Expert consensus on management of metabolic disease in Chinese liver transplant recipients
Tian Shen, Li Zhuang, Xiao-Dong Sun, Xiao-Sheng Qi, Zhi-Hui Wang, Rui-Dong Li, Wen-Xiu Chang, Jia-Yin Yang, Yang Yang, Shu-Sen Zheng, Xiao Xu
Abstract Metabolic disease, including diabetes mellitus, hypertension, dyslipidemia, obesity, and hyperuricemia, is a common complication after liver transplantation and a risk factor for cardiovascular disease and death. The development of metabolic disease is closely related to the side effects of immunosuppressants. Therefore, optimization of the immunosuppressive regimen is very important for the prevention and treatment of metabolic disease. The Chinese Society of Organ Transplantation has developed an expert consensus on the management of metabolic diseases in Chinese liver transplant recipients based on recent studies. Emphasis is placed on the risk factors of metabolic diseases, the effect of immunosuppressants on metabolic disease, and the prevention and treatment of metabolic diseases.selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: htt p://creativecommons.org/licenses/by-nc/4.0/
Key words: Liver transplantation; Metabolic disease; Diabetes mellitus; Hypertension; Dyslipidemia; Hyperuricemia; Obesity; Immunosuppressive agents; Consensus
FOREWORD
Thanks to mature surgical techniques and standardized perioperative and long-term management, the survival rate of Chinese liver transplant recipients has gradually improved. According to the Report on the Medical Quality of Liver Transplantation in China in 2018[1], mortality within 1 wk after liver transplantation decreased from 3.7% in 2015 to 2.2% in 2018; the 3-year cumulative survival rate was 78.51% in liver transplant recipients with benign end-stage liver diseases and 75.87% in those with hepatocellular carcinoma who met Hangzhou Criteria. This means that the survival in Chinese liver transplant recipients has reached the international leading level as described in the annual data report 2018 of Organ Procurement and Transplant Network/Scientific Registry of Transplant Recipients[2]. However, chronic diseases after liver transplantation, including metabolic disease, chronic kidney disease, and cardiovascular diseases, are increasing year by year. Metabolic complications, including diabetes mellitus, hypertension, dyslipidemia, obesity, and hyperuricemia, are common after liver transplantation.
Relevant reports show that the incidence rates of diabetes mellitus, hypertension, dyslipidemia, hyperuricemia, and obesity are 30%-40%[3], over 50%[4], 40%-66%[5], 14%-53%[6-9], and 18%-30%[10,11], respectively, and tend to increase with time after liver transplantation[12]. Metabolic disease is closely associated with the development of chronic kidney disease, infections, and cardiovascular diseases and greatly affects the quality of life and long-term survival of recipients[13,14]. However, metabolic disease can be prevented and treated through early intervention.
This consensus is aimed at providing recommendations for the prophylaxis and treatment of metabolic disease in Chinese liver transplant recipients to improve the long-term survival of the recipients.
RECOMMENDATIONS FOR THE PROPHYLAXIS AND TREATMENT OF METABOLIC DISEASE IN LIVER TRANSPLANT RECIPIENTS
Effective immunosuppressive therapy is essential for ensuring the long-term survival of liver grafts after liver transplantation[14], but long-term use of immunosuppressive agents can lead to or aggravate post-transplant metabolic disease. Different immunosuppressive agents have different effects on metabolic disease. Calcineurin inhibitors (CNIs), including tacrolimus (TAC) and cyclosporine A (CsA), are often associated with hypertension, diabetes mellitus, dyslipidemia, and hyperuricemia; glucocorticoids are associated with hypertension, diabetes mellitus, obesity; mammalian target of rapamycin (mTORi) inhibitors are associated with dyslipidemia. However, mycophenolic acids (MPA), represented by mycophenolate mofetil (MMF), and antibody drugs such as rabbit antithymocyte globulin and basiliximab have no effect on metabolic disease (Table 1). Studies have found that basiliximab induction, combined with MMF and the glucocorticoid-free or early withdrawal regimen, or MMF combined with the dose-reduced CNI can decrease the occurrence of immunosuppressive agent-caused metabolic disease and adverse effects by ensuring immunosuppressive efficacy in liver transplant recipients[15-21]. Therefore, based on the improvement of dietary structure and lifestyle, metabolic disease should be well managed through individualized selection of appropriate immunosuppressive agents at a minimum dose according to clinical characteristics of recipients and the use of additional drugs when necessary. It requires involvement of the follow-up doctors of liver transplant recipients when the immunosuppressive regimens need adjustment.
Recommendation 1: The prophylaxis and treatment of post-liver transplantation metabolic disease should be based on changes in dietary habits and lifestyle, paying attention to the adverse effects of immunosuppressive agents, advocating personalized medication. The regimen with basiliximab induction and glucocorticoid-free or CNI minimization containing MPA is feasible.
Prophylaxis and treatment of diabetes mellitus
Post-transplant diabetes mellitus (PTDM) includes pre-existing diabetes mellitus and new-onset diabetes after transplantation (NODAT) in liver transplant recipients. PTDM is a common complication that occurs after solid organ transplantation[15]. In recent years, a considerable number of recipients are diagnosed as diabetes after transplantation because preoperative diagnosis of diabetes has not been standardized. In this case, it cannot be determined whether the patients develop NODAT. As a result, PTDM has been widely used. The 2019 diagnostic criteria for diabetes established by the American Diabetes Association are: fasting plasma glucose (FPG) ≥ 7.0 mmol/L (126 mg/L), 2 h blood glucose during oral glucose tolerance test ≥ 11.1 mmol/L (200 mg/dL), glycated hemoglobin (HbA1c) ≥ 6.5%, or random plasma glucose ≥ 11.1 mmol/L (200 mg/dL) (Table 2)[22,23]. The incidence of pre-transplantation diabetes mellitus is about 33%[24], and that of NODAT is 30%-40%[3]. PTDM is mainly characterized by insulin resistance with chronic progression on symptoms. It presents both the characteristics of type 2 diabetes mellitus (T2DM) and serious complications of type 1 diabetes, such as ketoacidosis[22]. PTDM increases the risks of rejection, infection, cardiovascular events, and death[23,25]. The 2017 Consensus on Managing Modifiable Risk in Transplantation recommends that the blood glucose control target after liver transplantation should be: fasting blood glucose < 6.7 mmol/L (120 mg/dL), peak blood glucose < 8.88 mmol/L (160 mg/dL), or HbA1c < 7%[26].
The main mechanisms of PTDM include decreased insulin sensitivity and β cell failure. PTDM is associated with many factors, such as hepatitis C virus infection, family history of diabetes mellitus, body mass index, immunosuppressive agents,etc. Among immunosuppressive agents, glucocorticoids, CNIs, and mTORi (including sirolimus and everolimus) are the most important pathogenic factors[3,27]. See Figure 1 for details. Studies have shown that the blood concentration of TAC higher than 8 ng/mL at 3 mo after liver transplantation is an independent risk factor for PTDM[28]. On the other hand, polymorphism of diabetes-related genes in donors and recipients has also been confirmed to be associated with PTDM. At present, it is believed that genetic background of T2DM will greatly increase the risk of diabetes. Molecular genetics of diabetes mellitus is more and more widely studied and has been applied in early genetic diagnosis, clinical treatment, and primary prophylaxis of diabetes mellitus. Gene diagnosis provides important clues for the study of diabetes pathogenesis. The studies of the First Affiliated Hospital of Zhejiang University and Shanghai Jiao Tong University School of Medicine have pointed out that ADIPOQ rs1501299[29]and SUMO4 rs237025[30]of the donor and the recipient are associated withPTDM, which will contribute to better managing PTDM.
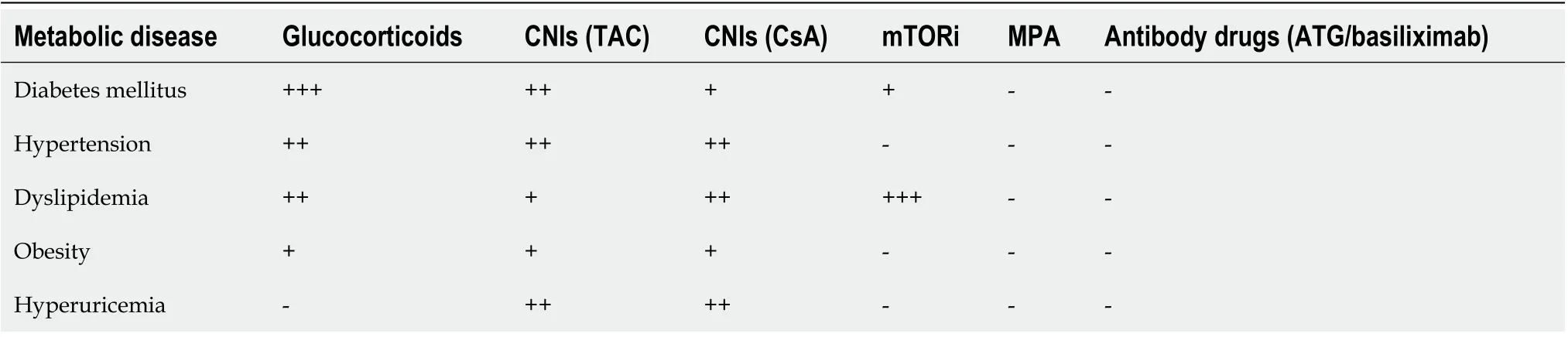
Table 1 Adverse effects of immunosuppressive agents on post-liver transplant metabolic disease
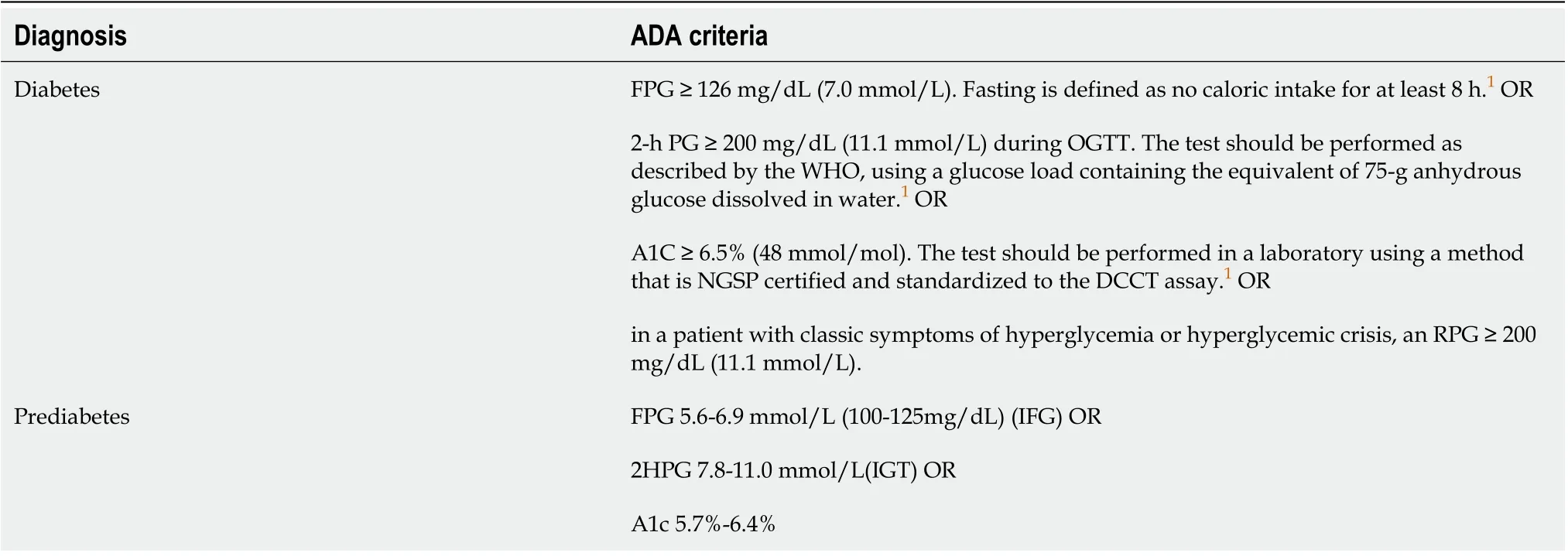
Table 2 2019 diagnostic criteria for diabetes and prediabetes by American Diabetes Association[24,25]
For diabetic liver transplant recipients, the treatment should be based on diet therapy, lifestyle modification, including exercise and weight loss (for obese recipients), and further adjustment of the immunosuppressive regimen and appropriate use of hypoglycemic drugs. Some studies have shown that the risk of NODAT increases by 5%[31]with a prednisone dose increase of 0.01 mg/kg. Compared with conventional glucocorticoid-based regimens, glucocorticoid-free or early withdrawal regimens can significantly reduce PTDM[32,33], while the use of basiliximab and MPA makes the glucocorticoid-free or early withdrawal regimen safe and feasible. Several other studies have shown that the fasting blood glucose level and glycosylated hemoglobin level in liver transplant recipients with PTDM decreased significantly after conversion from TAC to CsA[34,35]. For liver transplant recipients with poor blood glucose control [expressed as continuously elevated blood glucose level (> 11 mmol/L) and HbA1c (> 9%)], conversion from TAC to CsA or the combination of MMF and low-dose TAC is recommended[22,26].
In the early post-transplant period, insulin should be adopted in the presence of obvious hyperglycemic symptoms or markedly elevated glycosylated hemoglobin level before liver function is normal[36]. According to the available evidence, it is essential and safe to keep the average blood glucose < 10 mmol/L and HbA1c < 8% in the first week after liver transplantation while using insulin as a prophylaxis strategy[37]. Moreover, insulin is the best choice when using high-dose glucocorticoids. With the extension of post-transplantation time, the oral hypoglycemic agents can be started when the insulin dose is reduced to less than 24 units daily. Oral hypoglycemic agents need to be selected based on the renal function of the recipients. Biguanide drugs, such as metformin, which are cleared mainly through the kidneys, can only be safely applied in recipients with estimated glomerular filtration rate above 60 mL/min/1.73 m2, while sulfonylureas, such as glipizide and glimepiride, can be used in both and are more preferred for recipients with impaired renal function[38].

Figure 1 Risk factors for post-liver transplant diabetes mellitus[3]. PTDM: Post-transplant diabetes mellitus; CNI: Calcineurin inhibitor; mTORi: Mammalian target of rapamycin; HCV: Hepatitis C virus; CMV: Cytomegalovirus.
Recommendation 2: The blood glucose target after liver transplantation is: Fasting blood glucose < 6.7 mmol/L (120 mg/dL), peak blood glucose < 8.88 mmol/L (160 mg/dL), or HbA1c < 7.0%.
Recommendation 3: Minimization of the dose of glucocorticoids and MPA combined with CNI reduction can reduce the occurrence of PTDM. For liver transplant recipients with poorly controlled blood glucose expressed as continuously elevated blood glucose (> 11 mmol/L) and HbA1c levels (> 9%), converting TAC to CsA is recommended.
Recommendation 4: Insulin is recommended when glucocorticoids are used intravenously and the liver function is not fully recovered. When the dose of insulin is reduced to less than 24 units per day, oral hypoglycemic drugs are recommended if the liver function is normal. Metformin or sulfonylureas can be used in recipients with normal renal function, while sulfonylureas, such as glipizide and glimepiride, are preferred in recipients with impaired renal function.
Prophylaxis and treatment of hypertension
Hypertension is defined as: In the absence of antihypertensive medications, systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg measured three times on different days. Hypertension occurs in more than 50% of liver transplant recipients, and the incidence increases over years with the prolongation of the survival[4]. About 47% of the recipients develop hypertension at 1 to 3 mo after transplantation[39]. Hypertension is a major risk factor for renal insufficiency[40]and cardiovascular disease[41]after liver transplantation. The 2017 Consensus on Managing Modifiable Risk in Transplantation group recommends that the blood pressure target should be under 130/80 mmHg[27], while for recipients with renal injury, it should be below 125/75 mmHg[42].
Hypertension after liver transplantation has complex causes and is associated with multiple factors. The risk of post-operative hypertension increases significantly in obese or diabetic recipients[43,44].
Immunosuppressive agents such as CNIs and glucocorticoids are the main risk factors for new-onset hypertension after liver transplantation. The main mechanism of CNI-induced hypertension is to induce increased systemic circulatory resistance and further influence renal blood flow while glucocorticoids increase vascular resistance and myocardial contractility mainly through their mineralocorticoid effect[14]; see Table 3 for the specific mechanisms. Other risk factors include elderly age, geneticbackground,etc[45].
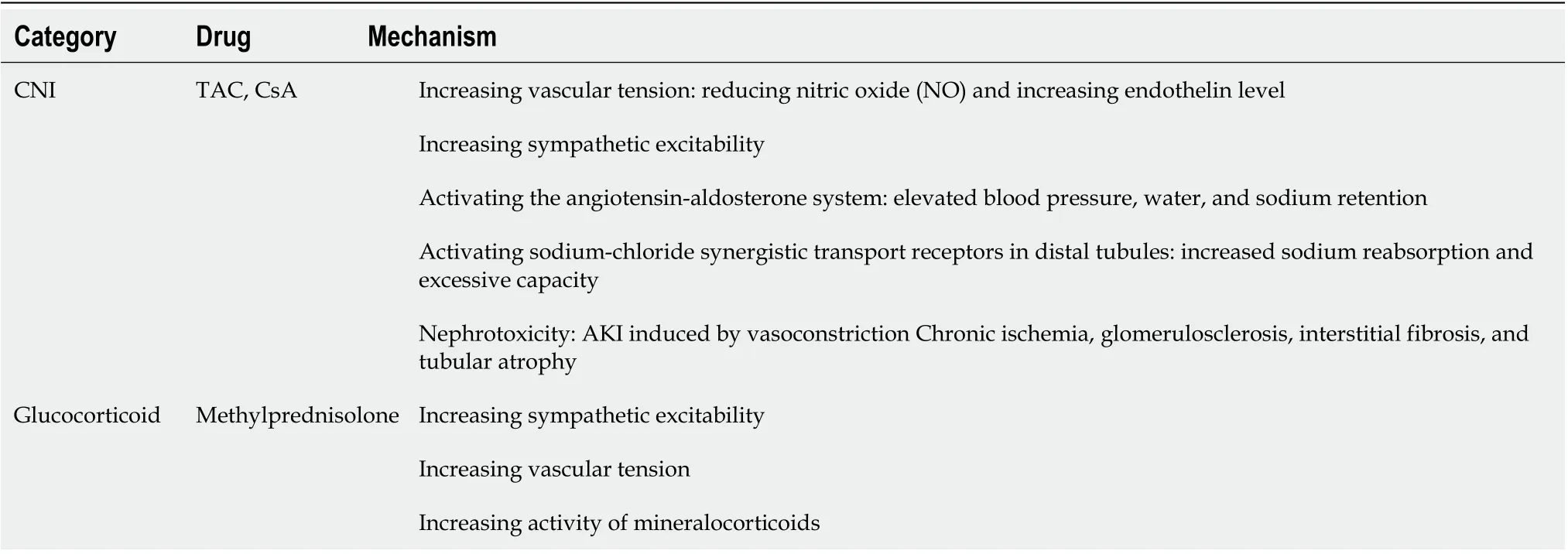
Table 3 Relevant mechanisms of hypertension after liver transplantation induced by common immunosuppressive agents[42]
To control post-liver transplant hypertension, firstly, physicians should comprehensively assess the risk factors associated with the recipients. The existing risk factors should be avoided, and active intervention measures such as changing the unhealthy lifestyle, limiting salt in diet, controlling body weight, taking appropriate sports activities,etc., should be taken[43]. Personalized immunosuppressive regimens should be formulated based on the constitutions of the recipients. The risk of hypertension caused by immunosuppressive agents can be reduced to a certain extent by adjusting the immunosuppressive regimen, for example, minimizing the immunosuppressive agents (CNIs and glucocorticoids) that may cause hypertension. The risk of hypertension caused by CsA is higher than that caused by TAC[46]. However, the regimen of MPA represented by MMF combined with CNI reduction regimen can significantly reduce the risk of new-onset hypertension after liver transplantation, and the glucocorticoid-free or early withdrawal regimen can also significantly reduce the incidence of new-onset hypertension after liver transplantation[47-50].
If the target blood pressure cannot be achieved by changing the lifestyle and adjusting the immunosuppressive regimen, antihypertensive drugs should be used[42]. Common antihypertensive drugs include calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), β-blockers, and diuretics. Because CCBs can directly counteract the vasoconstriction induced by CNIs, they are often used as first-line agents in recipients without proteinuria. Dihydropyridine CCBs (such as nifedipine, amlodipine, nicardipine,etc.,) are preferred due to their relatively decreased medication interaction as compared with the nondihydropyridine CCBs[51]. However, nondihydropyridine CCBs (verapamil and diltiazem) should be used cautiously because they can significantly increase the bioavailability of CNIs. For hypertensive recipients with proteinuria, ACEIs and ARBs can be used as first-line drugs as they can reduce proteinuria[47], but they should be used cautiously when renal function is significantly impaired. The treatment of hypertension with diuretics is mainly suitable for recipients with excessive circulatory blood volume during the early post-transplant period[52]. If the blood pressure cannot be controlled at an appropriate level through single-agent therapy, CCBs and ACEIs or ARBs can be used conjunctively.
Recommendation 5: The blood pressure target after liver transplantation is: Blood pressure < 130/80 mmHg and target blood pressure < 125/75 mmHg for recipients with renal injury.
Recommendation 6: The regimen with minimization of glucocorticoids and the combination of MPA and CNI reduction can reduce post-liver transplant hypertension.
Recommendation 7: CCBs, ACEIs, and ARBs should be used as first-line antihypertensive agents. ACEIs and ARBs are suitable for liver transplant recipients with proteinuria.
Prophylaxis and treatment of dyslipidemia
Chinese Society of Organ Transplantation Standard for the Management of Blood Lipids in Solid Organ Transplant Recipients 2019 points out that dyslipidemia refers to the abnormal elevation of total cholesterol (TC), triglycerides (TG), or low density lipoprotein cholesterol (LDL-C) or reduction of high density lipoprotein cholesterol (HDL-C) in the blood. The normal blood lipid levels in the Chinese population are: TC < 5.18 mmol/L (200 mg/dL), TG < 1.7 mmol/L (150 mg/dL), LDL-C < 3.37 mmol/L (130 mg/dL), and HDL-C ≥ 1.04 mmo1/L (40 mg/dL). With an incidence of 40%-66%, post-liver transplant dyslipidemia is one of the important risk factors for cardiovascular disease. The treatment aim dyslipidemia is LDL-C < 2.59 mmol/L (100 mg/dL), and it is LDL-C < 1.8 mmol/L (70 mg/dL) in those with cardiovascular risk factors[5].
Risk factors for dyslipidemia include dietary habits, age, body mass, other metabolic complications (such as diabetes, hypertension, obesity,etc.), genetic factors, and drugs. After liver transplantation, the use of immunosuppressive agents, especially mTORi, CNIs and glucocorticoids, is the main cause of dyslipidemia. Different CNIs have different effects on blood lipid levels. CsA and mTORi can more effectively reduce blood lipid levels than TAC and mTORi glucocorticoids, respectively. mTORi increases blood lipid levels by increasing liver lipid synthesis, reducing lipid clearance, and inhibiting insulin and insulin-like growth factor pathway[53]. CNIs increase blood lipids mainly by reducing bile acid synthesis, down regulating LDL receptor function, inhibiting cholesterol clearance, inducing cholesterol synthesis, and promoting the conversion of very low-density lipoprotein cholesterol to LDL[5].
For the treatment of dyslipidemia after liver transplantation, changing lifestyle and dietary habits and adjusting the immunosuppressive regimen are preferred. Changes in lifestyle and dietary habits include quitting smoking, limiting salt and alcohol consumption, reducing the intake of saturated fatty acids and cholesterol, choosing foods that can reduce LDL-C, such as phytosterols (2 g/d) and soluble fibers (10-25 g/d), reducing weight (by 5%-10% for recipients who are overweight or obese), and regular physical exercise, including adequate medium-intensity exercise to consume at least 836.8 kJ of calories every day. Of all the immunosuppressive agents, MPA represented by MMF has no impact on blood lipids. Some studies have shown that withdrawal or reduction of CNIs on the basis of MMF will lead to decreases of TC and TG[54]. The dyslipidemia induced by the immunosuppressive regimen of MMF combined with TAC is significantly lower than that induced by mTORi combined with TAC[21]. Refractory dyslipidemia (currently not clearly defined) is generally considered to include the following situations: severe dyslipidemia, such as severe elevation of TG level (≥ 5.65 mmol/L or 500 mg/dL) or (and) LDL level (≥ 4.92 mmol/L or 190 mg/dL); or those who still have elevated TG or LDL level after regular lipid-lowering therapy[55]. For refractory dyslipidemia or dyslipidemia confirmed to be caused by immunosuppressive agents, adjusting the immunosuppressive regimen, such as discontinuing mTORi, replacing CsA with TAC, or using the regimen of reduced CNIs combined with MMF, should be considered[15].
If blood lipid levels cannot be controlled by changing dietary habits, exercising more, and adjusting the immunosuppressive regimen, drugs should be used. Statins are the preferred treatment for hypercholesterolemia, but adverse effects, especially potential hepatotoxicity and myopathy, should be guarded against. Most statins (except hydrophilic statins: pravastatin, fluvastatin) and CNIs are metabolized by cytochrome P450. Therefore, during the use of statins, it is necessary to monitor the plasma concentration of immunosuppressive agents and adjust the dose timely. Simvastatin and CNIs have an obvious interaction in the metabolic process, so simvastatin is not recommended in liver transplant recipients. For hypertriglyceridemia recipients with a normal cholesterol level, fish oil is preferred. If its effect is still unsatisfactory, xyloheptanoic acid or fenofibrate can be added[5]. For recipients with normal liver function, lipid-regulating drugs can be continued. However, such drugs should be discontinued for recipients with liver enzymes over three times higher than the normal values, and liver function should be monitored to identify the causes of abnormal liver function before deciding whether to use lipid-lowering drugs.
Recommendation 8: The blood lipid target after liver transplantation is: LDL-C < 2.59 mmol/L (100 mg/dL). For recipients with cardiovascular risk factors, it is LDL-C < 1.8 mmol/L (70 mg/dL).
Recommendation 9: For liver transplant recipients with dyslipidemia, reducing and withdrawing glucocorticoids should be considered; mTORi should be carefully used and blood lipid indicators should be monitored closely. For refractory dyslipidemia or dyslipidemia caused by immunosuppressive agents, the immunosuppressive regimen should be adjusted; physicians can consider discontinuing mTORi, converting CsA to TAC, or adopting the regimen of reduced CNIs combined with MPA.
Recommendation 10: Statins are the preferred treatment for hypercholesterolemia. Liver function, creatine kinase, and concentration of immunosuppressive agents should be closely monitored before and after using statins.
Recommendation 11: Fish oil is the preferred treatment for hypertriglyceridemia. Xylene heptanoic acid or fenofibrate can be added if fish oil fails to achieve a satisfactory effect.
Prophylaxis and treatment of hyperuricemia
Chinese Nephrologist Association Clinical Practice Guidelines for the Diagnosis and Treatment of Hyperuricemia in Patients with Kidney Diseases 2017 points out that hyperuricemia (HUA) refers to two fasting serum uric acid (SUA) measurements on different days being higher than 420 μmol/L for men and postmenopausal women and higher than 360 μmol/L for non-menopausal women[56]under normal purine dietary conditions. The incidence of hyperuricemia after liver transplantation ranges from 14% to 53%[6-9]. Hyperuricemia can cause gout, uric acid stones, and renal injury and is closely related to T2DM and hypertension, cardiovascular disease, chronic kidney disease,etc[6,7]. Chronic nephropathy caused by hyperuricemia after liver transplantation is one of its main hazards[7]. The hyperuricemia control target: for patients with hyperuricemia complicated with cardiovascular risk and cardiovascular disease, SUA should be lower than 360 μmol/L; for patients with gout, SUA should be lower than 300 μmol/L[57].
Post-liver transplant hyperuricemia may be related to immunosuppressive agents, HUA history, and diuretics[6,7]. It has been confirmed that hyperuricemia is associated with immunosuppressive agents, mainly CNIs (CsA and TAC), which can decrease uric acid excretion by reducing glomerular filtration rate and increasing the renal tubular reabsorption of uric acid[58,59]. Some studies reported that the incidence of HUA in recipients treated with CsA after transplantation was much higher than those receiving TAC[59-61]. However, some other reports showed no significant difference in new-onset HUA between CsA and TAC[62].
The general treatment for hyperuricemia after liver transplantation includes changing the unhealthy lifestyle, such as adopting the low purine diet, drinking more water, alkalinizing urine properly, and exercising more. In addition, screening for related complications, cooperating with specialists, and actively managing metabolic and cardiovascular risk factors related to the elevation of the SUA level should be taken into consideration[56]. Drugs that can increase SUA should be avoided as much as possible. Some reports showed that SUA level decreased by combining MMF with reduced CNIs[63], converting CNIs to mTORi[64], or converting TAC in general dosage form to TAC in sustained-release dosage form[63].
If the general treatment fails to control hyperuricemia effectively, drugs should be used. According to the classification and diagnosis criteria of hyperuricemia, drugs that promote uric acid excretion, such as benzbromarone, fenofibrate, losartan, and those drugs that can inhibit uric acid production such as allopurinol, febuxostat and topiroxostat, can be selected[56]. These drugs can effectively reduce SUA level, with no impact on the concentration of immunosuppressive agents reported[6-8,57,65]. Allopurinol and benzbromarone should be used cautiously in recipients with severe renal insufficiency. Instead, febuxostat or topiroxostat can be used when renal insufficiency exists. However, when liver function is severely impaired, febuxostat or topiroxostat should be used cautiously. Antihypertensive and lipid-lowering drugs, losartan and fenofibrate, also can decrease uric acid, and they are preferred for liver transplantation recipients with hypertension or dyslipidemia[57].
As only observational studies have shown that both SUA and serum creatinine decreased with the uric acid-lowering therapy[6,8], additional studies are needed to confirm whether uric acid-lowering therapy can effectively improve the renal function of liver transplant recipients and reduce the mortality.
Recommendation 12: The long-term control target of SUA after liver transplantation is as follows: for patients with hyperuricemia complicated with cardiovascular risk factors and cardiovascular disease, SUA should be lower than 360 μmol/L; for patients with gout, SUA should be lower than 300 μmol/L.
Recommendation 13: Reduction or even withdrawal of CNIs after liver transplantation combined with MPA or mTORi can contribute to the reduction of SUA.
Recommendation 14: Drugs that promote uric acid excretion or inhibit uric acid production should be selected according to the classification and diagnostic criteria of HUA. Recipients with severe renal insufficiency can be treated with febuxostat or topiroxostat, and benzbromarone can be used when liver function is severely impaired. Losartan and fenofibrate can be used for recipients with hypertension and for those with dyslipidemia, respectively.
Prophylaxis and treatment of obesity
The World Health Organization defines obesity as body mass index (BMI) ≥ 30 kg/m2, and it is further classified into type I obesity (BMI 30-34.9 kg/m2), type II obesity (BMI 34.9-40 kg/m2), and type III obesity (BMI ≥ 40 kg/m2)[66]; when BMI is above 40 kg/m2, it is pathological obesity[67]. New-onset obesity after liver transplantation is defined as BMI ≥ 30 kg/m2[68]during the follow-up after transplantation. Studies showed that the incidence of obesity at 1 year and 3 years post transplantation in adult recipients was 23.7% and 30.6%[10,69]respectively, while it was 19% and 18%, respectively, in pediatric recipients[11]. Obesity is closely associated with the outcome of liver transplant recipients, and overall survival decreases in obese recipients[70], Moreover, new-onset obesity in liver transplant recipients is closely related to cardiovascular events, infections, and respiratory failure[68,71]. BMI after liver transplantation should be controlled below 30 kg/m2and within 25 kg/m2as far as possible.
Weight gain and subsequent obesity are driven by a complex interplay of genetic, physiological, behavioral, and environmental factors[72]. Obesity before transplantation is a very high risk factor for obesity after transplantation[73]. Other risk factors include genetic and psychological factors (such as depression), age, sex, race, dietary habits, exercise habits, immunosuppressive agents,etc[68,74]. For liver transplant recipients treated with immunosuppressive agents, the dose and duration of glucocorticoids are the main causes of weight gain[10,68]. The standard-dose CNIs cause more weight gain than reduced CNIs combined with MMF or mTORi[75]. It has been reported that TAC users are more likely to gain weight 1 year after liver transplantation than CSA users[69].
Obese recipients can control BMI below 25 kg/m2by changing their lifestyle (diet/exercise) and medication and by undergoing surgery when appropriate[27]. Reduction or discontinuation of glucocorticoids is recommended for high-risk recipients, and early conversion of TAC to CsA may also be considered[76]. In addition to improving lifestyle and adjusting immunosuppressive agents, drugs and weightloss surgery should be introduced in the early post-transplant period when necessary[77]. Drugs for obesity can be classified into non-central weight-loss drugs (with orlistat as the representative drug), central weight-loss drugs (sympathomimetic drugs, such as phentermine; 5-hydroxytryptamine drugs, such as lorcaserin), and hypoglycemic drugs (such as metformin, liraglutide)[78]. For liver transplant recipients with refractory obesity, it is difficult to achieve significant results through diet, exercise, and drugs. In these patients, weight-loss surgery has become the main treatment regimen, including Roux-en-Y gastric bypass surgery, gastric sleeve resection, and biliary-pancreatic shunt surgery[79,80]. In addition, endoscopic therapy can limit the contact between food intake and gastrointestinal mucosa, thus limiting absorption and achieving weight loss[77]. However, neither surgical nor endoscopic therapy has become a treatment standard for liver transplant recipients[77,80].
Recommendation 15: The BMI target after liver transplantation is < 25 kg/m2. The regimen of glucocorticoids minimization with reduced CNIs can contribute to reducing weight gain after liver transplantation. For obese recipients who have failed to respond to behavioral therapy and drug therapy, surgical treatment may be considered.
MONITORING OF METABOLIC DISEASE IN LIVER TRANSPLANT RECIPIENTS
For liver transplant recipients, monitoring of metabolic disease should be the focus. The immunosuppressive regimen should be adjusted timely according to disease situation. It should be evaluated at least every 6 mo to reduce the long-term toxicity of drugs, and the possible secondary cardiovascular events and renal impairment. Glucose, blood pressure, lipid, serum uric acid, and BMI should be taken as routine follow-up monitoring items after liver transplantation. Metabolic disease monitoring in liver transplant recipients is not substantially different from that in non-transplant patients. For diabetic recipients, lycosylated hemoglobin is the gold standard for longterm blood glucose control, while self-blood glucose monitoring is the primary measurement. The combination of glycosylated hemoglobin screening and oral glucose tolerance test is an ideal method with both examination and diagnostic efficiency[22]. Family blood pressure measurement is also encouraged for hypertensive recipients; LDL-C, TG, TC, and serum uric acid are the basic monitoring items for liver transplant recipients with dyslipidemia and hyperuricemia[15,81]; long-term monitoring of body weight after liver transplantation is required.
For liver transplant recipients with diabetes mellitus, hypertension, dyslipidemia, hyperuricemia, or obesity, besides the basic monitoring items mentioned above, electrocardiogram, 24-h ambulatory blood pressure, coronary computed tomography angiography, B-mode ultrasonography of the carotid artery, urinary protein, ophthalmoscopy, bilateral renal ultrasound, joint X-ray or computed tomography, and other examinations should be performed (Table 4). Through these examinations on target organs, cardiovascular and cerebrovascular diseases, chronic kidney disease, retinopathy, gouty arthritis, and other diseases that may be secondary to metabolic disease can be promptly detected and diagnosed, so as to improve the long-term survival of liver transplant recipients. The efficacy of the treatments on metabolic disease should be evaluated every 3 mo[82]. Standardized monitoring needs the attention of transplantation physicians and the cooperation of recipients.
Recommendation 16: For long-term survival of liver transplantation, attention should be paid to monitoring of metabolic disease.
Recommendation 17: The immunosuppressive regimen should be evaluated at least once every 6 mo in order to reduce long-term toxicity and should be adjusted as needed.
Recommendation 18: Blood pressure, lipid, glucose, serum uric acid, and BMI should be monitored every 3 mo in the first year after liver transplantation, and every year after that, and the efficacy of the treatment for metabolic disease should be evaluated every 3 mo.

Table 4 Monitoring of metabolic disease after liver transplantation
 World Journal of Gastroenterology2020年27期
World Journal of Gastroenterology2020年27期
- World Journal of Gastroenterology的其它文章
- Histopathological landscape of rare oesophageal neoplasms
- Details determining the success in establishing a mouse orthotopic liver transplantation model
- Modified percutaneous transhepatic papillary balloon dilation for patients with refractory hepatolithiasis
- Serum ceruloplasmin can predict liver fibrosis in hepatitis B virusinfected patients
- Acceptance on colorectal cancer screening upper age limit in South Korea
- Transarterial chemoembolization with hepatic arterial infusion chemotherapy plus S-1 for hepatocellular carcinoma
