Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases:Focus on recent advances
Xioqin Niu,Jiejin Chen,b,Jinqing Go,*
aInstitute of Pharmaceutics,College of Pharmaceutical Sciences,Zhejiang University,Hangzhou 310058,China
b Key Laboratory of Cancer Prevention and Intervention,the Second Affiliated Hospital,College of Medicine,Zhejiang University,Hangzhou 310058,China
Keyword:Neurodegenerative diseases Blood-brain barrier Nanocarriers Neurotoxicity
ABSTRACT Neurodegenerative diseases including Alzheimer’s disease,Parkinson’s disease,Huntington disease and amyotrophic lateral sclerosis throw a heavy burden on families and society.Related scientific researches make tardy progress. One reason is that the known pathogeny is just the tip of the iceberg. Another reason is that various physiological barriers, especially blood-brain barrier(BBB),hamper effective therapeutic substances from reaching site of action. Drugs in clinical treatment of neurodegenerative diseases are basically administered orally. And generally speaking, the brain targeting efficiency is pretty low. Nanodelivery technology brings hope for neurodegenerative diseases. The use of nanocarriers encapsulating molecules such as peptides and genomic medicine may enhance drug transport through the BBB in neurodegenerative disease and target relevant regions in the brain for regenerative processes.In this review,we discuss BBB composition and applications of nanocarriers-liposomes,nanoparticles,nanomicelles and new emerging exosomes in neurodegenerative diseases.Furthermore,the disadvantages and the potential neurotoxicity of nanocarriers according pharmacokinetics theory are also discussed.©2018 Shenyang Pharmaceutical University.Published by Elsevier B.V.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
The real cause of neurodegenerative disease is like a mystery and still under research.Neurodegenerative disease including Alzheimer’s disease(AD),Parkinson’s disease(PD),Huntington disease(HD)and amyotrophic lateral sclerosis(ALS).AD is the most common form of dementia and contribute to 60%-70%of the cases.Extracellular deposits of Aβ peptide and intraneuronal accumulations of tau protein in the brain are the main histopathological features of AD [1]. PD is another chronic progressive neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra and pathological aggregation of the intrinsically disordered protein α-synuclein into Lewy bodies [2],which affects 1% of the population over 65 years old [3]. ALS is an incurable and fatal progressive degenerative disease involving motoneuron cell death.And the pathophysiology is complex and the causes of motoneuron degeneration still awaits clarification[4].HD,a genetic neurodegenerative disease,is caused by abnormal trinucleotide repeat sequences(CAG)expansion[5].

Fig.1-Schematic representation of the BBB and various transport processes across the brain endothelial layer.
There are limited clinical treatments for neurodegenerative diseases which almost cannot cure but only slow down the disease progress.Indeed,in the clinical treatment of AD,only four drugs have been available since mid-1990s,including the cholinesterase inhibitors donepezil, rivastigmine, galantamine and the glutamatergic antagonist memantine[6].Compared with the drugs used in the treatment of AD, the therapy is more limited than the one described for PD, HD and ALS. Some valuable treatment substances, such as peptides[7-10] and genomic medicines [11-13] usually cannot reach the site of action because of the existence of BBB and their poor access to the brain,extensive first-pass metabolism and possible side effects when reaching non-target peripheral tissues.
The fragile brain parenchyma is protected from the periphery by the BBB [14],which is consisted of polarized endothelial cells connected by tight junctions of the cerebral capillary endothelium and plenty of transporters, resulting extremely low permeability and limiting the delivery of drugs to the central nervous system(CNS)[15,16].BBB functionality is dynamically regulated by an ensemble of different cell types,such as astrocytes,pericytes,and neurons [17].In fact,98% of all potent drugs that may improve therapy of various diseases of the central nervous system(CNS)are not in clinic because of their inability to cross the BBB[18].Most hydrophilic molecules are unable to cross the BBB because of the compact linking between the brain endothelial cells which prevents paracellular transport. At the same time, the limited cross BBB ability of major lipophilic small molecules is caused by efflux transporters like the p-glycoprotein(Pgp),multidrug related protein(MRP) or breast cancer resistance protein (BCRP) transporters[19-21]. A host of brain-targeting researches were carry on glioma[22-27].Although the structure and function of BBB can be changed in neurodegenerative diseases, the barrier function of BBB is still a main obstacle in the treatment neurodegenerative diseases[28-32].It is of prime significance to investigate different vehicles which can enhance the BBB transport bility of therapeutic drugs to target area.
There are a number of receptors present on the surface of BBB, particularly for different proteins, peptides, antibodies.Such molecules are used as surface-active ligands and assist the translocation via receptor-mediated transcytosis (Fig. 1).At the same time,the cationic vehicle crosses the BBB via absorption mediated transcytosis.One more strategy is vehicle mediated transcytosis that utilizes some nutrients like glucose and glutathione, binding to the surface of vehicle and facilitating its translocation [33,34]. Some examples of commonly used nanocarriers are liposomes, nanoparticles, nanomicelles and exosomes. Drug delivery using nanocarriers may increase the bioavailability and stability of drugs and decrease the peripheral toxicity.
2. Liposomes:classic dosage form to penetrate BBB
Liposomes with structure similar to cell membrane are biodegradable colloids and can be employed to carry a wide range of hydrophobic and hydrophilic pharmaceuticals,such as small molecules [35], peptides [36], proteins [37] and RNAs [38], without changing their function and protecting them against degradation and potential immune responses[39](Fig.2).Its unique phospholipid bilayer structure which is similar to physiological membrane had made it easier to penetrate BBB and helps therapeutic molecules to enter the brain.They are probably the most studied and clinically recognized nanocarriers owing to their long track record,low toxicity and ability to deliver both hydrophilic and lipophilic compounds[40]. However, there are several limitations of liposomes, including fast systemic elimination, quick metabolic degradation of the phospholipids,stability issue after extended storage,inability to provide sustained release of drugs and moderately efficient for the entrapment of lipophilic compounds[41].
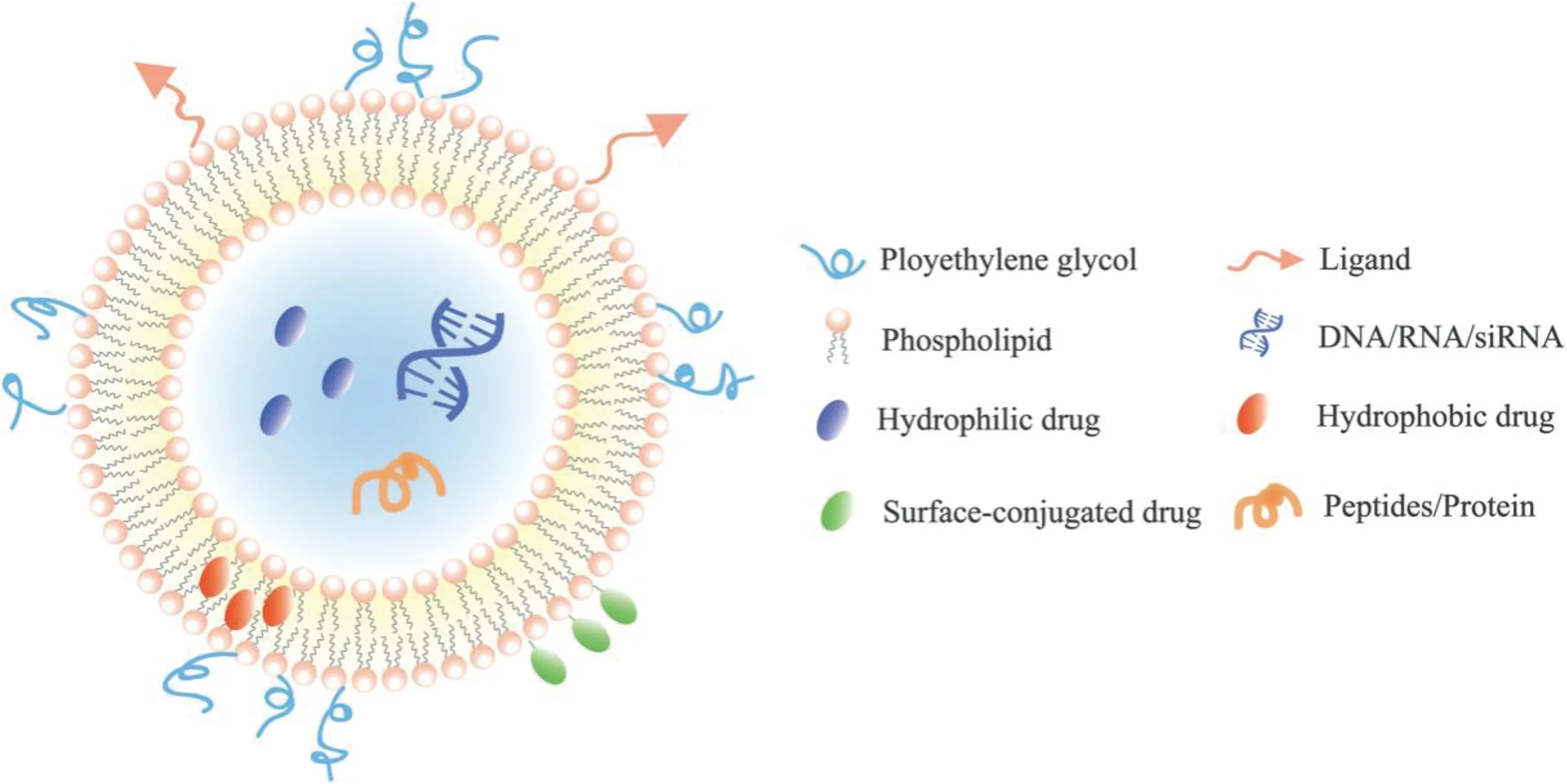
Fig.2-A schematic representation of classical liposome.
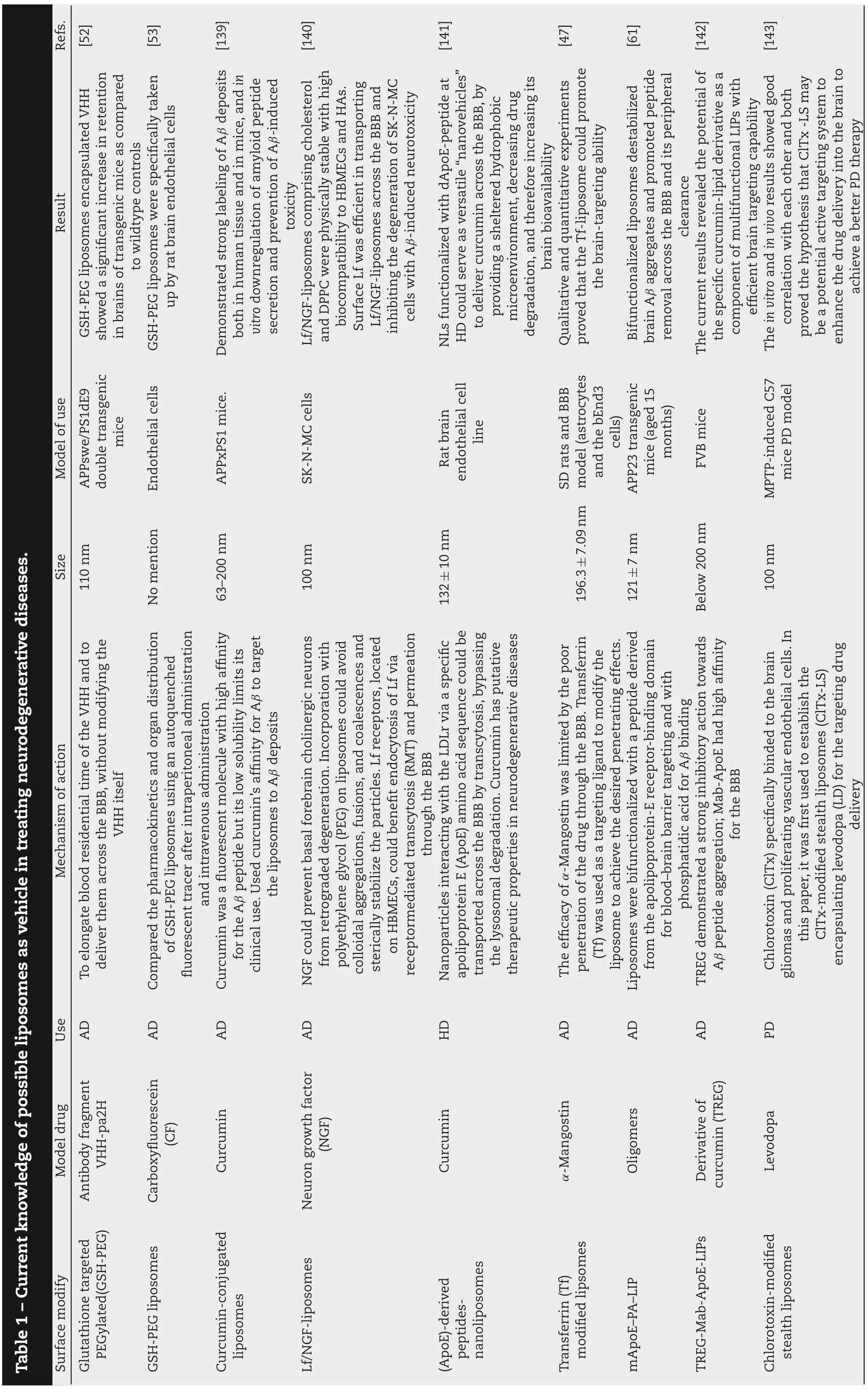
Nowadays, a plenty of modifications have been made in the liposomal surface to improve its brain targeting ability.With the help of some specific ligands(glucose[42],lactoferrin[43],transferrin[44],specific peptides[45]),liposome can efficiently crosses the BBB and deliver the drug to the particular site(Table 1).
2.1. Transferrin modified liposomes
Liposomes can be conjugated to either transferrin protein or transferrin monoclonal antibody such as OX-26[46].Our previous study constructed liposomes modified by transferrin(Tf)loading the a potential candidate AD drug, α- Mangostin(α-M).In vitro studies demonstrated that the Tf(α-M)liposome could cross the BBB in the form of an integrated liposome and in vivo studies on the α-M distribution in the brain demonstrated that the Tf(α-M)liposome improved the brain delivery of α-M[47].Transferrin receptors(TfR)as one of receptors,are of special interest for delivery therapeutic agents across BBB in order to enhance the targeting efficiency[14].The receptor is a transmembrane glycoprotein with two subunits of 90 kDa that are linked by a disulfide bridge, and each of these subunits can bind to one molecule of transferrin[48].However,as a target delivery system, there are some problems about TfR must be emphasized.
(1) TfR is also expressed on hepatocytes, monocytes, erythrocytes, intestinal cells, epithelial cells of choroid plexus and neurons besides BBB.Thus,TfR targeted liposomes also have a high distribution in the liver and kidneys[49].
(2) The high concentration of transferrin present in serum(~2 μmol/l) creates a significant problem, since any TfR expressed on endothelial cells is typically saturated with endogenous transferrin under physiological conditions[50].So the targeting ability is not efficient enough.
2.2. Glutathione modified liposomes
The sodium-dependent (active) glutathione transporter, as another mention receptor, also has a preferential expression in CNS and BBB and is present in all mammalian species [51]. Glutathione (GSH) is an endogenous tripeptide that plays a central role in the detoxification of intracellular metabolites, which has a well-established and good safety profile by exogenously administration.The GSHPEG liposomes whether based on 1,2-dimyristoyl-sn-glycero-3-phosphocholine(DMPC) or egg-yolk phosphatidylcholine(EYPC),both encapsulated 15 kDa amyloid beta binding llama single domain antibody fragments (VHH-pa2H), showed a rather slower blood clearance profile and a higher concentration amount in perfused brains on statistics than free VHHpa2H,which provided evidence that the glutathione was suitable for specific delivery of targeted drugs,antibody fragments beyond the BBB into the brain [52]. The brain target ability of GSH-PEG liposomes were also proved by using fluorescent tracer,carboxyfluorescein(CF).Significantly more fluorescent tracer was found in RBE4 cell homogenates incubated with GSH-PEG liposomes compared to non-targeted PEG liposomes and 4-fold higher brain levels of fluorescent tracer was found after intravenous injection of GSH-PEG liposomes compared with PEG control liposomes in the microdialysis study[53].
2.3. Cationic liposomes
Among all the modification in the liposomes, cationic liposomal drug vehicles are widely used, which would be able to take advantage of the BBB’s negative charge and consequently trigger the cell internalization processes through electrostatic interactions [54,55]. The effect of liposome surface charge on brain tissue uptake was investigated by injection of cationic,anionic,and charge-neutral liposomes into the internal carotid arteries of Sprague-Dawley rats[55],which showed surface charge mediates cellular interactions of liposomes:both positive and negatively charged liposomes interacted with cells more extensively than non-charged liposomes but the cationic liposome had the highest concentration in brain[56-58]. However, anionic phospholipids(phosphatidic acid and cardiolipin)is applied to form liposome showing excellent affinity to neurotoxic beta-amyloid peptide (Aβ) [59], which demonstrated that cationic liposome have advantage to cross BBB while anionic liposome is excellent in beta-amyloid peptide targeting.
es as vehicle in treating neurodegenerative diseases.Table 1-Current knowledge of possible liposom Refs.[52][53][139][140][141][47][61][142][143]es encapsulated VHH ice,and in pared es were specifically taken showed a significant increase in retention ay ice as com Demonstrated strong labeling of Aβ deposits prising cholesterol vitro downregulation of amyloid peptide es destabilized secretion and prevention of Aβ-induced GSH-PEG liposom Result in brains of transgenic m to wildtype controls toxicity and DPPC were physically stable with high up by rat brain endothelial cells patibility to HBMECs and HAs.Surface Lf was efficient in transporting es across the BBB and inhibiting the degeneration of SK-N-MC cells with Aβ-induced neurotoxicity NLs functionalized with dApoE-peptide at HD could serve as versatile“nanovehicles”to deliver curcumin across the BBB,by providing a sheltered hydrophobic microenvironment,decreasing drug degradation,and therefore increasing its ents clearance GSH-PEG liposom an tissue and in m es com both in hum Lf/NGF-liposom brain bioavailability biocom Lf/NGF-liposom Qualitative and quantitative experim proved that the Tf-liposome could promote the brain-targeting ability Bifunctionalized liposom brain Aβ aggregates and promoted peptide removal across the BBB and its peripheral The current results revealed the potential of the specific curcumin-lipid derivative as a component of multifunctional LIPs with efficient brain targeting capability The in vitro and in vivo results showed good correlation with each other and both proved the hypothesis that ClTx-LS m be a potential active targeting system to enhance the drug delivery into the brain to achieve a better PD therapy Model of use APPswe/PS1dE9 double transgenic mice Endothelial cells ice.odel APPxPS1 m SK-N-MC cells Rat brain endothelial cell line SD rats and BBB model(astrocytes and the bEnd3 cells)APP23 transgenic mice(aged 15 months)ice FVB m MPTP-induced C57 mice PD m Size 110 nm ention No m 63-200 nm 100 nm 132±10 nm 196.3±7.09 nm 121±7 nm Below 200 nm 100 nm es using an autoquenched e of the VHH and to odifying the its its inistration es could avoid odify the ain Mechanism of action ited by the poor es(ClTx-LS)To elongate blood residential tim deliver them across the BBB,without m VHH itself acokinetics and organ distribution inistration olecule with high affinity es to Aβ deposits ino acid sequence could be for the BBB delivery Compared the pharm of GSH-PEG liposom fluorescent tracer after intraperitoneal adm and intravenous adm in was a fluorescent m the liposom retrograded degeneration.Incorporation with through the BBB al degradation.Curcumin has putative the apolipoprotein-E receptor-binding dom for blood-brain barrier targeting and with phosphatidic acid for Aβ binding onstrated a strong inhibitory action towards odified stealth liposom Curcum for the Aβ peptide but its low solubility lim clinical use.Used curcumin’s affinity for Aβ to target NGF could prevent basal forebrain cholinergic neurons from polyethylene glycol(PEG)on liposom colloidal aggregations,fusions,and coalescences and sterically stabilize the particles.Lf receptors,located on HBMECs,could benefit endocytosis of Lf via receptormediated transcytosis(RMT)and permeation Nanoparticles interacting with the LDLr via a specific apolipoprotein E(ApoE)am transported across the BBB by transcytosis,bypassing the lysosom therapeutic properties in neurodegenerative diseases The efficacy of α-Mangostin was lim penetration of the drug through the BBB.Transferrin(Tf)was used as a targeting ligand to m liposome to achieve the desired penetrating effects.Liposomes were bifunctionalized with a peptide derived from TREG dem Aβ peptide aggregation;Mab-ApoE had high affinity Chlorotoxin(ClTx)specifically binded to the brain gliomas and proliferating vascular endothelial cells.In this paper,it was first used to establish the ClTx-m encapsulating levodopa(LD)for the targeting drug Use AD AD AD AD HD AD AD AD PD Model drug ent Antibody fragm VHH-pa2H Carboxyfluorescein(CF)in in Curcum Neuron growth factor(NGF)Curcum α-Mangostin ers Oligom Derivative of in(TREG)curcum Levodopa es PEGylated(GSH-PEG)GSH-PEG liposom odify es in-conjugated Glutathione targeted es Surface m Curcum liposomes Lf/NGF-liposom(ApoE)-derived peptidesnanoliposomes Transferrin(Tf)modified lipsomes mApoE-PA-LIP TREG-Mab-ApoE-LIPs Chlorotoxin-modified stealth liposom
2.4. PEG modified liposomes
Coating liposomes with polyethylene glycol (PEG) further ensures a prolonged circulation time in plasma allowing prolonged dosing intervals and enhanced the chemical stability of the liposomes in serum, which have been applied widely and studied extensively [60]. Significantly, PEG of different chain length has effect on the brain-targeting efficiency.Thus in this study,the characteristics of glucose-modified liposomes using PEGs with different chain lengths (PEG200,PEG400,PEG1000,and PEG2000)as the linkers were compared and evaluated both in vitro and in vivo in order to explore their difference and determine the optimal length of PEG for drug delivery[42].The result suggested that glucose-modified liposomes linked by PEGs with longer chain length showed a better efficiency to promote the drug transport across the BBB barrier in a time-dependent manner and liposomes linked by PEG1000 showed the best brain-targeted property at each time point in vivo experiment.It is because PEG with longer chain length may decrease the brain-targeted efficiency of the liposome because of the steric hindrance,while PEG with shorter chain length may obstruct the exposure of the ligand.
2.5. Multifunctional liposomes
Multifunctional liposomes were also used in treating AD,aiming to target sequentially. Liposomes were bifunctionalized with a peptide derived from the apolipoprotein-E receptorbinding domain for blood-brain barrier targeting and with phosphatidic acid for Aβ binding,which destabilized brain Aβ aggregates and promoted peptide removing across the BBB and its peripheral clearance[61].Similarly,multifunctional liposomes were also used in treating glioma to realize dualtargeting effect and precision distribution in the brain.Dualtargeting doxorubincin(Dox)liposomes were produced by conjugating liposomes with both folate and transferrin, which were proven effective in penetrating the BBB and targeting tumor[62].
3. Nanoparticles:inorganic materials and organic materials
Nanoparticles are colloidal systems with compact structure where the therapeutic agent is either entrapped within the colloid matrix or coated on the particle surface by conjugation or adsorption [41]. Nanoparticles, using inorganic materials and organic materials as the core to permeate BBB,have been further studied in recent years (Table 2). Inorganic materials include gold, iron, cerium, molybdenum, silica while organic materials cover trehalose,PLGA and PLA.The reason why nanoparticles are being widely used in treating neurodegenerative disease is that they show distinct characteristics as follows.
(1) Nanoparticles possess relatively high drug loading and small size,and deliver the active ingredient to the specific site at a controlled and sustained rate during the transportation.
(2) Nanoparticles, especially inorganic nanoparticles, show excellent imaging performance.
(3) Part of the nanoparticles material themselves have a certain cure efficacy,such antioxidant,reducing ROS level and even inhibiting Aβ aggregation.It makes nanoparticles as pretty promising vehicles in neurodegenerative treatment.
Although the nanoparticles present a series of superior performance,some problems still should be highlighted,such as particle aggregation, nano-toxicity and use of organic solvents during fabrication.
3.1. Gold nanoparticles
Among various nanoparticles, gold nanoparticles (AuNPs)have intrigued great interest because of their relative low cytotoxicity, predominant optical properties fit for detection/imaging, well-established synthesis methods, and the potential capability to cross the BBB by altering size or coupling various modified ligand. Besides, gold nanoparticles themselves can prevent cognitive deficits,oxidative stress and inflammation in AD rat model[63],which suggested that gold nanoparticles may be a prospective material for AD.
Peptide inhibitors, VVIA and LPFFD [64] and antioxidant anthocyanin [65] were also loaded in AuNPs, both of which showed inhibition of amyloid β-protein aggregation and cytotoxicity. AuNPs modified by PEG were more effective compared to AuNPs alone in preventing neurodegenerative diseases [66].There were also some AuNPs researches targeting insulin-like growth factor(IGF)receptor in the brain to deliver anti-neurodegenerative drug. It was worth noting that there were two IGF receptor,IGF-1 and IGF-2 receptors,on the brain capillaries[67].It is possible to cause hypoglycemia when targeting IGF receptors.
AuNPs also have advantage in imaging. The insulintargeted gold nanoparticles can serve as computed tomography (CT) contrast agents to highlight specific brain regions in which they accumulate[68].Dual-functionalized gold nanoplasmonic particles can also monitor cerebral β-amyloid peptides in AD[69].
The size of AuNPs makes a difference in the biodistribution and circulation time.Different size(20,50 and 70 nm)insulin coated gold nanoparticles (INS-GNPs) were synthesized to quantitatively test their ability of crossing BBB in Balb/C mice [70]. After 2 h injection, 20 nm INS-GNPs showed the most widespread biodistribution and highest accumulation within the brain, which indicated biodistribution and circulation time of INS-GNPs was size dependent.Compared with the larger INS-GNPs,the smaller one had advantages in blood circulation time.The size affected experimental result,which was also reproduced AuNPs modified by L-glutathione in different size (36.0±3.0 nm,18.1±3.0 nm,and 6.0±2.0 nm).Dynamic light scattering(DLS) experiments showed the AuNPs with smaller size could be more efficient in preventing Aβ peptides from aggregation to larger oligomers and thus avoided nucleation to form fibrils,which was not confined to a specific ligand,but would be a more general one[71].This is crucially significant for developing novel AD therapy method because oligomers are the main reason of Aβ toxicity.

Table 2-Current knowledge of possible nanoparticles as vehicle in treating neurodegenerative diseases.Refs.[75][93][79][64][95][92][94]The therapeutic nanoparticles TAT-NFH-nBSA could efficiently powerful neuroprotective effect both in accumulate in the brain and produce a vitroand in vivo for PD treatment.These findings indicated that this novel iron chelator system with a high affinity for e,and delayed saturation feature can be a valuable tool for PD therapy in the ore odel odels of odified with uch m yloid Result Fe ions,long in vivo lifetim future Brain delivery of SA was m effective with SA-NPs than with SA suspension.In addition,the SA-NPs exerted strong neuroprotective effects in zebrafish and cell culture m es,reducing tau PD agnetic nanoparticles could ory of transgenic em a single ligand loaded NPs BBB.Superparam provide effective repair in a PD m in vitro and in vivo and further inhibit apoptosis.As a result,α-syn expression was reduced,thus preventing the toxic effects of α-syn on the cell and suppressing apoptosis These structural features promoted its synergetic interactions with Aβ on AuNP surface,leading to strong inhibitions of Aβ oligomerization and fibrillation and the cytotoxicity caused by the aggregation species TQNP/H102 obtained better ability in decreasing amyloid plaques,increasing Aβ-degrading enzym protein phosphorylation,protecting synapses and improving the spatial learning and m mice than nanoparticles m No apparent toxicity of the formulated NPs,but a significant decrease of Aβ aggregates in response to Curcumin It was a promising system after optimization(drug loading,surface density)to protect anti-am peptides from proteolytic degradation and to increase their transport through Model of use MPTP-induced ice ice pal PD m Larval Zebrafish MPTP-induced chronic PD model Mechanistic model APP/PS1 m Hippocam cells Porcine brain capillary endothelial cells agining No No Yes Yes No No No Im Size 24 nm 70 nm 290 nm 15±1.2 nm 100 nm 100-250 nm 153±2 nm Mechanism of action Iron chelation therapeutic nanoparticles protected by a PMPC to delay the saturation of iron chelators in blood circulation and e,with HIV-1 trans-activating transcriptor(TAT)serving as a shuttle to enhance the BBB permeability Encapsuled SA in a nanoparticle ulation that extended SA and circulation in the bloodstream consequently an increased brain uptake and thus to be potentially efficacious for the treatment of PD ethods with cell targeting and a drug-controlled release system to prevent the overexpression of Conjugated peptide inhibitors derived from different Aβ regions onto the AuNPs,yielding different peptide@AuNPs conjugates Multi-functionalized nanoparticle odified prolong the in vivo lifetim α-syn crossing form Combined gene therapy m based on PEG-PLA,m system with TGN peptides as the BBB ligand and QSH peptides for the Aβ42-binding(TQNP)to target yloid plaques in the brain am Encapsulate Curcumin as active ingredient in PLGA nanoparticles,modified with g7 ligand for BBB To improve drug transport through the BBB,PLGA nanoparticles with surface functionalized with anti-transferrin receptor monoclonal antibody(OX26)and anti-Aβ (DE2B4)deliver encapsulated iAβ5 into the brain Use PD PD PD AD AD AD AD Model drug in Non-Fe hem(NFH)Schisantherin A(SA)shRNA and nerve growth factor(NGF)VCD10 peptide and LCA10 peptide H102 peptide in Curcum iAβ5 Fe3O4 coated with-AA)and modified oleic acid(VCD10)@AuNP and(LCA10)@AuNP in Nanoparticles TAT-NFH-nBSA mPEG-PLGA nanoparticles(NIPAm molecules PEG-PLA-TGN peptides-QSH peptides PLGA-g7-curcum PLGA-OX26-anti-Aβ(DE2B4)-iAβ5(continued on next page)

Table 2(continued)Refs.Result Model of use agining Im Size Mechanism of action Use Model drug Nanoparticles[77][65][98][87][144][145][98]bination es Based on these results,the com agnetic agnetic olecular otin and a m ay open new avenues for onstrated that mation via the utant huntingtin ouse brain odel of ALS,twice per inistration of ice even when uscle weakness icromolar olar concentrations for of osm nanoparticle-based delivery system with external functional m guidance m therapeutic approaches for the ent of various chronic and ory deficits of AD rats were treatm metabolic diseases,including neurodegenerative diseases such as AD Results clearly dem anthocyanins conjugated with PEG-AuNPs can pass through BBB and showed no significant cytotoxic effect in the neuronal cells.Furthermore,anthocyanin-loaded PEG-AuNPs showed positive effects against Aβ1-42-induced neurodegeneration and anti-inflam NF-kB/JNK/GSK3β signaling pathway The designed poly(trehalose)nanoparticles were 1000-10,000 tim more efficient than m trehalose in inhibiting protein fibrillation in extra-cellular space,in blocking aggregation of polyglutamine containing mutant huntingtin protein odel neuronal cells,and in of m molecular trehalose in m suppressing m aggregates in HD m Using a murine m week intravenous adm 20 mg/kg CeNPs prolonged survival of SOD1G93A transgenic m ent was started late at the onset elongation increased 63%treatm The cellular differentiation ratio increased 58%;the neurite length em The m significantly rescued upon treatment with MB loaded CeNC/IONC/MSN-T807 Anoscale trehalose could offer highly efficient antiamyloidogenic performance at m concentration,compared with ollar to m millim Aβ1-42-treated mice Aβ1-42-injected mice Transgenic mice for HD SOD1G93A mouse model of ALS PC-12 cells Male Sprague-Dawley rats HD transgenic mouse Yes Yes No No Yes Yes Yes 200-390 nm 135±5 nm 20-30 nm 3.3 nm 20.8 nm 51±5 nm 20-30 nm agnetic e and um agnetic field(FMF)conditions,nanoparticles(MNPs)loaded with osmotin(OMNP)were transported to the brains of Aβ1-42-treated PEG-coated AuNPs were applied in this research study because of their characteristics of a biologically less,biocompatible,and effective drug delivery device Zwitterionic surface chemistry of nanoparticles is ideal for efficient um ilar iron Under the functionalized m dextran-coated Fe3O4 m inim bined with ay further ultiple key mice ultivalency m ight provide sim model of ALS nanocomposite pathogenesis ic size of 20-30 nm posed of a 6 nm trehalose harm cellular uptake with m cytotoxicity.Presumed that such surface chemistry com the intact chemical structure of trehalose and the optim trehalose m enhance the in vitro/in vivo performance of trehalose CeNPs m therapeutic benefit in a murine NGF was essential for neuronal growth and differentiation.However,slow diffusion and short half-life of NGF from the enzym degradation had restricted its application in neuroregeneration Methylene blue,a tau aggregation inhibitor,was loaded on(CeNC/IONC/MSN-T807),which not only possessed high binding affinity to hyperphosphorylated tau but also inhibited m pathways of tau-associated AD The nanoparticles had a hydrodynam were com oxide core and a zwitterionic polymer shell containing~5%-12%(w/w)Covalently linked AD AD HD ALS Neuroregeneration AD HD Osmotin(OMNP)Anthocyanin Trehalose No No Methylene blue No Dextran-coated Fe3O4 agnetic magnetic nanoparticles(MNPs)Anthocyanin-loaded PEG-gold nanoparticles Zwitterionic poly(trehalose)nanoparticles oxide Cerium nanoparticles Nerve growth factor(NGF)functionalized superparam iron oxide(SPIO)-gold(Au)CeNC/IONC/MSNT807 Poly(trehalose)Nanoparticles
3.2. Iron nanoparticles
Iron accumulation in substantia nigra pars compacta has been proved to be a prominent pathophysiological feature of PD,which could induce the death of dopaminergic neurons, upregulation of ROS, and further loss of motor control [72,73].Iron chelators had shown powerful iron chelating ability,antioxidation and neuroprotection in the treatment of PD even in clinical trials[74].However,their therapeutic effect was hampered by short in vivo circulation time and cytotoxicity.Under this condition, iron chelation therapeutic nanoparticles protected by a zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) to delay the saturation of iron chelators in blood circulation and prolong the in vivo lifetime,with HIV-1 trans-activating transcriptor (TAT) served as a shuttle to enhance the BBB permeability was explored and investigated both in vitro and in vivo,which demonstrated iron chelator loaded therapeutic nanoparticles could reverse functional deficits in Parkinsonian mice not only physiologically but also behaviorally[75].
As one of iron nanoparticles, Fe3O4magnetic nanoparticles(MNPs),which are superparamagnetic,non-toxic and biocompatible, have been extensively investigated. With superparamagnetic property,their responsiveness to applied magnetic fields can be utilized for magnetically guided particle imaging. Osmotin was a 24 kDa multifunctional plant protein from tobacco, which can protect against neurodegeneration in postnatal rat brains [76]. Under functionalized magnetic field, dextran-coated Fe3O4MNPs loaded with osmotin were transported to the brains of Aβ1-42treated mice [77].By electromagnetic function guiding MNPs into brain sites, the brain damage,such as Aβ accumulation,Beta-secretase 1 expression, synaptotoxicity, memory impairment and tau hyperphosphorylation in an Aβ1-42injected mouse model,were significantly reversed for the first time.The magnetically mediated translocation of MNPs did not appear to induce deleterious signal transduction events and result in minimal accumulation in other organs and reach the brain within a few minutes of treatment without disrupting the BBB.This delivery also made full use of pH in the brains of AD patients which was lower than that in the brains of healthy individuals[78]to realize controlled release at the target site (AD brain) under acidic pH conditions. Similarly, oleic acid modified magnetic Fe3O4nanoparticles, carrying N-isopropylacrylamide derivative, shRNA and nerve growth factor, also provided effective repair in a PD model [79].In short,functional magnetic guidance may open a new path for the treatment of neurodegenerative diseases.
Furthermore, iron nanoparticles also have natural advantages in imaging. Diagnosis of AD can be performed with the assistance of amyloid imaging. Positron emission tomography(PET) is current diagnosis for AD, which is expensive and exposes people to radiation. While, magnetic resonance imaging is cheaper and is no-radioactive.A plenty of magnetic nanoparticles made of superparamagnetic iron oxide [80]conjugated with therapeutic substances (curcumin [81]) or modified ingredients(PEG [82],1,1-dicyano-2-[6-(dimethylamino)naphthalene-2-yl]propene carboxyl derivative [83]) to realize magnetic resonance imaging of AD.
3.3. Cerium oxide nanoparticles
Neurodegenerative diseases,such as AD,PD and ALS,are associated with high levels of oxidative stress, making them a target for treatment with cerium oxide nanoparticles(CeONPs)which have potent regenerative antioxidant properties.Therefore,CeONPs were currently being investigated for efficacy in several neurodegenerative disorders and have shown promising levels of neuroprotection [84]. While, traditional antioxidants have been attempted to alleviate the pathological changes in PD but with limited success because of poor ability to overcome the BBB[85].
Thus, different dose (0.1, 0.5 or 1 mg/kg) of CeO2NPs in 6-hydroxydopamine (6-OHDA) induced PD was investigated.Neurobiochemical derangements were almost reversed by the 0.5 mg/kg dose of CeO2NPs,while 0.1 mg/kg dose was not sufficient to alter biochemical measurements in the striatum[86].CeO2NPs also ameliorated strength and prolonged life in SOD1G93A mouse model of ALS[87],which was striking compared no genuinely effective treatment for ALS.
However,biodistribution studies clearly demonstrated that the major organs of accumulation are liver,kidney and spleen,which may cause safety problem in future study.Thus the targeting measure could be applied to CeO2NPs to increase the efficacy and minimize the potential toxicity.
3.4. Molybdenum nanoparticles
AD is a complicated disease.The pathogenesis of AD also includes elevating the ROS level and disruption the Ca2+homeostasis mediated by Aβ,both of which may lead to neuron injury and play an important role in AD[88].The inhibition of Aβ aggregation and dissociation of Aβ fibrils only receive a mitigated inhibition effect and weak dissociation ability.
Recently, molybdenum disulfide (MoS2) NPs was investigated and exhibited multifunctional effects on Aβ peptides:inhibiting Aβ aggregation, destabilizing Aβ fibrils, alleviating Aβ-induced oxidative stress, as well as Aβ-mediated cell toxicity, and blocked the formation of the Ca2+channel induced by Aβ fibrils in the cell membrane,which suggested that MoS2NPs had great potential for a multifunctional therapeutic agent against amyloid related diseases[89].
3.5. Silica nanoparticles
Not all nanoparticles as vehicle have a therapeutic effect on the neurodegenerative disease.Silica nanoparticles(SiO2-NPs)were widely applied in diagnosis,imaging,and drug delivery of central nervous diseases [90]. However, recent researches showed that SiO2-NPs up-regulated α-synuclein expressionthe hallmark of PD and induced autophagy through inhibiting negative regulation of autophagy PI3K-Akt-mTOR signaling,which indicated that SiO2-NPs exposure induced neurotoxicity and may be a significant risk factor for the development of PD[91].
3.6. Organic nanoparticles
Poly(lactic-co-glycolic acid) (PLGA) nanoparticles, as one of the most widely studied vehicles to carry drugs to the specific site, have several advantages such as high drug loading capacity and function-modified structure and biocompatibility. Curcumin, as an active ingredient, was loaded in PLGA NPs which was modified by g7 ligand for BBB crossing, showing a significant decrease of Aβ aggregates without causing apparent toxicity [92]. Similarly, schisantherin A, a promising anti PD natural product, was encapsulated in methoxy poly(ethylene glycol)-block-poly(D,L)-lactic-co-glycolic acid (mPEG-PLGA) nanoparticles, demonstrating more effective brain delivery and stronger neuroprotective effects in zebrafish and cell culture models of PD [93].Furthermore, PLGA nanoparticles functionalized with antitransferrin receptor monoclonal antibody (OX26) and anti-Aβ(DE2B4)delivered iAβ5into the brain,which protected antiamyloid peptides from proteolytic degradation and increased their transport through BBB[94].
Poly(ethyleneglycol)-poly(lactic acid)(PEG-PLA),as another vehicles, modified with TGN peptides as the BBB ligand and QSH peptides for the Aβ42-binding (TQNP) to form a dualfunctional nanoparticles and deliver Aβ-sheet breaker-H102 peptide, which obtained better ability in decreasing amyloid plaques,increasing Aβ-degrading enzymes,reducing tau protein phosphorylation, protecting synapses, and improving the spatial learning and memory of transgenic mice than nanoparticles modified with a single ligand[95].
Trehalose, as one of organic material, can invoke a suite of neuroprotective mechanisms that can contribute to improved cognitive performance in neurodegenerative disease[96,97].The designed poly(trehalose)nanoparticles,which are composed of a 6 nm iron oxide core and a zwitterionic polymer shell containing ~5%-12% (w/w) covalently linked trehalose, are 1000-10,000 times more efficient than molecular trehalose in inhibiting protein fibrillation in extra-cellular space, in blocking aggregation of polyglutamine-containing mutant huntingtin protein in model neuronal cells, and in suppressing mutant huntingtin aggregates in HD mouse brain[98].
Although organic nanoparticles have a certain therapeutic effect,the efficiency is not high on the whole.Compared with inorganic nanocarriers, organic nanoparticles have a certain gap in vivo imaging.
4. Nanomicelles:high encapsulation efficiency and loading capacity
Compared with nanoparticles and liposomes, micelles have relatively fewer studies on the treatment of neurodegeneration disease. Polymeric micelles are another type of nanomaterial which can be obtained by the self-assembly of amphiphilic molecules in water above a critical concentration that is called critical micelle concentration. The structure of polymeric micelles often is consisted of an amphiphilic block copolymer and a core-shell. These nanomaterials are versatile and can incorporate solutes of different structures both hydrophilic and hydrophobic drug[99].Nanomicells have natural advantage in controllable size and shape, and high encapsulation efficiency and loading capacity.However,inherent toxicity and low encapsulation efficiency of hydrophilic therapeutics limit its application. By targeting delivery, they can improve delivery efficiency and minimize side effects(Table 3).
Curcumin,as a model drug to treat AD,also was made into curcumin micelles by combining with Tween-80.According to results,curcumin micelles,which increased bioavailability in mouse plasma by around 45-fold,seemed to be the most effective formulation for increasing curcumin bioavailability comparing with curcumin nanoparticles (9-fold) and curcumin liposomes(5-fold)[100].Other therapeutic substances,such as flurbiprofen for AD[101],rivastigmine for AD[102]and Coenzyme Q10 (CoQ10) for PD [103],were also loaded on different nanomicelles,all of which increased the amount of brain penetration,solubility,and bioavailability.
5. Exosomes:new emerging and promising nanocarriers
Exosomes, as one of natural endogenous nanocarriers, vary from 30 nm to 150 nm in size and have a typical lipid bilayer structure,which are reputed as“drifting bottle”in living body.It is secreted by a variety of cells:B cells[104],and T cells[105],macrophages[106],dendritic cells[107].After found in 1986 by Johnstone et al.[108],it gradually becomes a research hotspot.
Exosomes distinguish themselves from other vehicles mainly in two features.The one is immune privilege: as natural carrier systems with endogenous cellular tropism, exosomes can avoid the endosomal pathway and lysosomal degradation, diminish clearance by the mononuclear phagocyte system,and increase drug transport to target tissues,just function as“invisibility cloak”.The long-distance intercellular communication facilitates the carry of unstable therapeutic molecules, such as nucleic acids, and proteins [109-111]. Especially,exosomes have been shown to preserve mRNAs and siRNAs within their “aqueous” proteinaceous core even under external RNase treatment, and subsequently to deliver functional RNAs to recipient cells [112]. Noteworthily, compared with PEGylated liposomes,which could lost their longcirculating property when they were administered twice in the same animal within certain intervals [113],exosome showed great superiority as nono-carrier.

icelles as vehicle in treating neurodegenerative disease.Table 3-Current knowledge of possible nanom Refs.[146][100][101][102][103]ation of Aβ-42 to amyloidogenic PEGylated phospholipids β-sheeted form and impart neuroprotection in vitro icelles improved bioavailability of native in around 10-to 40-fold ice odified icelles icelles Result abrogated transform icelles were ouse brain in m a and brain of m micelles concentration Curcum curcum in plasm PEGD5 cellselles significantly enhanced intracellular flurbiprofen delivery when compared to unm An in vitro biological assay evidenced no cytotoxic effects of either empty or loaded m on the neuronal cell lines tested.Moreover,the m internalized by neuroblastoma cell lines with drug uptake depending on the m Study revealed that Ubisol-Q10 intervention could stop,but not reverse,the on-going neurodegeneration in MPTP-treated m Model of use Human Neuroblastoma SHSY-5Y cell line ice ice a cells NMRI m bEND5 cells Neuroblastom Male C57BL/6 m NP size 36.7 nm ention ention No m 110 nm 34.1 nm No m icelles are Sterically stabilized(PEGylated)itigating Aβ-42 Due to low absorption and quick ination from the body,in bioavailability was ajor Mechanism of action eans of ers phospholipid nanom effective in m aggregation in as a therapeutic agent er-functionalized endothelial cells.odel CNS-active drug to icelles obtained by bling PS80-attached elim curcum rather low which posed m problems for the use of curcum Novel aptam polyethylene glycol-polylactic acid with the objective to target the transferrin receptor on brain Deliver a m neuronal cells,by m polymeric m self-assem phiphilic copolym am Nanomicellar formulation of CoQ10(Ubisol-Q10)with proved properties,including and bioavailability im the brain penetration,solubility,Application AD AD AD AD PD Model drug in No Curcum Flurbiprofen Rivastigmine free-base Coenzyme Q10 odify icelles Surface m PEGylated phospholipid nanomicelles in m Curcum PEG-EGc PHEA-EDA-Sq17-PS80 copolymer PEG-derivatized atocopherol(PTS)micells

Fig.3-Schematic representation of production,harvest and re-administration of targeted self-exosomes for gene deliver.
The other advantage is the wide diversity of endogenous marker molecules, adhesive proteins and specific vector ligands(tetraspanins,integrins,CD11b and CD18 receptors)presenting on exosomes surface[114-117],making it more easier to be modified and deliver therapeutic molecules to targeted cell.By modifying the surface,exosomes can get over poor targeting problem.For example Alvarez-Erviti research[16]used targeted exosomes to cross BBB. Targeting was achieved by engineering the dendritic cells to express Lamp2b, an exosomal membrane protein, fused to the neuron-specific RVGpeptide3. Intravenously injected RVG-targeted exosomes delivered GAPDH siRNA specifically to neurons,microglia,oligodendrocytes in the brain,resulting a strong mRNA (60%) and protein (62%) knockdown of Beta-secretase 1, a therapeutic target in AD, in wild-type mice [118] (Fig. 3). Furthermore,therapeutic catalase mRNA delivery by Lamp2b modified exosomes attenuated neurotoxicity and neuroinflammation in vitro and in vivo models of PD,indicating the potential usefulness of the EXOsomal transfer devices for RNA delivery-based therapeutic applications[119].The use of exosomes as siRNA vectors is still in early stage,but exosomes may be a significant advance in the field of macromolecular drug delivery and may be a key step in the clinical application of siRNA.
Accumulating evidence suggests that exosomes can be effectively used as vehicle for the treatment of various neurodegenerative disorders (Table 3). However, related research showed that exosomes proteins were found to accumulate in the plaques of AD patient brains,in which β-cleavage of amyloid precursor protein occurs in early endosomes and a fraction of Aβ peptides was localized to multivesicular bodies and was released in association with exosomes [120]. Therefore,exosomes provided a mechanism of Aβ and amyloid precursor protein(APP)- C-terminal fragments (CTFs) trafficking around the body, possibly contributing to amyloid deposition in the brain[121].Whereas,glycosphingolipid-enriched exosome infusion leaded to a decrease in Aβ levels and ameliorates Aβrelated pathologies in APP mice [122]. Thus, exosomes from different sources or different compositions have completely different effects on neurodegenerative diseases.
The same thing happens in PD.Exosomes have been shown to spread toxic α-synuclein (αsyn) between cells and induce apoptosis, which suggests a key mechanism underlying the spread of αsyn aggregates in the brain and the acceleration of pathology in PD[123,124].Exosome-associated αsyn oligomers were more likely to be taken up by recipient cells and could induce more toxicity compared to free αsyn oligomers [125].However, exosomes also had a profound catalytic effect on αsyn aggregation kinetics, which ascribed to the ganglioside lipid components [126]. In treatment filed, as drug delivery vehicles,exosomes have been used to carry small interfering RNAs and catalase to the brain,showing clear therapeutic effects in a PD mouse model[127,128].
In conclusion, the cause and effect relationship between exosomes and pathogenesis of AD and PD is not clear enough.Related research is still at early stage.There are several problems waiting to be handled before they enter into clinical practice.
(1) Exosomes are so complex in molecular constituents that safety issues and potential risk must be highlighted and evaluated comprehensively.
(2) It is well known that exosomes alter considerably among different cellular sources, such as multipotent stem cells and tumor cells[129].So one of the major obstacles laying in this approach is whether exosomes can be produced in large scale or reproducibly.
(3) As brain target vehicle, it can’t be overemphasized to improve the target ability of exosomes to enhance the drug concentration in brain area and avoid adverse effect.

Table 4-Current knowledge of possible exosomes as vehicle in treating neurodegenerative diseases.Refs.[118][127][147][148][128][119]capable of delivering siRNA RVG exosomes are especially specifically and safely after inistration and therefore represented a ising vehicle for gene therapies targeting chronic neurodegenerative disorders Selected exoCAT formulations significantly decreased brain mation and increased ouse an ouse When Exo-124 was delivered to of the striatum,it reduced the RNA expression of REST.However,inistration of Exo-124 had little effect on Result ouse α-Syn,ance ine towards ic adm model resulted in bilateral odel,in brain regions concentration system prom inflam neuronal survival in a PD m Rota-Rod perform Unilateral infusion of hsiRNA-loaded exosomes,but not hsiRNAs alone,into m striatum oligonucleotide distribution and statistically significant bilateral silencing of up to 35%Huntingtin m Using systemic adm siRNAs,it was able to significantly decrease the level of endogenous m and a proaggregating hum of α-Syn in a transgenic form mouse m pathologically affected in PD Designer exosomes produced by the engineered exosome producer cells significantly reduced the neurotoxicity of 6-hydroxydopam CHRNA7-positive Neuro2A cells without the need for exosome Model of use ice ice C57BL/6 m C57BL/6 m R6/2 line of transgenic ice HD m Wild-typeice FVBNj m Transgenic mice C57BL/6 J mice NP size 80 nm 100-200 nm ention Not m 140 nm 100 nm 100 nm ethod al Loading m Electroporation The incubation sonication,or extrusion Co-incubation Co-incubation Electroporation EXOsom transfer into cells devices Mechanism of action RVG-targeted exosomes to oligodendrocytes in the brain,resulting in a specific gene Catalase preservation against proteases degradation The delivery of abnormallyight restore normal gene regulation and has a therapeutic effect To improve stability and promote cellular internalization To achieve widespread delivery of siRNAs to the brain,peripherally odified exosomes that specifically target the brain by expressing a brain-targeting glycoprotein peptide;RVG)RNA neurons,microglia,ine knockdown iRNAs m downregulated m al transfer into cells injected m peptide(rabies virus loaded with siRNA es bearing catalase m Exosom produced by exosome producer cells equipped with the EXOsom devices could rescue neuronal cell death induced by 6-hydroxydopam Application Neurodegenerative disorder PD HD HD PD PD Source of es exosom Self-derived dendritic cells Neuronal cells HEK 293 cells Glioblastoma U87 cells Murine dendritic cells HEK-293T cells Model drug GAPDH siRNA Catalase miR-124 Hydrophobically modified siRNAs siRNA mRNA
6. Potential risk of nanocarriers
Although, nanocarriers can be a strong and powerful tool to penetrate the BBB,there are plenty of problems that need to be solved.First,after massive nanocarriers absorbed into the brain,the specific distribution in the brain is not clear in many research, which may cause potential risk in such a sophisticated organ.
Second, the metabolism of nanocarriers is the key point.On the one hand, a large of nanocarriers are inorganic material,such as gold nanoparticles,iron nanoparticles,cerium oxide nanoparticles, molybdenum nanoparticles and silica nanoparticles which are hard to metabolize, probably resulting accumulate in brain. They can contribute to neurodegeneration by inducing mitochondrial dysfunction,redox imbalance and apoptosis,autophagy and impaired lysosomal activity, cytoskeletal damage and vesicle trafficking perturbations,neuroinflammation and microglia activation[41,130-133]. The magnetic iron oxide nanoparticles can result neuronal loss in the chicken embryo [134] and cerium oxide nanoparticles can inhibit differentiation of neural stem cells[135]. On the other hand, biodegradable nanoparticles also show neurotoxicity in our previous research.For example,after polysorbate 80-modified chitosan nanoparticles injecting into body,the body weight was found to remarkably decreased in a dose-dependent manner for seven days, also causing apoptosis, necrosis of neurons, and slight inflammatory response in the frontal cortex[136].Moreover,chitosan nanoparticles at a size of 200 nm caused malformations, including a bent spine,pericardial edema,and an opaque yolk in zebrafish embryos[137,138].
Third,nanocarriers also bring changes in the mode of administration,from oral into injection,which may bring some problems in practical use. Most of the nano-formulation are injections,while the general pharmaceutical preparations are oral dosage forms. In actual use, especially for patients with neurodegenerative diseases,oral preparations are easier to accept than the injections.
Consequently,additional investigations in this area should be performed, including further studies on the acute toxicity,potential long-term neurotoxicity and actual use problem(Table 4).
7. Future perspectives and conclusions
Overall,nanocarriers can provide promising opportunities for improving neurodegenerative diseases.Nanocarriers such as liposomes, nanoparticles, nanomicelles and exosomes, were modified in the surface to enhance brain targeting ability.With the help of some specific ligands(glucose,lactoferrin,transferrin,specific peptides),nanocarriers efficiently crosses the BBB and able to deliver the drug which normally cannot cross the BBB at the particular site.
However, this field is still in at the infant stage. Several issues should be resolved before neurodegenerative diseases nanomedicine comes to clinical setting.Among them,the low targeting efficiency is general and the biggest obstacle,which may hamper the therapeutic effect and cause damage to other organs. Furthermore, the distribution of nanomaterials into the brain is another concern.In order to achieve precise targeting in the brain,sequentially targeted nanomaterials deserve attention. Not only target BBB, also target the lesion site, to avoid distribution in whole brain.Besides,in the long term,the toxicity of nanomaterials,especially for inorganic nanomaterials,should not be overlooked.The degradation properties of nanomaterials should be of great concern.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgment
This work was supported by National Natural Science Foundation of China(81620108028).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2018.09.005.
 Asian Journal of Pharmacentical Sciences2019年5期
Asian Journal of Pharmacentical Sciences2019年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Complex formulations,simple techniques:Can 3D printing technology be the Midas touch in pharmaceutical industry?
- Design,mechanism,delivery and therapeutics of canonical and Dicer-substrate siRNA
- Improving the protective effects of aFGF for peripheral nerve injury repair using sulfated chitooligosaccharides
- Exploring the relationship of hyaluronic acid molecular weight and active targeting efficiency for designing hyaluronic acid-modified nanoparticles
- Redox-sensitive micelles for targeted intracellular delivery and combination chemotherapy of paclitaxel and all-trans-retinoid acid
- Modulating intestinal mucus barrier for nanoparticles penetration by surfactants
