrated to be safe and a feasible treatment in a clinical trial[10].Previous studies indicate that aFGF decreases the number of apoptotic neurons,inhibits the inflammatory response, and improves motor functional recovery after nerve injury[11-13].We previously demonstrated that aFGF regulates the neuronal microenvironment including neurotransmitter release, Aβ pathology, oxidative stress, and signals that mediate apoptosis [14,15]. Yet, the actual therapeutic utility of aFGF is limited by its short half-life,instability in vivo,and undesirable side effects at high systemic concentrations.
Numerous studies have investigated potential biological materials to protect growth factors from inactivation,improve therapeutic delivery to the target area,and provide a microenvironment for nerve repair.Extracellular FGF signals are transduced through fibroblast growth factor receptors(FGFR)in an interaction that requires heparan sulfate proteoglycan(HSPG)and/or Klotho-type co-receptors[16,17].Thus,heparin is often added to growth factor-containing materials to form a hydrogel that immobilizes and protects the high-affinity heparinbound growth factor from degradation, facilitates growth factor release in a sustained fashion, and enhances growth factor binding affinity to receptors on the cell surface[18-23].For example, Wang et al. designed a novel thermo-sensitive aFGF-infused heparin-poloxamer hydrogel to deliver aFGF to the injured spinal cord in a localized and sustained manner[24]; however, heparin has profound side effects as an anticoagulant. Therefore, the development of an ideal material for growth factor delivery that improves binding and stability while minimizing unwanted side effects remains a challenge.
Chitosan oligosaccharide (COS) is the oligomerized product of enzyme-digested chitosan and is widely be used in the pharmaceutical,food,biotechnology,and agricultural sectors [25-27]. Low molecular weight COS molecules are water soluble,nontoxic,biocompatible,and biodegradable [28].The hemostatic, antibacterial, anti-inflammatory, and cell stimulatory activities of COS contribute to its applications.Furthermore,the availability of hydroxy groups on the COS backbone(e.g., hydroxy and amino groups) is useful for modification with alkyl,carboxymethyl,and sulfated groups to improve its solubility,reactivity,biological activities,and degradability under specific physiological conditions[29].According to earlier studies,sulfated chitosan oligosaccharide(SCOS)has heparinlike properties and a similar skeletal structure to heparin.The 6-sulfate group SCOS might play a key role in protein absorption,while the 2-/3-sulfates are thought to be responsible for its protein binding specificity [30].Taken together,these data suggest that SCOS can protect growth factors against degradation [31]; however, no report to date has investigated the interaction of SCOS and aFGF.
In the present study, we prepared SCOS as a sulfated glycosaminoglycan analog with well-defined sulfation sites(3,6-O-sulfated chitooligosaccharides) and investigated its protective effects on aFGF in a cellular assay. We subsequently formulated an injectable thermo-sensitive hydrogel and assessed accumulative aFGF release, growth factor protection, effects on sciatic nerve repair, acute toxicity, and biocompatibility in rats and mice. These results provide important evidence for the therapeutic utility of an aFGF-SCOS composite hydrogel in peripheral nerve injury.
2. Materials and methods
2.1. Materials
COS (molecular weight, 3.5×103Da) was purchased from Zhejiang Jinke Pharmaceutical Co., Ltd (Hangzhou, China).Poloxamer was obtained Germany BASF Corporation (Ludwigshafen, Germany). Reference standard aFGF was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). aFGF stoste(5 mg/ml, 2.34×107IU/ml, NO. 20150429) was synthesized in our laboratory.An aFGF enzyme-linked immunosorbent assay kit was purchased from Wuhan Huamei Biotechnology Co.,Ltd(Wuhan, China). Fetal bovine serum (FBS), trypsin, and Dulbecco’s modified essential medium (DMEM) were purchased from America Gibco Corporation (New York, USA). An electronic BW-YLS-3E instrument was purchased from Shanghai Ruanlong Technology Co., Ltd (Wuhan, China). A LE7406 hot plate analgesia meter was purchased from America Harvard Apparatus Corporation (USA). RSC96 cells were purchased from the Chinese Academy of Sciences(Shanghai,China).
Dimethylformamide was provided from Shanghai Demao Chemical Co.,Ltd (Shanghai,China).Chlorosulfonic acid was purchased from Hubei Jusheng Technology Co., Ltd (Wuhan,China).Absolute ethyl alcohol,sodium hydrate,and Na2S2O4were obtained from Guangzhou Youyi Chemical Co., Ltd(Guangzhou,China).All reagents were analytical grade.
2.2. Animals
Specific pathogen-free male Sprague-Dawley rats(250±20 g)and KM mice(20±2 g,male and female in a 1:1 ratio)with certificate no. 44007200028693 were supplied by the Guangdong Medical Laboratory Animal Center (Guangdong, P.R. China).Rats and mice were kept in different animal room respectively with constant temperature (25±2°C) and humidity(55%±10%)on a 12-h light/dark cycle with free access to water and food.The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Jinan University (ethical review no. 20130301003) and all experiments were conducted in accordance with the National Institutes of Health guide for the care and use of Laboratory animals(NIH Publications No.8023,revised 1996).
2.3. Synthesis of SCOS

Fig.1-The synthetic and Characterization of sulfonated chitosan oligosaccharide(SCOS).(A)Synthetic scheme of SCOS.(B)The infra-red spectrum(IR spectrum)of COS(red)and SCOS(blue).(For interpretation of the 参考文献 to color in this figure legend,the reader is referred to the web version of this article.)
SCOS was synthesized using the method of Gamzazade et al.with some modifications (Fig. 1A) [32]. Briefly, 1.5 g COS was added to 60 ml DMF solution,followed by the addition of 1.2 ml dichloroacetic acid. The mixture was reacted for 24 h. Then,10 ml chlorosulfonic acid was slowly added to 60 ml DMF solution at 0-4 °C to prepare transparent colorless sulfonated reagent. The sulfonated reagent was heated to 60 °C and slowly added to the COS solution and reacted for 2 h. Then,100 ml distilled water was added and the pH was adjusted to 7.0 with 20% NaOH solution,followed by centrifugation of 4000 rpm.The supernatant was washed with ethanol to obtain the precipitate.Then,the precipitate was dissolved in distilled water and dialyzed for 4 d.Finally,the solution in the dialysis bag was concentrated and freeze-dried to yield SCOS.The precipitate was dissolved in distilled water,dialyzed exhaustively with distilled water,and lyophilized.The resultant light brown powder was easily dissolvable in distilled water.The product was characterized by infrared testing and an elemental analysis at room temperature.The results indicated the successful introduction of sulfonated groups at the 3 and 6 positions of COS. Characteristic absorptions derived from sulfate groups on the infrared spectrum at 800,1240,and 1350/cm were assigned to C-O-S,S=O,and S-N (Fig.1B),respectively [33].The results of elemental analysis indicated 12.8% sulfur content and a degree of sulfation value of 2.1(Table.1).
2.4. Cell culture
The RSC96 cell line was obtained from the Cell Bank of the Chinese Academy of Sciences.The cells were cultured in highglucose Dulbecco’s modified Eagle’s medium(DMEM)containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin under a humidified atmosphere of 5% CO2at 37 °C. The culture media were replaced every day and the cells were subcultured for experimental procedures at 80%-90%confluence.
2.5. H/R injury model
The H/R model was prepared following previously published methods with modifications[34,35].The in vitro H/R model was established by adding sodium hydrosulfite (Na2S2O4) to thecultured cells. The cells were treated with 8 mM Na2S2O4at 37 °C in 5% CO2for 6 h, and then the culture media were replaced with normal media for an additional 12 h to generate a reoxygenated condition.

Table 1-Elemental analysis of COS and SCOS.

Table 2-Administration of different proportions of aFGF and SCOS.
2.6. Experimental protocols
(1) To determine the effect of aFGF or SCOS alone on the release of LDH from RSC96 cells after Na2S2O4 injury,and then selected the optimal concentration,cells were treated respectively with different concentrations of SCOS (0,1,5,10,20,40,80 nmol/l) or aFGF(0,0.25,0.5,1,2,4,8,16 nmol/l)for 24 h after HR treatment.
(2) To investigate the effect of different ratios in combination of aFGF and SCOS on the repair of damaged cells, complete medium containing different ratios of aFGF and SCOS (as shown in Table 2) was added respectively for 24 h after HR treatment.And the combination of heparin(3 nmol/l)and aFGF was used as positive control.
2.7. LDH assay
Cytotoxicity induced by aFGF with or without SCOS was assessed by the quantification of LDH release into the culture medium.After the treatment in 2.6,RSC96 cells were washed 3 times with ice-cold phosphate-buffered saline (PBS), suspended in cold lysis buffer, and shaken at 4 °C for 2 h. LDH activity in cell supernatants was determined using a commercially available kit from the Jiancheng Bioengineering Institute(Nanjing,China).
2.8. Preparation and characterization of aFGF/SCOS thermo-sensitive hydrogels
Poloxamer gels are in the liquid state at refrigerated temperatures of 4-5°C and become a gel at physiological temperatures,thereby limiting drug diffusion.This unique property is known as “reverse thermal gelation” and is of significant interest for liquid suppository systems. Poloxamer hydrogel is more practical as an injectable hydrogel vehicle for drug delivery[36].Briefly,20 g poloxamer(407)and 5 g poloxamer(188)were slowly added into 75 ml water for injection and refrigerated at 4°C.And then 7 g glycerol,3 g propylene glycol and 0.5 g Sorbic acid were added to the mixture solution after the solution is completely swelling.And the solution was refrigerated at 4°C after sterilization in a high temperature sterilizing oven.The aFGF and sulfonated chitosan oligosaccharides are mixed in a molar ratio of 1:5 with the gel in a liquid state and uniformly added to the matrix to obtain a clear and transparent aqueous solution (operating on an ice water bath at 4 °C),and the concentration of aFGF is 10 μg/ml(50 000 IU/ml).The solution was free flowing at 4 °C and transformed into a gel at 32 °C(Fig.3A).Furthermore,the solid hydrogel remained stationary when the sample vial was inverted,verifying the high structural strength of the injectable thermo-sensitive hydrogel at physiological temperature [37]. The micromorphology of the prepared aFGF thermo-sensitive hydrogel was observed by scanning electron microscopy(SEM).
2.9. In vitro release profile
A volume of 5 ml liquid hydrogel was added to a dry tube and incubated for 20 min at 37°C to facilitate complete formation of the hydrogel. Then, 2 ml PBS was slowly added to the hydrogel and placed on a shaker (50 r/min) at 37 °C.Every 12 h,the supernatant was aspirated and 2 ml PBS buffer was added back to the hydrogel. The concentration of aFGF was measured in the obtained supernatants using an enzyme-linked immunosorbent assay kit.
2.10. Activity of aFGF released from aFGF/SCOS thermo-sensitive hydrogels
The activity of aFGF in composite hydrogels was analyzed by MTT assay in Balb/c 3T3 cells. Briefly, cells in the logarithmic growth phase were seeded onto 96-well plates (3000 cells/well/200 μl) and grown for 24 h at 37 °C (n=6 wells per group).The culture medium was discarded and aFGF thermosensitive hydrogel was diluted and added to wells for 3 days.On day 3, MTT solution (20 μl, 5 mg/ml final concentration)was added to each well and the assay was completed as described above.
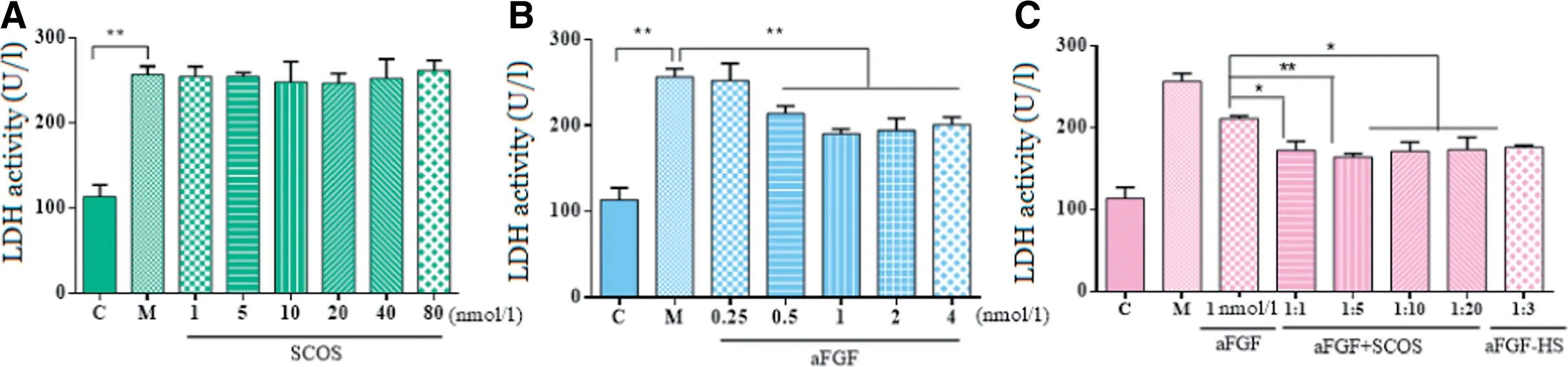
Fig.2-(A)LDH levels in RSC96 cells exposed to Na2S2O4 following treatment with SCOS.(B)aFGF and(C)aFGF+SCOS,C:Control group,cells with no treatment served as the control.M:model group,cells were subjected to HR by Na2S2O4.Data are shown as mean±SD of three independent experiments performed(*P <0.05, **P <0.01).LDH,lactate dehydrogenase;HR,hypoxia/reoxygenation.
2.11. Sciatic nerve injury assay of aFGF/SCOS thermo-sensitive hydrogels
Rats were divided into 7 groups:a control group,sham operation group,model group,blank hydrogel group,aFGF(80 μg/kg)alone thermo-sensitive hydrogel group, and 2 different concentrations of aFGF in aFGF/SCOS thermo-sensitive hydrogel groups(40,80 μg/kg,n=8).Total 30 μl thermo-sensitive hydrogel were injected at the site of the severed sciatic nerve.Mechanical paw withdrawal thresholds and thermal withdrawal latencies were measured to study repair of the transected sciatic nerve.
2.12. Toxicity and biocompatibility studies
2.12.1. Acute toxicity test in vivo
KM mice were divided by sex and subsequently into a dose group and a control group(n=8).At 24 h before treatment,fur was removed from a 4-6 cm2patch on both sides of the back and denuded animals were treated with composite hydrogel containing aFGF (three groups:0, 2500 and 250 000 IU/g) and SCOS.All animals were allowed ad libitum access to food and water for 14 d and observed regularly for mortality or changes in behavior (irritation, restlessness, respiratory distress, abnormal locomotion,and catalepsy).
2.12.2. In vivo biocompatibility
The composite hydrogel was injected into mice subcutaneously to analyze host responses to the gel and in situ gel degradation. Briefly, 8-week-old KM mice weighing 18-22 g were injected subcutaneously with 100 ml of hydrogel solution (n=8) on both sides of the back under sterile conditions[38].Saline(100 ml)was injected as a control.Mice were euthanized and samples were harvested at 7,14,and 21 days after injection.Tissues were fixed in 10%neutral buffered formalin for 15 min and subjected to histological analysis. Tissue sections were stained using standard protocols for hematoxylin and eosin. No significant lymphocytic infiltration or foreignbody granulation was observed.
2.13. Statistical analysis
All numerical data were expressed as the mean±SD(standard deviation)at least 3 independent experiments.The SPSS 10.0 software package was used for statistical tests. The trial involved multiple dosing groups and MTT data were compared between groups using one-way ANOVA followed by Tukey’s test.The threshold for statistical significance was P <0.05
3. Results and discussion
3.1. SCOS improves the protective effects of aFGF in RSC96 cells
SCOS alone did not have any significant effects on LDH release in RSC96 cells exposed to HR injury(P >0.05).In contrast,aFGF(0.25,0.5,1,2,or 4 nmol/l)significantly decreased supernatant LDH activity compared to the HR control group(P <0.05).Combination of aFGF (1 nmol/l) with SCOS (molar ratio of aFGF:SCS were 1:1,1:5,1:10,or 1:20)had a larger negative effect on LDH release than did corresponding concentrations of aFGF alone(P <0.05).Therefore,aFGF protected RSC96 cells against HR injury, and this effect was enhanced by combination of aFGF with SCOS. A comparison of aFGF: SCOS molar ratios revealed that LDH release was lowest when the molar ratio was 1:5.
Some evidence suggests that the interaction of FGF with heparan sulfate is critical for FGF activity, and that heparan sulfate has lower binding affinity for aFGF than heparin due to the presence of a 6-sulfate group [39]. A comparison of aFGF(1 nmol/l):SCOS(1:5)to aFGF: heparin (1:3) did not reveal any significance differences(Fig.2).
3.2. Characterization of the aFGF/SCOS thermo-sensitive hydrogel
aFGF/SCOS thermo-sensitive hydrogel was sliced, sprayed,and observed under SEM at various magnifications.As shown in Fig. 3A, the hydrogel formed upon heating to 37 °C and melted to a liquid when cooled to 4 °C. This sol - gel transition first allowed good mixture of aFGF into the poloxamer solution at 4 °C. Then, gelation at 37 °C facilitated successful loading. The inner structure of the aFGF thermosensitive situ hydrogel was highly porous and interconnected,which allowed aFGF to diffuse through the 3D network and realize slow release(Fig.3B).

Fig.3-Characterization of thermo-sensitive hydrogel.(A)aFGF thermo-sensitive hydrogel formation diagram.(B)The scanning electron microscope(SEM)analysis show the aFGF thermo-sensitive hydrogel is porous crisp structure.(C)The cumulative release of active aFGF from aFGF/SCOS thermo-sensitive hydrogel and aFGF hydrogel of without SCOS;(D)The cumulative dissolution of aFGF/SCOS hydrogel and aFGF hydrogel of without SCOS.
The dissolution rate of thermo-sensitive hydrogel (A) and the cumulative release of aFGF (B) are shown in Fig. 3C and 3D. The results suggested that the release behavior of the thermo-sensitive hydrogel was consistent with the zero-order kinetic equation. The dissolution and dissociation curves of the aFGF/SCOS thermo-sensitive hydrogels had a good linear relationship, indicating that the release of aFGF was likely controlled by dissolution.
The bioactivity of aFGF in the thermo-sensitive hydrogel was detected by MTT assay. The bioactivities of aFGF were 4.56×104,4.54×104,and 4.47×104IU/ml for 3 hydrogel samples;these values were close to the labeled amount(4.68×104IU/ml).
3.3. Sciatic nerve injury assay
Peripheral nerves can regenerate to compensate for functional deficits; however, clinical and experimental evidence shows that regeneration is usually difficult to achieve with unsatisfactory results,especially after severe injury[40-42].In our study,we used a sciatic nerve transection model to simulate severe injury.Mechanical paw withdrawal thresholds and thermal withdrawal latencies were measured at 1, 4, 10, and 25 days post-injury (Fig. 4A and 4B). The nerve transection model increased mechanical paw withdrawal thresholds and thermal withdrawal latencies from 4 d post-injury,and then thresholds gradually recovered during the 25-day study period. Treatment with aFGF alone and aFGF/SCOS thermo-sensitive hydrogels (aFGF 40 or 80 μg/kg) decreased withdrawal thresholds and latencies at all time points compared to the model group (P <0.01). Especially aFGF/SCOS thermo-sensitive hydrogel decreased paw withdrawal thresholds from 117.75±8.38(g,4 d)to 65.74±3.39(g,10 d),but aFGF alone group were 140.58±27.54(g,4 d)to 89.12±5.60(g,10 d)(P <0.05, n=8). The thermal withdrawal latencies decreased from 11.61±2.26 (s,4 d) to 2.37±0.67 (s,10 d),however aFGF alone group were 17.69±1.47 (s, 4 d) to 4.65±1.73 (s, 10 d)(P <0.05 n=8). These findings suggest that the aFGF/SCOS thermo-sensitive hydrogel promoted sciatic nerve repair.

Fig.4-Sciatic nerve protection of aFGF/SCOS thermo-sensitive hydrogel.(A)The paw withdrawal threshold to mechanical stimulation of rats in different groups.(B)The thermal withdrawal latency of rats in different groups.(C)The healing condition of severed sciatic nerve in different group.(a)Sham;(b)Model;(c)Blank hydrogel;(d)aFGF(40 μg/kg)thermo-sensitive hydrogel;(e)aFGF(40 μg/kg)/SCOS thermo-sensitive hydrogel;(f)aFGF(80 μg/kg)/SCOS thermo-sensitive hydrogel.Data are shown as mean±SD of 8 independent experiments performed.
Additionally, nerve healing was observed in aFGF/SCOStreated mice compared to model mice on Fig.4C and 4D.The image shown in Fig.4 was sampled from at least 8 replicates and was consistent with behavioral observations.
3.4. Acute toxicity
Treatment with the aFGF/SCOS thermo-sensitive hydrogel(2500 and 250 000 IU/g bioactivity) had no significant effects on mouse body weight and did not induce any clinical signs of toxicity either immediately or during the post-treatment period.These findings suggested that the substance was nontoxic. Furthermore, there were no visible effects on the liver,heart,spleen,kidney,or pancreas(Fig.5A).
3.5. Biocompatibility
In order to investigate host responses to the aFGF/SCOS thermo-sensitive hydrogel,we examined changes in response to the highest possible concentration of aFGF/SCOS thermosensitive hydrogel.All the animals survived without evidence of malignant infection or abscess at the injection sites.Tissues adjacent to the polymer showed native histological structure with regular muscle alignment and cell morphology at 7, 14,and 21 d post-injection (Fig.5B).Histology did not reveal any signs of an inflammatory reaction,indicating that the hydrogel had good biocompatibility.
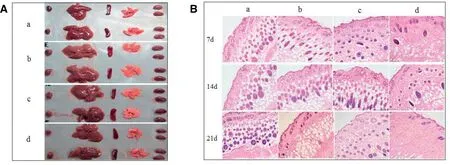
Fig.5-Quality safety evaluation of aFGF/SCOS thermo-sensitive hydrogel.(A)The appearance morphology of heart,liver,spleen,lung and kidney of mice in acute toxicity test(g,mean±SD,n=8).(B)Histopathological analysis of skin treated with aFGF thermo-sensitive hydrogel in biocompatibility test(H&E×100):(a)Control;(b)Blank Hydrogel;(c)aFGF(2500 IU/g)/SCOS thermo-sensitive hydrogel;(d)aFGF(250 000 IU/g)/SCOS thermo-sensitive hydrogel.
In brief, our findings suggested that aFGF/SCOS (3, 6- sulfate) had the greater ability to promote nerve injury repair compared to aFGF or SCOS alone.Pretreatment with aFGF and SCOS dramatically decreased LDH release after injury compared to pretreatment with aFGF or SCOS alone (P <0.01).SCOS increased the efficiency of nerve repair and recovery of function of aFGF in rats with sciatic nerve injury compared with aFGF alone (P <0.05). One possible explanation for this observation is that the 6-sulfate group of SCOS (3,6-sulfate) bound aFGF to promote stability while the 3-sulfate group exerted effects on cellular adhesion and spreading.Alternatively,our observation may be related to the electrostatic interaction of negatively charged sulfonic acid groups with positively charged lysine or arginine residues in aFGF.
As everyone knows, heparin is often used as a bridge to modify GFs to achieve their combinatorial activity, but there are a few unwanted side effects such as thrombocytopenia and bleeding occurred when heparin applied to the clinic[43].Bickler P, et al. reported three cases of bleeding at femoral and sciatic catheter sites in patients receiving a single daily dose of enoxaparin(40 mg)[44].Exogenous heparin might inhibit BMP-2 osteogenic bioactivity and reduces BMP-2 signaling. Short-term use of heparin after bone fracture even can delay bone healing and long-term treatment with heparin can increase the risk of osteoporosis[45].The side effects of heparin could be ignored in the transcutaneous medication for its poor absorptivity.But if it was used in vivo administration,we should consider the risk of systemic administration.In our research, we used aFGF in-situ forming techniques to repair peripheral nerve injure,which need to inject drugs into body.So we should consider the risk of systemic administration of heparin.We have looking for alternative products of heparin.SCOS has a similar skeletal structure to heparin, which can replace heparin to achieve certain biological functions of heparin and avoid its side effects. Fungal sulfated chitosan was more effective in the decrease of platelet adhesion in comparison with heparin,which meant sulfated chitosan maybe reduce the risk of thrombocytopenia and bleeding [46]. Ding et al. also reported that sulfated chitosan can promote the neural differentiation of ESCs [47].Based on the above,SCOS would have superiority than heparin in nerve injury healing field.
According to the results of in vivo and in vitro, the aFGF/SCOS thermo-sensitive hydrogel could load and stabilize aFGF and performed a series study of sciatic nerve injury efficiency in vivo.Irrespective of the molecular mechanism of aFGF in nerve injury healing details,enhancement of restore by aFGF/SCOS thermo-sensitive hydrogel are likely to be(i) for the similar skeletal structure to heparin, SCOS could specific bind with aFGF for a complete synergy of action, (ii)the hydrogel formed upon heating to 37 °C and melted to a liquid when cooled to 4 °C. We can load aFGF at 4 °C, which is very advantageous to kept bioactivity stable of aFGF, (iii)the inner structure of the aFGF thermo-sensitive hydrogel was highly porous and interconnected,which provided effectively guaranteed to stabilize loaded proteins, facilitate aFGF binding and releases slowly.
On the other hand,aFGF plays a pivotal role in regulating fibroblasts, which are central to wound healing. The role of aFGF in wound healing and the development of relevant preparations have been relatively mature, but the function and application of aFGF in nerve injury healing has not been deeply explored. In our laboratory, aFGF has been used to repair injured nerve and obtained some interesting results[15,48-50]. Exogenous administration of haFGF has been shown to prevent degeneration and apoptosis of neurons.aFGF is involved in the regulation of synaptic plasticity, processes attributed to learning and memory through improving cholinergic nerve functions. aFGF also can repaired human spinal cord injury in a clinical trial.In our Lab’s latest research,aFGF binds to its receptor,activating the PI3K pathway,leading to CREB activation followed by the splicing of XBP1u mRNA to XBP1s mRNA in the ER.aFGF upregulates ADAM10 to attenuate the Alzheimer Phenotype of APP/PS1 Mice through the PI3KCREB-IRE1a/XBP1 Pathway[15].But aFGF how to repair sciatic nerve is still uncertain. Additional studies are required to confirm the present observations and clarify the mechanism of action of our novel aFGF/SCOS thermo-sensitive hydrogel.
Nerve injury is still incompletely clear, and the specific medicine and therapeutic measure are also totally unavailable yet.So it has many advantages,and remarkable and practical clinical significance to expand the application of aFGF in nerve injury.
4. Conclusion
Acidic fibroblast growth factor(aFGF)plays an important role in biological regeneration. Yet, its clinical application thus far has been limited by a short half-life and instability in vivo.SCOS is readily prepared by modifying COS with chlorosulfonic acid to realize combination with aFGF to delayed diffusion and protect growth factor bioactivity. We tested the protective and nerve-regenerative effects of a thermosensitive hydrogel employing poloxamer as the skeleton material and aFGF combined with SCOS. aFGF contained in thermo-sensitive hydrogel was released with dissolution of the hydrogel,protected against HR injury in RSC96 cells,and promoted repair of the injured rat sciatic nerve. The present work provides a novel therapeutic delivery platform for treating peripheral nerve injury.
Declaration of interest
The authors report no conflicts of interest. The authors are responsible for the content and writing of this paper.
Acknowledgments
This work was supported by the Science and Technology Program of Guangzhou (201508020001), the Major Scientific and Technological Special Project of the Administration of Ocean and Fisheries of Guangdong Province (Yuecainong, 2017, no.17), the Operating fund of Guangdong Provincial Key Laboratory of Bioengineering Medicine (No. 2014B030301050), a project of the Research Development and Industrialization of Guangdong Province (2013B090500046), and the Guangdong Province Higher Vocational Colleges and Schools Pearl River Scholar Funded Scheme(2012).
 Asian Journal of Pharmacentical Sciences2019年5期
Asian Journal of Pharmacentical Sciences2019年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Complex formulations,simple techniques:Can 3D printing technology be the Midas touch in pharmaceutical industry?
- Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases:Focus on recent advances
- Design,mechanism,delivery and therapeutics of canonical and Dicer-substrate siRNA
- Exploring the relationship of hyaluronic acid molecular weight and active targeting efficiency for designing hyaluronic acid-modified nanoparticles
- Redox-sensitive micelles for targeted intracellular delivery and combination chemotherapy of paclitaxel and all-trans-retinoid acid
- Modulating intestinal mucus barrier for nanoparticles penetration by surfactants
