miR-361-3p调节恶性胶质瘤细胞功能的研究
郭小叶 冒平 王佳 廉海平 王伟 白晓斌 宋锦宁
[摘要] 目的 研究miR-361-3p在恶性胶质瘤(GB)细胞中的功能。 方法 培养LN299和U87MG细胞及人正常星形胶质细胞(NHA)。miR-361-3p的mimic或inhibitor及MTCP1的siRNA(si-MTCP1)转染U87MG细胞。RT-PCR和Western blot检测目标基因水平的变化。CCK-8和Caspase3試剂盒分别检测细胞的增殖和凋亡。荧光素酶基因报告实验检测miR-361-3p和MTCP1基因的结合。 结果 与NHA比较,miR-361-3p在LN299和U87MG中低表达。U87MG转染mimic可抑制MTCP1的表达和细胞增殖率,升高Caspase3活性,并减少WT-MTCP1的荧光素酶活性。U87MG转染inhibitor增加细胞增殖率,而转染si-MTCP1后细胞凋亡增加。 结论 miR-361-3p可能通过抑制MTCP1来抑制GB细胞的增殖并促进凋亡。
[关键词] 恶性胶质瘤;细胞增殖;凋亡;miR-361-3p
[中图分类号] R739.41 [文献标识码] A [文章编号] 1673-7210(2019)10(a)-0007-05
Studies of miR-361-3p in regulating the function of glioblastoma cells
GUO Xiaoye MAO Ping WANG Jia LIAN Haiping WANG Wei BAI Xiaobin SONG Jinning
Department of Neurosurgery, the First Affiliated Hospital of Xi′an Jiaotong University, Shaanxi Province, Xi′an 710061, China
[Abstract] Objective To study the role of miR-361-3p in glioblastoma (GB) cells. Methods LN299, U87MG and normal human astrocytes (NHA) were cultured. miR-361-3p mimic or inhibitor and MTCP1 siRNA (si-MTCP1) were transfected into U87MG cells, respectively. Gene expression was tested by RT-PCR and Western blot. Cell proliferation and apoptosis were measured by CCK-8 and Caspase3 kits, respectively. The binding of miR-361-3p and MTCP1 were detected by luciferase reporter assay. Results Relative to NHA, miR-361-3p levels were lower in LN299 and U87MG. Transfected with mimic, MTCP1 levels and the proliferation of U87MG cells were decreased, and the Caspase3 activity was increased, and luciferase activity in WT-MTCP1 was decreased. Furthermore, transfected with inhibitor, the proliferation of U87MG cells were increased, while the apoptosis rate in the si-MTCP1 group was increased. Conclusion MiR-361-3p may inhibit the proliferation and promote apoptosis of GB cells by inhibiting MTCP1.
[Key words] Glioblastoma; Cell proliferation; Apoptosis; miR-361-3p
恶性胶质瘤(glioblastoma,GB)是一种起源于大脑或脊髓胶质细胞的肿瘤。GB的治疗通常采用手术、化疗和放疗的综合方法,但复发率较高[1]。基因靶向治疗是GB治疗的新方法[2]。研究证实,微小RNA(miRNA)在GB的发生过程中发挥着关键作用,可调节细胞增殖,介导细胞生长和关键激酶的表达[3]。最新研究[4]发现miR-361-3p可以抑制视网膜母细胞瘤的增殖和干性特征。然而,miR-361-3p在GB中的详细功能和机制尚不清楚。本研究探讨miR-361-3p及其靶基因成熟T细胞增殖1(mature T cell proliferation 1,MTCP1)与GB的增殖和凋亡的关系。
1 材料与方法
1.1 细胞系和培养
人正常星形胶质细胞(normal human astrocytes,NHA)和GB细胞系LN299和U87MG购自美国典型培养物保藏中心(American Type Culture Collection,ATCC,Manassas,USA)。使用含10% FBS的DMEM培养基于37℃、5%CO2培养箱中培养。
1.2 RNA寡核糖核苷酸构建和脂转染
miR-361-3p mimic(mimic)、miR-361-3p inhibitor(inhibitor)和MTCP1 siRNA(si-MTCP1)购自Gemma Biology公司(中国上海)。U87MG细胞转染实验分组:control组、mimic组及阴性对照组(mimic-NC組)、inhibitor组及其阴性对照组(inhibitor-NC组)、si-MTCP1及其阴性对照组(si-NC组)。使用Lipofectamine 2000脂质体转染系统进行细胞转染,随后在37℃、5%CO2和95%湿度下孵育8 h。
1.3 RT-PCR和Western blot检测miR-361-3p和MTCP1的表达
用Trizol试剂提取各组细胞总RNA,对RNA进行定量分析后,用反转录试剂盒合成cDNA,用PCR仪对cDNA进行扩增。根据实时定量PCR试剂盒对其进行定量分析。
Western blot:收集细胞并加入1 mL RIPA裂解液,抽提总蛋白。通过10% SDS-PAGE凝胶电泳分离蛋白质并转移到聚偏二氟乙烯膜上。用5%脱脂牛奶封闭1 h后,将膜与一抗MTCP1(Abcam,ab128358, 1∶1000)或GADPH(Abcam,ab131385,1∶1000)在4℃下孵育过夜。通过增强的化学发光法(Western Biotechnology Corporation)使免疫反应条带显色。
1.4 CCK-8检测细胞增殖
将细胞消化后,移入15 mL离心管,1500 r/min离心5 min(离心半径60 cm),将细胞转入6孔板培养。48 h后使用CCK8检测细胞活力。10 μL浓度为5 mg/mL的CCK8溶剂添加到96孔板,避光孵育2 h后在450 nm处检测吸光度[5-6]。
1.5 细胞凋亡率检测
使用细胞凋亡酶联免疫吸附测定(ELISA)试剂盒(Roche Diagnostics,德国)检测细胞凋亡率。细胞裂解30 min后将细胞质溶解物转入双抗涂抹的细胞板中,加入Anti-DNA-POD和Anti-histone-biotin 孵育2 h。用酶标仪检测490 nm处吸光度值。
1.6 荧光素酶报告基因测定
基于TIANAMP基因组DNA试剂盒(TIANGEN Biotech,中国)的操作手册,提取DNA用于构建荧光素酶报告载体。使用miR361-3p或阴性对照组NC和荧光素酶报告载体分别共转染后48 h,收集细胞并使用双荧光素酶报告分析系统(E1910)(Promega,中国)检测样品的荧光素酶活化。
1.7 统计学方法
数据采用SPSS 22.0软件进行分析,计量资料以均数±标准差(x±s)表示,多组比较使用单因素方差分析,组间两两比较采用Bonferroni法。两组计量资料比较采用t检验。以P < 0.05为差异有统计学意义。
2 结果
2.1 miR-361-3p在GB细胞中的表达
与NHA比较,LN299和U87MG细胞中的miR-361-3p降低,差异有统计学意义(P < 0.05);另外,与LN299比较,U87MG细胞中的miR-361-3p降低(P < 0.05)(图1)。
与NHA比较,*P < 0.05;与LN229比较,#P < 0.05。NHA:人正常星形胶质细胞
2.2 miR-361-3p抑制MTCP1
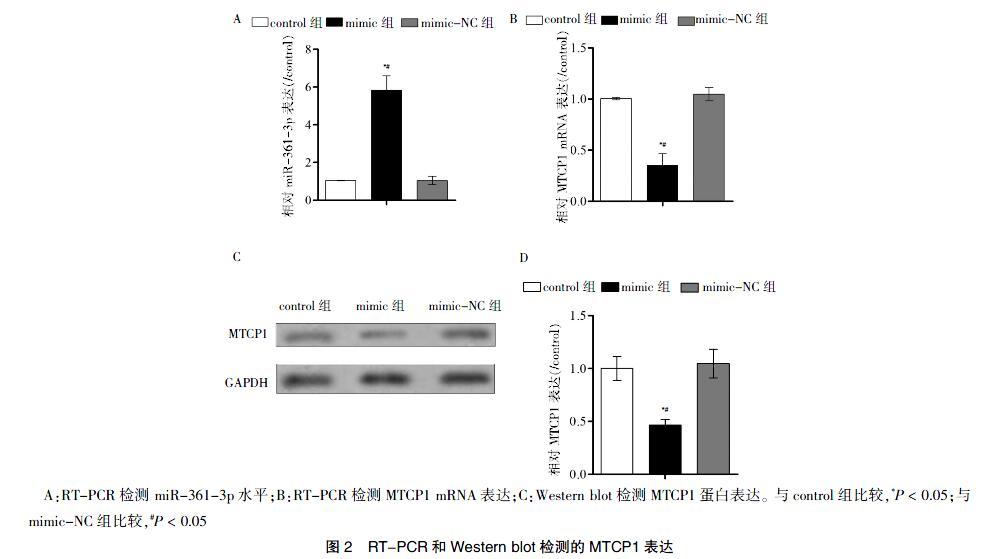
与control组或mimic-NC组比较,mimic组中miR-361-3p升高,差异有统计学意义(P < 0.05)(图2A),且MTCP1的mRNA表达水平及蛋白水平均降低,差异有统计学意义(P < 0.05)(图2B~D)。
2.3 miR-361-3p抑制GB细胞的增殖
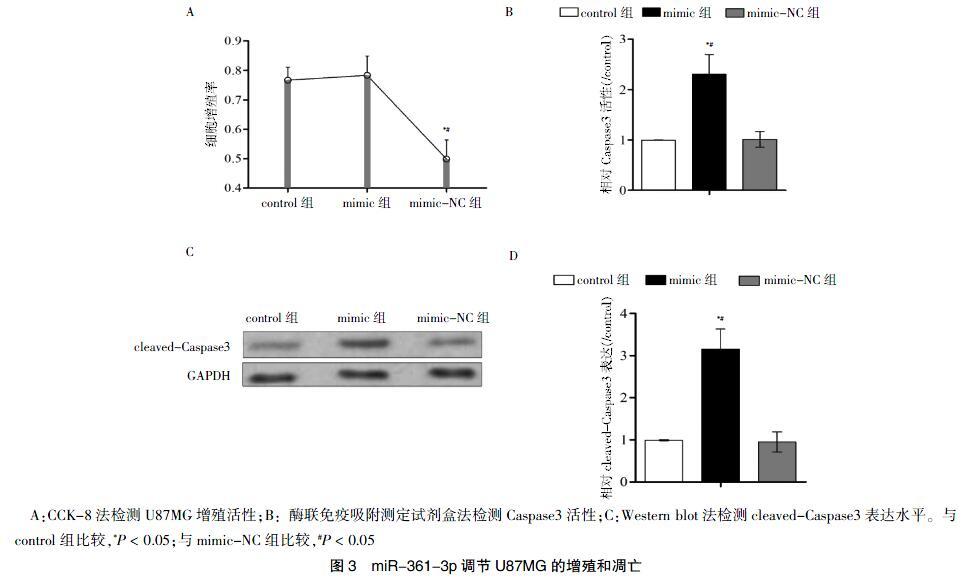
转染48 h后,mimic组的细胞增殖率低于control组及mimic-NC组,差异有统计学意义(P < 0.05)(图3A)。
2.4 miR-361-3p促进Caspase3活性
mimic组的Caspase3活性高于control组及mimic-NC组,差异有统计学意义(P < 0.05)(图3B);另外mimic组的cleaved-Caspase3的表达水平高于control组及mimic-NC组,差异有统计学意义(P < 0.05)(图3C~D)。
2.5 miR-361-3p通过下调其靶基因MTCP1调节GB细胞的增殖和凋亡
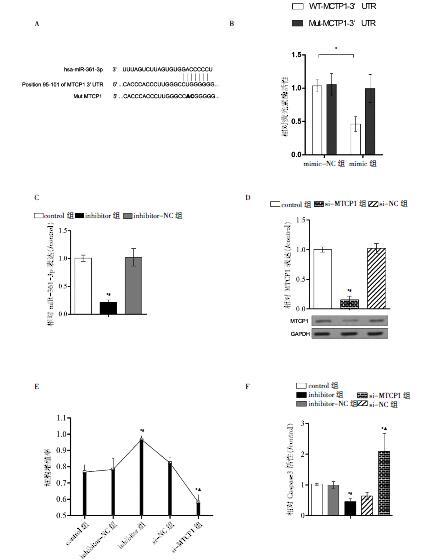
miR-361-3p与MTCP1 mRNA的3′-UTR区域结合位点的序列如图4A所示。另外,与mimic-NC组比较,mimic组中WT-MTCP1的荧光素酶活性降低,差异有统计学意义(P < 0.05)(图4B)。与control组和inhibitor-NC组比较,inhibitor组能抑制miR-361-3p,差异有统计学意义(P < 0.05)(图4C)。与control组和si-NC组比较,si-MTCP1组能抑制MTCP1表达,差异有统计学意义(P < 0.05)(图4D)。与control组或si-NC组比较,si-MTCP1组的细胞增殖能力减弱,但与control组或inhibitor-NC组比较,inhibitor组增殖活性增强,差异有统计学意义(P < 0.05)(图4E)。与control组或inhibitor-NC组比较,inhibitor组的Caspase3活性减弱,差异有统计学意义(P < 0.05);而与control组或si-NC组比较,si-MTCP1组的Caspase3活性增加,差异有统计学意义(P < 0.05)(图4F)。
3 討论
GB由大脑或脊髓起源,并随着神经细胞增殖和凋亡等多种功能的失调,细胞侵袭性增加,形成转移特征,从而使肿瘤恶变[7-9]。已报道多种与恶性发展相关的miRNA,且大量关于miRNA的促进或抑制细胞的增殖和凋亡仍然是目前研究的热点,例如miR-622[10]、miR-548b[11]、miR-21和miR-1224-5p[12]等。miRNA是抗GB的一类潜在靶点[13]。本研究提示miR-361-3p可能通过抑制MTCP1来抑制GB细胞的增殖,并促进凋亡。
miR-361-3p与各种癌症(如非小细胞肺癌、胰腺导管腺癌、胶质母细胞瘤)的增殖和凋亡有关[4,14-15]。研究证实miR-361-3p在非小细胞肺癌中表达降低,并发挥抑癌的作用[14]。另外,研究发现miR-361-3p靶向双特异性磷酸酶2抗体(DUSP2)能调节细胞外调节蛋白激酶(ERK),并抑制胰腺导管腺癌的上皮间质转化[15]。而且miR-361-3p在视网膜母细胞瘤中表达降低,并抑制视网膜母细胞瘤细胞的增殖和干性[4]。与以上研究结果类似,本研究中miR-361-3p在U87MG和LN299细胞系中的表达降低,而且过表达miR-361-3p可以抑制GB细胞的增殖活性,增强细胞凋亡标志物Caspase3活性,此结果进一步提示miR-361-3p在包括GB在内的多种肿瘤中可能扮演抑癌基因。
miR-361-3p往往可调节多种靶基因,如胰岛素样生长因子1(insulin-like growth factor 1,IGF1)[16]、TNF基因[17]、转录活性p73基因[18]和促卵泡激素(FSH)[19]。研究表明,MTCP1能与关键癌症调节基因AKT1相互作用[20],推测其具有重要的肿瘤调节作用。另外,MTCP1已被证实可在GB细胞中被miR-126靶向抑制[21]。本研究同样发现MTCP1在GB细胞中可被miR-361-3p靶向抑制,且si-MTCP1能抑制GB细胞的增殖,并促进细胞凋亡。与本研究结果类似,有研究发现,在GB中si-MTCP1可以抑制GB肿瘤组织的生长和GB细胞的转移[21],提示miR-361-3p通过抑制MTCP1进而抑制GB细胞的增殖并促进凋亡。
综上所述,本研究提示miR-361-3p抑制GB细胞的增殖并促进凋亡,对MTCP1的抑制是miR-361-3p发挥功能的关键机制之一。本研究提供了miR-361-3p对GB抑癌作用的体外实验证据,揭示新的miR-361-3p/MTCP1分子组合对GB细胞增殖和凋亡的影响,为GB靶向治疗的动物及临床研究提供了一个新靶点。
[参考文献]
[1] Brandner S,Jaunmuktane Z. Neurological update:gliomas and other primary brain tumours in adults [J]. J Neurol,2018,265(3):717-727.
[2] Friedmann-morvinski D,Narasimamurthy R,Xia Y,et al. Targeting NF-κB in glioblastoma:A therapeutic approach [J]. Sci Adv,2016,2(1):e1501292.
[3] Tian Y,Nan Y,Han L,et al. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma [J]. Int J Radiat Oncol,2012,40(4):1105-1112.
[4] Zhao D,Cui Z. MicroRNA-361-3p regulates retinoblastoma cell proliferation and stemness by targeting hedgehog signaling [J]. Exp Ther Med,2019,17(2):1154-1162.
[5] Wang Y,Wang Y,Li J,et al. CRNDE,a long-noncoding RNA,promotes glioma cell growth and invasion through mTOR signaling [J]. Cancer Lett,2015,367(2):122-128.
[6] Liu S,Jin J,Jia YG,et al. Glycopolymers Made from Polyrotaxanes Terminated with Bile Acids:Preparation,Self-Assembly,and Targeting Delivery [J]. Macromol Biosci,2019,19(4):1800478.
[7] Dong X,Jin Z,Chen Y,et al. Knockdown of long non‐coding RNA ANRIL inhibits proliferation,migration,and invasion but promotes apoptosis of human glioma cells by upregulation of miR-34a [J]. J Cell Biochem,2018,119(3):2708-2718.
[8] Ng HK,Sun DT,Poon WS. Anaplastic oligodendroglioma with drop metastasis to the spinal cord [J]. Clin Neurol Neuro,2002,104(4):383-386.
[9] Otani Y,ichikawa T,Kurozumi K,et al. Fibroblast growth factor 13 regulates glioma cell invasion and is important for bevacizumab-induced glioma invasion [J]. Oncogene,2018,37(6):777.
[10] Sestini S,Boeri M,Marchiano A,et al. Circulating microRNA signature as liquid-biopsy to monitor lung cancer in low-dose computed tomography screening [J]. Oncotarget,2015,6(32):32868.
[11] Yang Y,Mei Q. miRNA signature identification of retinoblastoma and the correlations between differentially expressed miRNAs during retinoblastoma progression [J]. Mol Vis,2015,21:1307.
[12] Qian J,Li R,Wang YY,et al. MiR-1224-5p acts as a tumor suppressor by targeting CREB1 in malignant gliomas [J]. Mol Cell Biochem,2015,403(1/2):33-41.
[13] Rao SA,Santosh V,Somasundaram K. Genome-wide expression profiling identifies deregulated miRNAs in malignant astrocytoma [J]. Modern Pathol,2010,23(10):1404.
[14] Chen W,Wang J,Liu S,et al. MicroRNA-361-3p suppresses tumor cell proliferation and metastasis by directly targeting SH2B1 in NSCLC [J]. J Exp Clin Canc Res,2016,35(1):76.
[15] Hu J,Li L,Chen H,et al. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma [J]. Cell Death Dis,2018, 9(8):807.
[16] Cannavicci A,Zhang Q,Dai SC,et al. Decreased Levels of MicroRNAs-28-5p,-361-3p and Increased Insulin-Like Growth Factor 1 mRNA Levels in Mononuclear Cells from Hereditary Hemorrhagic Telangiectasia Patients [J]. Can J Physiol Pharm,2019,97(6):562-569.
[17] Lago TS,Silva JA,Lago EL,et al. The miRNA 361-3p,a Regulator of GZMB and TNF Is Associated With Therapeutic Failure and Longer Time Healing of Cutaneous Leishmaniasis Caused by L.(viannia)braziliensis [J]. Front Immuno,2018,9:2621.
[18] Tang L,Zhao B,Zhang H,et al. Regulation of nonylphenol‐induced reproductive toxicity in mouse spermatogonia cells by miR-361-3p [J]. Mol Reprod Dev,2017,84(12):1257-1270.
[19] Ye RS,Li M,Li CY,et al. miR-361-3p regulates FSH by targeting FSHB in a porcine anterior pituitary cell model [J]. Reproduction,2017,153(3):341-349.
[20] Laine J,Knstle G,Obata T,et al. Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family [J]. J Biol Chem,2002,277(5):3743-3751.
[21] Han L,Liu H,Wu J,et al. miR-126 Suppresses Invasion and Migration of Malignant Glioma by Targeting Mature T Cell Proliferation 1(MTCP1)[J]. Med Sci Monit,2018, 24:6630-6637.
(收稿日期:2019-04-15 本文編辑:张瑜杰)

