Potential synergic mechanism of Wutou-Gancao herb-pair by inhibiting efflux transporter P-glycoprotein
Yufei He , Zihong Wei , Ying Xie , Xiulin Yi , Yong Zeng , Yzhuo Li ,**,Chngxio Liu ,,* Shenyng Phrmceuticl University, Chin
b State Key Laboratory of Drug Delivery Technologies and Pharmacokinetics, Tianjin Institute of Pharmaceutical Research, China
c Tianjin Institute of Pharmaceutical Research New Drug Assessment Co. Ltd, China
d State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau SAR, China
Keywords:
ABSTRACTWutou-Gancao herb-pair is extensively used to attenuate the toxicity and enhance the efficacy of aconite.In this study, potential synergic mechanism of the herb pair was investigated by utilizing multiple approaches. In silico and in vitro Caco-2 cell models were applied to study the potential binding mode of bioactive ingredients existing in liquorice with P-glycoprotein(P-gp), as well as the inhibition effects on P-gp. Additionally, anti-inflammatory activity of aconitine (AC) combined with active ingredients of liquorice, as well as pharmacokinetic patterns of AC after co-administration was investigated. Antiinflammatory effect of AC (1 mg/kg) in rats was enhanced in combination with bioactive ingredients of liquorice (10 mg/kg). In the meanwhile, the exposure of AC in vivo was altered, in terms of Cmax and AUC. For instance, the Cmax and AUC were increased to 1.9 and 1.3 folds, respectively, when used in combination with liquiritigenin. The in silico study revealed the potential binding mode with outward facing conformation of P-gp. The resulting data obtained from transport of rhodamine-123 (Rh-123)across Caco-2 cell monolayer further indicated that the function of P-gp was inhibited by chemicals in liquorice. The synergic effect was therefore proposed to be attributed to inhibition of P-gp by liquorice since AC has been demonstrated to be the substrate of P-gp. The resuls revealed that potential synergic mechanism of Wutou-Gancao herb-pair by inhibiting function of key efflux transporter P-gp to enhance the exposure of AC in systematic circulation,and further the anti-inflammatory effect,which helps clarify the compatibility rationale of these two herbs.
1. Introduction
Aconite is a commonly used medicinal plant which is derived from the processed parent roots ofAconitum carmichaeliDebx.which exhibits many pharmacological actions, such as analgesic,anti-inflammatory, antiarrhythmic and cardiotonic actions [1-4].The chemical constitutes of aconite have been extensively investigated,and the majority of active/toxic compounds are determined to be alkaloids,either diester or monoester diterpenoid.Among all chemicals identified, aconitine (AC), hypaconitine and mesaconitine have been demonstrated to be the most potent ingredients,in terms of their bioactivity. In particular, AC as the most weighted molecule,exhibits direct impact on diverse systems such as central nervous and cardiovascular systems, presenting both efficacy and toxicity [5-12].
Liqurice is originally from the root of Glycyrrhiza uralensis Fisch,G. inflata Bat orG. glabraand also one of most widely used herbs,which is quite often applied in combination with aconite as herbpair, resulting in either toxicity reduction or efficacy enhancement of aconite [13-21]. The interaction of the herb-pair has also been studied. According to the literature, the acid-alkaline compounds interaction of liquorice and aconite play an important role in reducing toxicity of aconite [18]. For instance, the glycyrrhizic acid can precipitate with a variety of alkaloids existing in the aconite (including ester alkaloids) via carboxyl groups to form insoluble complexes,thus reducing the amount of ester alkaloids in the pharmaceutical liquid and attenuating the toxicity of aconite[22].Not only the reduction of toxicity of aconite through combined use of liquorice, but also the enhanced efficacy of aconite was observed.Chen et al.[23]and Xu et al.[24]reported that the LD50of mice after intragastric or intraperitoneal administration of aconite could be elevated by the compatibility of liquorice. In addition,liquorice is also able to enhance the tolerance and reverse adversereaction of animals which may be related to the effect of adrenocortical hormone activities of liquorice [16,22].
The bioavailability of oral drugs mainly depends on the small intestine absorption [25] and efflux transporters extensively expressed on the small intestinal epithelial cell which can directly affect absorption of xenobiotics. The P-gp efflux transporter[26-28] is located in the apical membrane of intestinal epithelial,hepatic, and renal tubular cells and obviously regulates the absorption,distribution,excretion,and further toxicity of xenobiotics,thus performing as the first protecting barrier for organisms. In addition to that,P-gp also acts as an important key in conferring the multi-drug resistance (MDR) phenotype to cancer cells [29], and through the modulation of P-gp, the pharmacokinetics of prescription drugs changes and the risk of drug-drug interactions(DDIs) increases [30]. While AC has been demonstrated to be absorbed via active transport mediated by efflux transporters[22,26,31,32]. The bioavailability of AC, however, was very limited,roughly 5%-10%after oral administration of pure chemical or water extract [33]. In addition, Ma [34] found that the accumulation of rhodamine at the basolateral side, as the probe substrate of P-gp,can be greatly enhanced after co-incubation with liquorice in a dose-dependent manner,which may be associated with inhibition of P-gp efflux transporters by liquorice. The effect of liquorice and its active ingredients on P-gp may therefore be critical for absorption process of AC and further its efficacy/toxicity by changed exposure in vivo. Consequently, it is worthy of being carefully investigated to better understand of compatibility of the herb-pair.
In this study, multiple approaches including in silico, in vitro and in vivo have been utilized to clarify the potential interactions between AC and active ingredients existing in liquorice. The in silico and Caco-2 cell model were adopted to study the inhibition of active ingredients of liquorice on P-gp. The investigations of the anti-inflammatory effects of AC on its own and combinations with those active ingredients of liquorice, as well as pharmacokinetics were performed after oral administration in rats.
2. Material and methods
2.1. Chemicals and reagents
AC (purity: 98.8%), liquiritin (purity: 98.5%), liquiritigenin (purity: 99.0%), licochalcone A (purity: 99.9%), isoliquiritin (purity:99.9%), isoliquiritigenin (purity: 99.5%) and glycyrrhetinic acid(purity:98.0%)were purchased from Vientiane Hengyuan Tech Co.,Ltd (China), while glycyrrhizic acid (purity: 99.0%) was obtained from Solarbio Science & Technology Co., Ltd (China). The chemical structures of these compounds are shown in Fig.1.The purity of the testing chemicals was authenticated by HPLC method. Indomethacin was purchased from Shanxi Yunpeng Pharmaceutical Co., Ltd(China). Verapamil was obtained from MCE. Complete Freund's Adjuvant and rhodamine-123 (Rh-123) were obtained from Sigma(USA). HPLC grade acetonitrile and formic acid for liquid chromatography were purchased from Fisher Scientific (USA).
2.2. Cell culture
Caco-2 cell from a human colon adenocarcinoma cell line was obtained from the cell bank of the Chinese Academy of Sciences(China) and maintained between passages 18-43. Caco-2 cells were cultured in DMEM supplemented with 10%fetal bovine serum and 1% antibiotic (10,000 U/mL penicillin, 10,000 μg/mL streptomycin) at 37°C in humidified air containing 5% CO2.
2.3. Animals
Adult male Sprague-Dawley rats (weight, 180-220 g) with jugular vein intubation were purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd(Certificate no.:SCXK(Jing)2016-0006,Beijing,China)for pharmacokinetic study,while adult male Sprague-Dawley rats(weight,140-160 g)were purchased for study of anti-inflammatory effects of AC. All animals were housed at the animal facility in Tianjin Institute of Pharmaceutical Research New Drug Assessment Co., Ltd and allowed to have access to food and water freely. All animal treatments were approved by the Institutional Animal Ethical Committee of Tianjin Institute of Pharmaceutical Research (Certificate no.: SYXK (Jin) 2016-0009,Tianjin, China).
2.4. Molecular docking
2.4.1. System setup
The active ingredients of liquorice, including flavone and pentacyclic triterpenoid were built from the scratch as ligands, their three-dimensional conformations were also generated.The crystal structure of human P-gp in the ATP-bound with outward-facing conformation (PDB ID: 6c0v) [35] obtained from PDB databank was chosen as the target for docking calculation.The bound ligand,ATP extracted from the complex, was taken as the reference to specify the active site for binding. The receptor was treated with Amber for molecular docking. The molecular geometries were optimized and the atom-centered point charges were calculated to fit the electrostatic potential using RESP20. The receptor was treated by leap module in Amber 12 with amber ff12SB (for macromolecule) and Generalized Amber Force Field (GAFF, for chemicals), the receptor was then saved in mol2 format prior to molecular docking.
2.4.2. Molecular docking
GOLD (Genetic Optimization for Ligand Docking) was used to perform molecular docking calculations at both sites. Each ligand was docked into the binding site for 10 times, starting from a different random population of ligand orientations and using the default settings by GA(genetic algorithm).All torsion angles in each compound were allowed for free rotation and the results of the different docking runs were ranked using Gold Score. In addition,early termination was allowed when the accepted RMSD of solutions was achieved.
2.5. Transport study with Caco-2 cell model
Rh-123 was taken as the probe substrate to investigate the potential effects of active ingredients existing in liquorice on function of P-gp. Bidirectional transport assays in Caco-2 cell were performed by adding compounds to both apical (A-to-B) and basolateral chamber (B-to-A). Cells were plated at 1×105cells/well density in 24-well plates,and incubated with 50 μM Rh-123 in the presence or absence of verapamil (50 μM), liquiritin (10 μM), liquiritigenin (10 μM), isoliquiritin (10 μM), isoliquiritigenin(10 μM),licochalcone A(10 μM),glycyrrhizic acid(10 μM)and glycyrrhetinic acid (10 μM).100 μL of samples were collected at 60 min from the chambers to study the transport. For fluorescence intensity detection, samples collected were detected in 96-well black plate by Microplate UV/VIS spectrophotometer (Varioskan Flash, Thermo Fisher Scientific,Waltham,MA).In all studies,measurements were performed in triplicate. The apparent permeability coefficients(Papp) were calculated by using the equation of Artursson and Karlsson:

wherePappis the apparent permeability coefficient (cm/s), ΔQ/Δt(μmol/s)is the rate at which the compound appears in the receiver chamber, C0(μM) is the initial concentration of the compound in the donor chamber,and A(cm2)represents the surface area of cell monolayer.

Fig.1. Chemical structures of aconitine and active ingredients in liquorice.
2.6. Anti-inflammatory effects of AC and mixture with active ingredients of liquorice
The SD rats were randomly divided into seven groups with eight rats in each. Apart from the untreated group, different combinations of AC and active ingredients in liquorice were orally administered to rats as follows with indomethacin as positive(1 mg/kg):1) AC group (1 mg/kg), 2) AC-liquiritin group (1 mg/kg and 10 mg/kg, respectively), 3) the AC-liquiritigenin group (1 mg/kg and 10 mg/kg, respectively),4) the AC-glycyrrhizic acid group (1 mg/kg and 10 mg/kg, respectively) and, 5) the AC-glycyrrhetinic acid group(1 mg/kg and 10 mg/kg,respectively).Prior to dosing,0.1 mL of Freund's adjuvant was given to each individual rat by intracutaneous administration at right hind foot.After oral administration,rats were inspected for edema,and hind paw volume was recorded by measuring paw volume at the following time points: 0,1, 2, 4 and 6 h.
2.7. Pharmacokinetic studies
The SD rats with jugular vein intubation were randomly divided into five groups with six rats in each. The rats received dosing through oral administration as follows:A)the AC(1 mg/kg),B)the mixture of AC(1 mg/kg)and liquiritin(10 mg/kg),C)the mixture of AC (1 mg/kg) and liquiritigenin (10 mg/kg), D) the mixture of AC(1 mg/kg)and glycyrrhizic acid(10 mg/kg),and E)the mixture of AC(1 mg/kg) and glycyrrhetinic acid (10 mg/kg). All chemicals were dissolved in 0.5%Na-CMC.Prior to dosing,animals were fasted for 12 h with water ad libitum. After oral administration, blood samples were collected via jugular vein tube (about 0.3 mL) into heparinized tubes at the following time points:0.25,0.5,1,1.5,2,4,6,10 and 24 h.Then,the blood samples were immediately centrifuged at 13000 rpm for 5 min at 4◦C,and the resulting plasma was collected and stored at -80◦C until analysis.
2.8. Determination of AC by LC-MS/MS
AC was analyzed by LC-MS/MS. A Waters Xevo TQD-Acquity UPLC H-Class Bio system was used. The LC conditions used were as follows: column, Acquity UPLC BEH C18 (2.1 mm×50 mm,1.7 μm);flow rate,0.3 mL/min;injection volume,10 μL;and column temperature, 40°C. The mobile phases consisted of 0.05% formic acid in water (phase A) and 100% acetonitrile (phase B). Column separation was performed using a gradient elution program:0-1min,15% phase B; 1-2.5 min, 30% phase B; 2.5-4 min, 45% B;4-4.1 min, 90% phase B; 4.1-6 min,15% phase B.
Data acquisition was performed using a Waters acquity Xeno TQD H-class bio spectrometer equipped with an ESI source and masslynx software (version 4.1). The parameters used for mass spectrometry were as follows: spray voltage, 3.5 kV; ion source temperature,150°C;sheath gas pressure,50 L/h;auxiliary gas flow,50 L/min;capillary temperature,400°C.Quantification was carried out by multiple reaction monitoring (MRM) in positive ionization mode. Sinomenine was used as an internal standard (IS). The transitions selected for quantification werem/z646→m/z586 for AC andm/z330→m/z181 for IS.The collision energy values for AC and IS were 34 and 30 V, respectively.
2.9. Pharmacokinetic analysis
Pharmacokinetic parameters were calculated by the software package DAS. The plasma concentration data was analyzed using non-compartmental analysis to obtain primary pharmacokinetic parameters,for instance,elimination half-life(t1/2),area under the concentration-time curve,maximum concentration(Cmax)and the time to reach the peak concentration (Tmax) mean values and standard deviations were also calculated. Statistical analysis was also performed. APvalue <0.05 was considered to be statistically significant.

Table 1GOLD scores of active ingredients of liquorice with outward facing conformation of human P-gp.
3. Results
3.1. Molecular docking
The docking results were clustered and then carefully analyzed through visualization. The GOLD scores are presented in Table 1.The docked poses at both sites were very similar. Taking site A as example, it was observed that flavones such as liquiritin, liquiritigenin, and others were able to form hydrogen bonds with residues tyrosine 401 and arginine 404 to locate their orientations.The tails of the molecules may also be able to build connections with serine 434 at the far end (Figs. 2 and 3 for docked poses). While pentacyclic triterpenoids, for instance, glycyrrhizinic acid and glycyrrhetinic acid, cannot form hydrogen bonds, in terms of their structure.The extensive hydrophobic contacts between side chains of residues and aromatic rings,however,contributed to stabilizing the molecules in the cavity. The docked poses revealed and scores given might suggest potential inhibition of active ingredients of liquorice by bound with P-gp.
3.2. The effect of active ingredients in liquorice on transport of Rh-123 across Caco-2 cell monolayer
Clearly,thePappvalues in the presence of testing substances and known inhibitors verapamil changed, by comparison with that of control. ThePappand RE values are presented in Table 2. The resulting data revealed that the concentrations of Rh-123 in Caco-2 cell from basolateral to apical side in the presence of liquiritin and other compounds declined to varying degrees, up to roughly 23%,indicating that transport of Rh 123 across membrane medicated by P-gp was inhibited (Figs. 4 and 5).
3.3. Anti-inflammatory effect of AC with/without active ingredients of liquorice in SD rats
The Freund's adjuvant was adopted to induce and assess the anti-inflammatory effect of AC with/without active ingredients of liquorice in SD rats with 1 mg/kg indomethacin as positive control.The edema appeared soon after adjuvant was administered. As shown in Figs. 6 and 7 and Table 3, the hind paw volume of each individual rat of untreated group rapidly increased from 1.7 mL at the beginning to approximately 2.6 mL until end of observation.The treated group was indicative that the edema was reduced significantly,and the anti-inflammatory effect of indomethacin and AC was comparable, in terms of the volume of edema. In addition,all treatment groups with combined use of active ingredients of liquorice exhibited more potent effect on edema induced than AC on its own to various degree,apart from the group with glycyrrhizic acid. The results confirmed the synergic effect of combination of the two herbs (Table 3, Figs. 6 and 7).
3.4. Effect of active ingredients of liquorice on the pharmacokinetics of AC in SD rats

Fig.2. Docked poses of active ingredients of liquorice into active sites of P-gp in outward facing conformation.(A)liquiritin,(B)isoliquiritin,(C)liquiritigenin,(D)isoliquiritigenin,(E) glycyrrhizic acid, (F) glycyrrhetinic acid, (G) licochalcone A. The docked chemicals were color coded.
The concentration of AC in rat plasma was quantified by the methods described previously. The pharmacokinetic parameters were then calculated by DAS program. The differences among treatment groups and controls were significantly different,whenPvalues were less than 0.05. The pharmacokinetic parameters are presented in Table 4.
The results of pharmacokinetic profile of AC were changed after combination use with chemicals existing in liquorice,according to the data obtained(Fig.8 and Table 4).The critical pharmacokinetic parameters,for instance Cmax,increased from 4.80±0.966 ng/mL to 5.77±0.831, 9.19±1.38, 5.73±0.700, 5.88±0.730 ng/mL, respectively, when co-administered with liquiritin, liquiritigenin, glycyrrhizic acid and glycyrrhetinic acid. Similarly, AUC(0-t)of AC was changed from 25.8±4.36 ng/mL*h to 30.0±6.63, 33.7±7.47,29.1±4.44 and 19.9±3.61 ng/mL·h, respectively. Additionally,other pharmacokinetic parameters were affected.For instance,the Tmaxof AC was slightly changed when combined with liquiritigenin and glycyrrhetinic acid from 1.08±0.376 h to 0.667±0.258 and 0.833±0.258 h, respectively, suggesting the combination may speed up the absorption process(Fig.8 for pharmacokinetic pattern of AC).

Fig. 3. Binding poses and interactions of active ingredients of liquorice within the active site of human P-gp in 2D mode.

Table 2The apparent permeation coefficient of rhodamine-123 across Caco-2 cell monolayer in the presence of verapamil and active ingredients of liquorice (n=3).
4. Discussion
The efflux transporters as the barrier play a vital role in transport of foreign compounds.Since AC and other DDAs have already been demonstrated to be the substrates of efflux transporter [32],for instance, P-gp, BCRP and Mrp2, the absorption process into blood and further other tissues and organs are remarkably affected by the transport process across the intestinal membrane. The reduced transport of Rh-123 was indicative of inhibition of P-gp by active ingredients of liquorice,even though the potency exhibited,in terms of the inhibitory effect of efflux of Rh 123, was moderate.This may still suggest that the accumulation of the substrate was therefore able to increase in systematic circulations.Further,DDAs,for instance, AC may increase its concentration and enhance efficacy caused by pharmacokinetic behavior changed, due to the combined used of active ingredients of liquorice,even unexpected toxicity may be exposed.

Fig. 4. Inhibitory of active ingredients of liquorice on transport of rhodamine-123 as probe substrate of P-gp across Caco-2 cell monolayer. The apparent permeation coefficients were presented in mean ± SD (n = 3). *P <0.05, **P <0.01, compared with control.

Fig.5. Histogram representation of the efflux ratios of rhodamine-123 in the presence of active ingredients of liquorice and verapamil. *P<0.05, **P<0.01, compared with control.

Fig. 6. The change of hind paw volume after oral administration. The rats were administered either aconitine at 1 mg/kg or aconitine in combination use with active ingredients of liquorice at 10 mg/kg, respectively. The indomethacin was chosen as positive control at 1 mg/kg. The untreated group was given saline. The data was present in mean value and error bar (n=6).
The efflux transporter P-gp acting as the most important barrier for controlling the entry of xenobiotics into tissues and organs powered by energy supply of ATP, especially for those toxic chemicals and drugs, has been extensively investigated. Its molecular structure has also been revealed by multiple approaches,for instance X-ray crystallography and electron cryo-microscopy[35-37]. In silico studies have been developed since then to clarify the binding features of small compounds with P-gp, due to the detailed structural information provided for understanding the potential mechanism of action [38]. A previous study by utilizing crystal structure of mouse P-gp (PDB code: 3G60) as the receptor for molecular analysis of a variety of active bioactive ingredients reported that 18β-glycyrrhetic acid was able to build hydrogeon bonds with P-gp,resulting in inhibition on its functions[30].Even though the sequence identity between mice and humans was up to 87%, the relevance of this model was always questioned. The prediction of binding mode should therefore be much more relevant with human P-gp. For the past decades, however, only inwardfacing conformation of P-gy was resolved. The recently determined outward-facing conformation of human P-gp provided a new insight into how ATP promotes substrate release and the movement of compounds across membrane. In this study, active ingredients of liquorice were taken for molecular docking calculations as to determine the binding potential. The resulting data suggested that the predicted solutions were able to fulfill the hydrogen-bond requirement to interact with the H-bond donor or acceptor specified on the Y401,R404 and S434 at the far end,which may help locate the orientation, even though the geometry and strength of the interactions varied.In addition to that,hydrophobic interactions were another key factor for those compounds bound to the protein.Aromatic rings existing overlapped with the sidechain of Y401 on the top of binding site to stabilize the ligand. All molecules tested presented very similar potential binding activities at both cavities. The compounds may take occupancy of the active sites of P-gp and further exhibit inhibition on function.
The transport of Rh-123,as the probe substrate,was chosen for investigating the inhibition of active ingredients of liquorice on efflux transporter P-gp. The resulting data suggested that either flavones or pentacyclic triterpene presented the inhibitory effect on transport of Rh 123. Additionally, AC has been demonstrated to be substrate of efflux transporters [32]; the results were supportive that the chemicals in liquorice are able to increase the accumulation of substrate of P-gp at basolateral side with moderate potency,further in systematic circulation.

Fig. 7. The comparison of hind paw volume of aconitine and combination with active ingredients of liquorice. *P <0.05, **P <0.01, compared with aconitine.

Table 3The hind paw volume (mL) of rats after administration of aconitine and combination with active ingredients of liquorice (n=8).
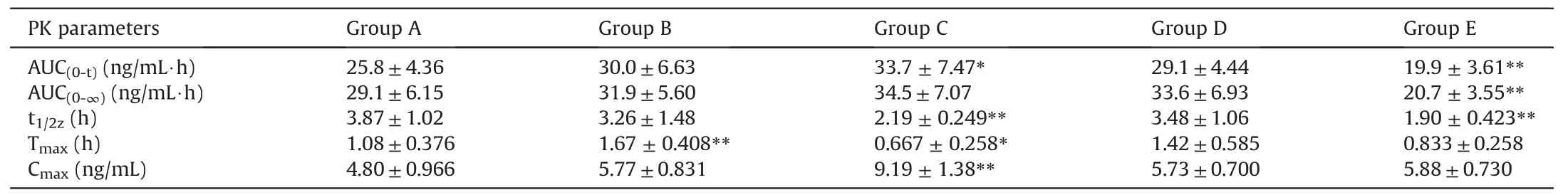
Table 4The pharmacokinetic (PK) parameters of aconitine alone or in combination of active ingredients of liquorice (n=6).
The representative chemicals of liquorice were chosen for further evaluation of the anti-inflammatory effect. By comparison with the hind paw volume of untreated group and treatment group with AC alone, the anti-inflammatory effect of those groups with combination use of chemicals in liquorice was more potent in short period time up to 6 h. Liquorice presented anti-inflammatory effects though the effect of AC was solid, in terms of its antiinflammatory and analgesic effects. The enhanced efficacy against inflammatory was therefore attributed to the increased level in blood or plasma.
The pharmacokinetic study was further supportive that the behavior of AC was affected by co-administration with chemicals of liquorice;in particular,the Cmaxand AUC(0-t)were enhanced,even though the pharmacokinetics of AC altered was limited. For instance,the Cmaxand AUC increased roughly 1.9 folds and 1.3 folds,respectively,by combination with liquiritigenin.The findings were consistent with the observation of its anti-inflammatory effect.The Tmaxvalues were also changed. The peak values were achieved early with liquiritigenin and/or glycyrrhetinic acid,suggesting that the rapid onset may be possible.Even though the differences were not significant, it may still have the potential effect. In the meanwhile, the peak values with liquiritin and glycyrrhizic acid were prolonged.It might be correlated with the metabolism process,for example those two chemicals may take effect after hydrolysis, for which further study is needed to confirm.

Fig. 8. The pharmacokinetic behaviors of aconitine alone or in combination with active ingredients of liquorice (n=6). (A) aconitine+liquiritin, (B) aconitine+liquiritigenin, (C)aconitine+glycyrrhizic acid, (D) aconitine+glycyrrhetinic acid.
5. Conclusions
In conclusion, the current study investigated the potential synergic effect of wutou-gancao herb pair by using the representative chemicals through multiple approaches. The understanding of compatibility of wutou-gancao herb pair was further expanded.The active ingredients of liquorice were able to inhibit the function of efflux transporter P-gp through Caco-2 cell model. The antiinflammatory effects of combination use of AC and chemicals in liquorice and pharmacokinetic behaviors were further investigated.The resulting data were supportive that enhanced efficacy and increased pharmacokinetic parameters after co-administration indicated the potential synergic effect of the herb pair through increased exposure in vivo via inhibiting the key efflux transporter.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by National Key Research and Development Program (Grant 2016YFE0121400), National Natural Science Foundation of China(Grant No.81430096),Macau Science and Technology Development Fund (006/2015/AMJ to Y.Xie), the program of Changjiang Scholars and innovative Research Team in University (No.IRT_14R41), and Tianjin Natural and Science Foundation(No.17YFZCSY01170).The authors are also very grateful for Mr.Chuan LI and Dr.Haizhi ZHANG for their technical assistance and helpful discussion.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2019.09.004.
 Journal of Pharmaceutical Analysis2020年2期
Journal of Pharmaceutical Analysis2020年2期
- Journal of Pharmaceutical Analysis的其它文章
- Recent advances and perspectives of nucleic acid detection for coronavirus
- Molecular immune pathogenesis and diagnosis of COVID-19
- Hollow fiber-based liquid phase microextraction followed by analytical instrumental techniques for quantitative analysis of heavy metal ions and pharmaceuticals
- Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019
- Erucic acid from Isatis indigotica Fort. suppresses influenza A virus replication and inflammation in vitro and in vivo through modulation of NF-κB and p38 MAPK pathway
- Metabolic profiling of four synthetic stimulants, including the novel indanyl-cathinone 5-PPDi, after human hepatocyte incubation
