Can contrast enhanced ultrasound differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma?
Jia-Yan Huang, Jia-Wu Li, Wen-Wu Ling, Tao Li, Yan Luo, Ji-Bin Liu, Qiang Lu
Abstract
Key words: Diagnosis; Contrast enhanced ultrasound; Hepatocellular carcinoma; Intrahepatic cholangiocarcinoma; Liver imaging reporting and data system
INTRODUCTION
Liver cancer is the sixth most common cancer worldwide and the fourth leading cause of cancer-related death[1]. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) account for approximately 95% of all primary liver cancers[2,3]. However, ICC is more likely to result in a worse prognosis[4], and the treatment for ICC is quite different from that for HCC in specific cases. Therefore, it is of paramount importance to differentiate these two entities for appropriate intervention and better judgment of prognosis.
Over the past decade, contrast-enhanced ultrasound (CEUS) has been recommended as a useful tool for the characterization of focal liver lesions by several international professional societies in Europe and Asia[5-9]. However, CEUS was removed from the updated American Association for the Study of Liver Diseases 2011 guidelines as a diagnostic technique for HCC[10]because a single-center study with a limited sample size reported that CEUS may misdiagnose ICC as HCC in cirrhosis patients[11]. ICC is more likely to display peripheral rim arterial phase hyper- enhancement (APHE) followed by early and marked washout in CEUS images compared with HCC[12-16]. However, some studies showed that the aforementioned CEUS patterns may be detected in some HCC cases as well[12,13,17-19], which adds to the difficulty in the differential diagnosis between the two entities.
The America College of Radiology released CEUS liver imaging reporting and data system (LI-RADS) for standardizing CEUS diagnosis of liver nodules in patients at risk for HCC[19,20]. In this system, the LR-M category represents malignancies but is not specific for HCC[20]. However, previous studies revealed a high sensitivity of LR-M criteria for diagnosing non-HCC malignancy but a quite low positive predictive value (PPV) because of a high proportion of HCC in this category[15,17,21]. Until now, the diagnostic accuracy of LR-M criteria in differentiating ICC and LR-M HCC (defined as HCC, categorized as LR-M according to CEUS LI-RADS) has not been fully studied. Hence, this study focused on analyzing the CEUS features of ICC and LR-M HCC and further evaluating the possibility and efficacy of LR-M criteria in differentiation between them. We also associated CEUS patterns with tumor markers to investigate the potential diagnostic efficacy.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board of West China Hospital of Sichuan University, and the requirement of written informed consent from patients was waived.
Patient selection
Patients with complete CEUS records together with pathologically confirmed ICC and LR-M HCC between January 2015 and October 2018 were included in this retrospective study. The patient selection flow chart is presented in Figure 1. In case of multiple lesions, the dominant tumor was chosen for analysis. Therefore, a total of 228 lesions were collected for analysis in this study.
Ultrasound examination
All enrolled patients underwent conventional ultrasound and CEUS examinations using a Philips IU 22 system (Philips Medical Solutions; Mountain View, CA, United States) with a C5-1 MHz convex transducer. The CEUS study was performed after conventional ultrasound examination of the liver. A 1.2-2.4-mL bolus injection of sulfur hexafluoride-filled microbubble contrast agent (SonoVue; Bracco, Milan, Italy) was administeredviaa 20-gauge angiocatheter needle placed in the antecubital vein, followed by flushing with 5 mL of 0.9% sodium chloride solution. After the completion of the SonoVue injection, the imaging timer was initiated simultaneously. The still images and video clips of CEUS examination were digitally stored for further evaluation.
Image analysis
The CEUS images were numbered randomly after deidentification and then reviewed by two radiologists (WL and JL) with more than 5 years of experience in liver CEUS examination independently. Both radiologists were blinded to the clinical information of the patients. Arterial phase enhancement, presence or absence of early washout, and washout degree of the liver nodules were analyzed. The APHE pattern refers to lesions that manifest as hyperechoic when compared with the surrounding liver parenchyma in the arterial phase. Rim APHE is a sub-type of APHE, where the enhancement is most pronounced in the periphery of the lesion. Washout refers to a lesion that presents a reduction in enhancement either in whole or in partvsthe surrounding liver parenchyma. Washout that occurs within 60 s is further termed “early washout”; otherwise, it is termed “late washout”. Marked washout is defined as a lesion that is virtually devoid of enhancement (so-called “punch-out”) within 120 s after contrast injection[22]. The enhancing feature of each lesion was analyzed, and the lesions were further classified into relevant categories according to the CEUS LI-RADS (2017 version) by both radiologists. If there was a discrepancy between the radiologists, arbitration from another senior radiologist (QL) with more than 10 years of experience in liver CEUS examination was performed. Meanwhile, the CEUS imaging features of lesions were recorded for further analysis.
Statistical analysis
Quantitative data are presented as the mean ± SD, and qualitative data are presented as absolute numbers and percentages. Enhancing patterns of the nodules in CEUS were compared by using theχ2test, while quantitative variables were compared using student’sttest and the Mann-Whitney test. Logistic regression was used to predict the correlation between LR-M characteristics, serum tumor markers, and ICC or LR-M HCC. The diagnostic capability of CEUS and tumor markers in differentiating between ICC and LR-M HCC was analyzed by receiver operating characteristic (ROC) curve analysis. The cut-off values of 100 U/mL and 20 ng/mL were used for the elevation of carbohydrate antigen 19-9 (CA 19-9) and alpha fetoprotein (AFP), respectively, as recommended by previous studies[23-27]. Interobserver agreement was evaluated by the two radiologists by calculating the κ-value. A κ value < 0.2 indicates poor agreement, 0.21 to 0.40 indicates fair agreement, 0.41 to 0.60 indicates moderate agreement, 0.61 to 0.80 indicates good agreement, and 0.80 to 1 indicates almost perfect agreement. Significance was defined asP< 0.05. Statistical analyses were performed using a statistical software package (MedCalc10.4.7.0, Ostend, Belgium).
RESULTS
A total of 228 patients with 228 pathologically confirmed lesions, including 99 ICCs and 129 LR-M HCCs, were included in this study. The clinicopathological data of the patients, including age, gender, nodule size, etiology, tumor markers, fibrosis stage, and pathological results, are presented in Table 1.
Interobserver agreement regarding the review of enhancing patterns in the arterial phase and portal/late phase showed good consistency, with κ values of 0.72 and 0.88, respectively. The tissue sample used for histological evaluation was obtained from surgical resection or percutaneous biopsy. Liver cirrhosis was found in 2% (2/99) of ICCs and 46.5% (60/129) of HCCs (P< 0.001). Chronic hepatitis B (CHB) was detected in 20.2% (20/99) of ICCs and 88.4% (114/129) of HCCs (P< 0.001), and intrahepatic duct dilatation was present in 17.2% (17/99) of ICCsvs2.3% (3/129) of HCCs (P< 0.001). In terms of tumor differentiation, poor, moderate, and well differentiation was found in 52.7% (68/129), 45.7% (59/129), and 1.6% (2/129) of LR-M HCCs,respectively. Regarding the tumor markers, CA 19-9 was significantly higher in ICC than in LR-M HCC [74.0 (41.9-136.5) U/mLvs18.8 (16.0-22.0) U/mL,P< 0.001], while AFP was significantly lower in ICC than in LR-M HCC [3.0 (2.7-3.5) ng/mLvs67.3 (18.0-146.7) ng/mL,P< 0.001].

Table 1 Demographic and clinicopathological information of 228 enrolled patients, n (%)
CEUS features of ICC and LR-M HCC
The CEUS image characteristics of ICC and LR-M HCC, including arterial phase enhancement pattern, washout onset timing, and washout degree are presented in Table 2. In the arterial phase, three types of enhancing patterns were illustrated: Homogeneous hyperenhancement, heterogeneous hyperenhancement, and rim hyperenhancement. Rim APHE was detected in 50.5% (50/99) of ICCsvs16.3% (21/129) of LR-M HCCs (P< 0.0001) (Figure 2-4). Arterial homogeneous hyperenhancement was observed in 15.2% (15/99) of ICCs and 37.2% (48/129) of LRM HCCs (P= 0.0004) (Figure 5). Early washout of contrast agent was illustrated in 93.4% (93/99) of ICCsvs96.1% (124/129) of LR-M HCCs (P> 0.05). Marked washout of contrast agent within 120 s was shown in 23.2% (23/99) of ICCsvs7.8% (10/129) of HCCs (P= 0.002). Of note, this feature did not show up alone in either of the two entities.
A comparison of the LR-M features between ICC and LR-M HCC is presented in Table 3. Rim APHE followed by early washout was the most frequent combination ofLR-M features, which was detected in 30.3% (30/99) of ICCsvs10.1% (13/129) of LRM HCCs (P= 0.0002). The presence of all three LR-M features in a nodule also showed a significant difference between the two entities (P= 0.0018).
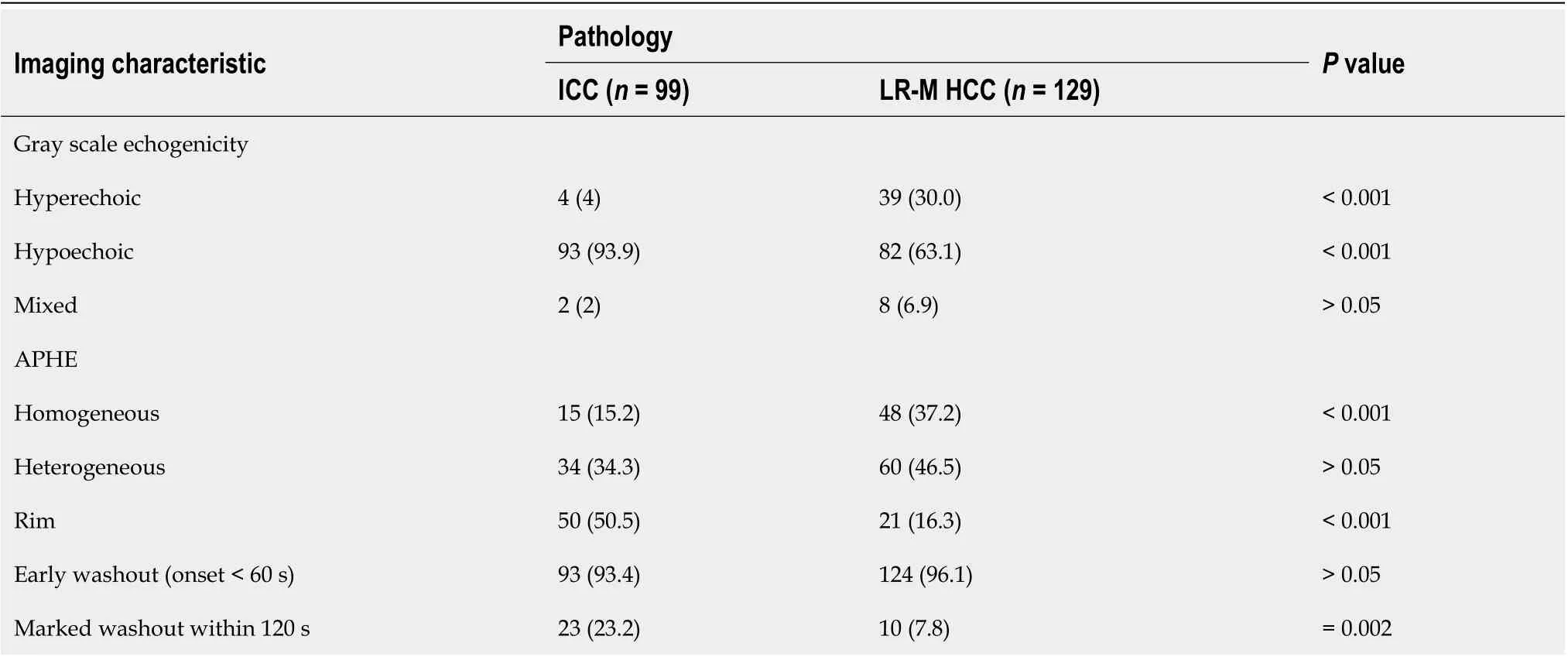
Table 2 Pre-contrast and contrast-enhanced ultrasound features of 228 lesions, n (%)
Taking rim APHE, early washout, marked washout, homogeneous hyperenhancement, CA 19-9, and AFP as independent variables, the regression analysis showed that rim APHE, CA 19-9, and AFP had significant correlations with ICC (r= 1.251, 3.075, and -2.767, respectively;P< 0.01). ROC curve analysis for the diagnostic performance of LR-M characteristics in differentiating ICC from LR-M HCC is presented in Table 4. Rim APHE presented the best diagnostic performance for ICC, and the area under the ROC curve (AUC) was 0.70 [95% confidence interval (CI): 0.63-0.76], with a sensitivity of 70.4% (95%CI: 58.4%-80.7%) and specificity of 68.8% (95%CI: 60.9%-75.9%). When rim APHE was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 (95% CI: 0.76-0.87) and 100% (95%CI: 86.8%-100%), respectively, with specificity decreasing to 63.9% (95% CI: 56.8%-70.5%).
DISCUSSION
The LR-M category of CEUS LI-RADS was generated for lesions that are malignant but not specific to HCC[20]. There was a significantly low PPV of LR-M for the diagnosis of non-HCC malignancy due to a high proportion of HCC cases in this category, leading to the recommendation of biopsy for all CEUS LR-M lesions[28,29]. In this retrospective study, we focused on ICC and LR-M HCC, which composed the majority of LR-M lesions, expecting to achieve a better understanding of the differential diagnosis between the two entities. Our study demonstrated that rim APHE and marked washout were more frequently observed in ICCs than in LR-M HCCs (50.5%vs16.3% and 23.2%vs7.8%, respectively;P< 0.01). Although early washout was the most common feature in both ICCs and LR-M HCCs, the rate difference of this feature between the two entities was not significant. Marked washout did not show up alone either in ICC or in LR-M HCC. Of note, rim APHE was a key feature, which showed a significant positive correlation with ICCs in our study. The AUC, sensitivity, and specificity of rim APHE for the differential diagnosis was 0.70, 70.4%, and 68.8%, respectively. When rim APHE was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 and 100%, with specificity decreasing to 63.9%.
Rim APHE was a symbolic wash-in pattern of ICC detected in 50.5% of ICC cases in the present study, which was in accordance with the rates of 43%-68.5% in previous reports[12-14,18]. Serum biomarkers, especially AFP and CA19-9, have been proven to be helpful for the diagnosis of HCC and ICC. In the study conducted by Chenet al[12], the investigators added CA 19-9 to their CEUS score nomogram to enhance the discriminatory power of the predictive model for the differentiation between ICC andHCC. We found that when using rim APHE plus CA 19-9 for the differential diagnosis, the AUC and sensitivity improved from 0.70 to 0.82 and 70.4% to 100%, respectively. However, rim APHE could be influenced by multiple factors, including tumor size, pathological constitution of a lesion, and liver background[18,30,31]. Small ICCs, especially those ≤ 2 cm, are rich in tumor cells with few fibrous tissues and no central necrosis[32], thus potentially mimicking the homogeneous hyperenhancement pattern of HCC[14,19,33,34]. Meanwhile, ICC showing rim APHE was more likely to be detected in livers without cirrhosis and chronic viral hepatitis[19,30,31,33]. In our study, chronic hepatitis B and cirrhosis were both more frequent in patients with LR-M HCCs than in those with ICCs (88.4%vs20.2% and 46.5%vs2%, respectively;P< 0.001). Similarly, in a recent study by Liet al[18], the authors proved that there was no significant difference in rim APHE, early washout, or marked washout between ICC patients with and without risk factors. All of these features were more frequent in ICCs than in HCCs, regardless of the risk factors].

Table 3 Comparison of the LR-M features between intrahepatic cholangiocarcinoma and LR-M hepatocellular carcinoma
In terms of washout pattern, previous studies indicated that ICC is prone to wash out earlier than HCC[12,13,15,34]. Although early washout was the most frequent feature of both ICCs and LR-M HCCs in this study, no significant difference was found in the rates of early washout between the two entities. This discrepancy may result from the difference in study subjects, as this study focused on LR-M HCC, which presented specific imaging features compared with typical HCC. The feature of washout within 60 s per LR-M criteria may be the primary reason why a substantial number of HCCs were classified as LR-M. In our study, 96.1% (124/129) of LR-M HCCs presented early washout, which is close to the results of 96% (214/224) in the study of Zhenget al[21]. Liuet al[13]found that the average washout time of ICCs was 27.5 s, compared with 70.1 s for HCCs (P< 0.05). Liet al[18]also reported that 90.7% and 92.7% of ICCs in patientswith and without risk factors, respectively, presented washout within 45 s. Thus, the early washout setting in LR-M may need to be further modified to address a considerable number of misdiagnosed HCCs.
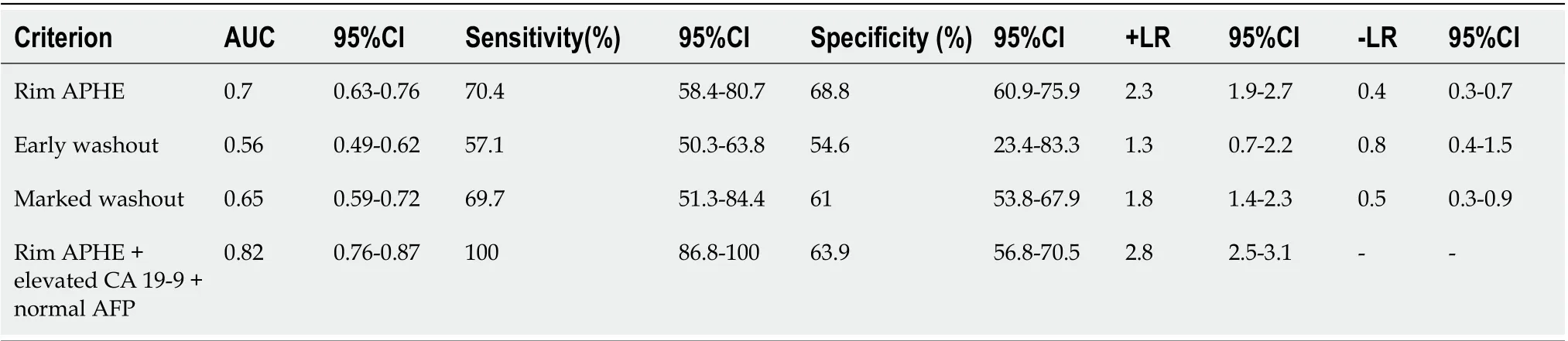
Table 4 The receiver operating characteristic curve analysis for diagnostic performance of contrast-enhanced ultrasound liver imaging reporting and data system LR-M characteristics in differentiation intrahepatic cholangiocarcinoma and LR-M hepatocellular carcinoma
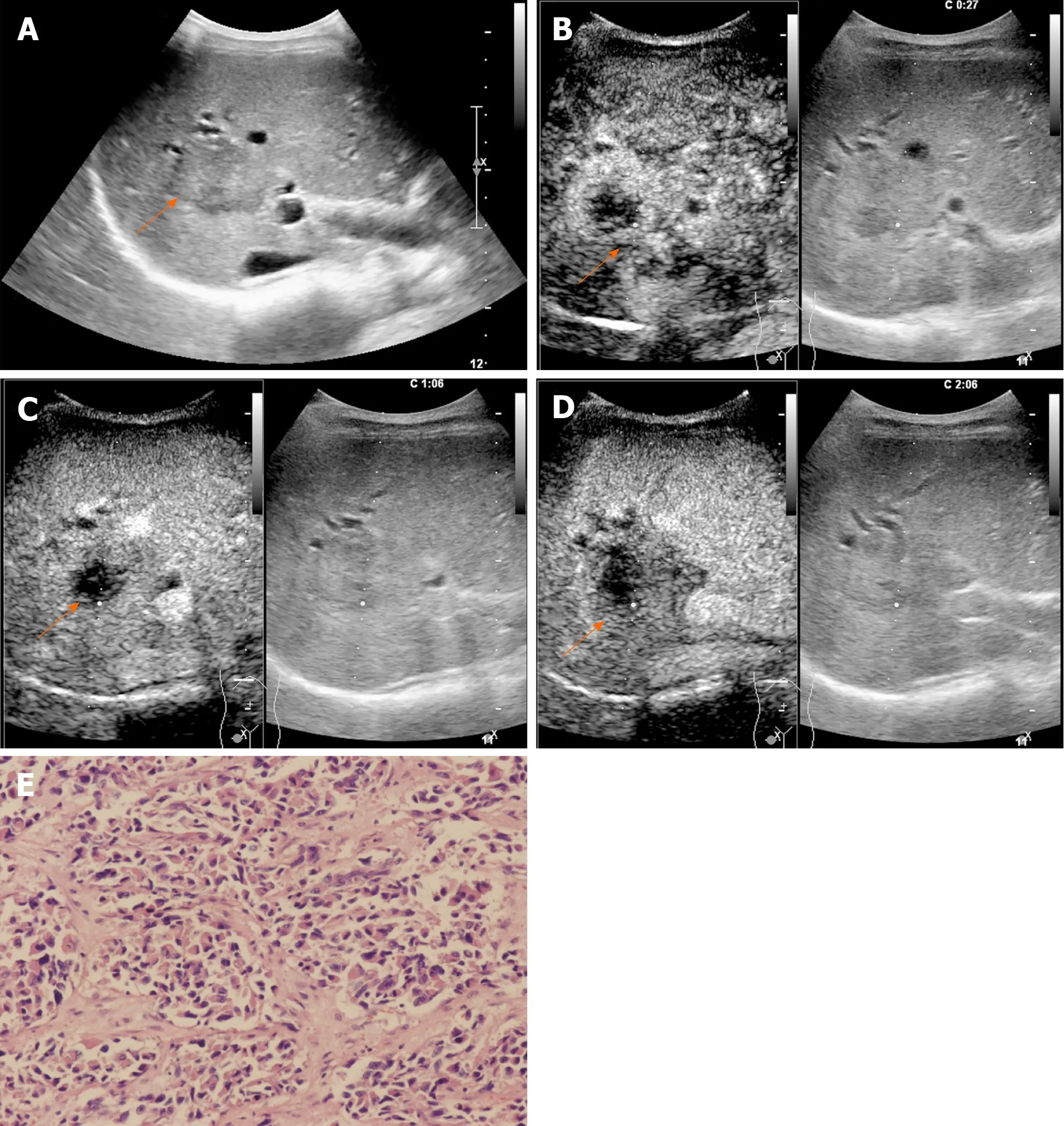
Figure 2 A 54-year-old female patient with a lesion categorized as LR-M. A: Conventional grayscale ultrasound detected a hypoechoic nodule (arrow) 3.6 cm in diameter in the right lobe of the liver; B: Rim arterial phase hyperenhancement (APEH) (arrow) in the arterial phase was demonstrated by contrastenhanced ultrasound; C and D: No washout (arrow) was observed in the early portal phase (by 60 s), and no marked washout (arrow) was observed by 126 s after SonoVue injection. This lesion was designated as LR-M because of rim APEH in the arterial phase; E: Poorly differentiated intrahepatic cholangiocarcinoma was confirmed by histopathology (hematoxylin and eosin staining, × 200).

Figure 3 A 46-year-old female patient with an LR-M lesion. A: A hypoechoic nodule (arrow) measuring 4.7 cm in diameter was identified in the left lobe of the liver by conventional grayscale ultrasound; B: Peripheral rim-like arterial phase hyperenhancement (arrow) in the arterial phase was demonstrated by contrastenhanced ultrasound; C: Early washout (arrow) was observed in the portal phase; D: No marked washout (arrow) was displayed until 155 s after SonoVue injection; E: Poorly differentiated ICC was confirmed by histopathology (hematoxylin and eosin staining, × 200).
Marked washout of contrast agent within 120 s was found more frequently in ICCs than in LR-M HCCs (P= 0.002) in this study. At the time point of 2 min, only 23.2% of the ICCs in our study showed marked washout, which is close to the rate of 25% reported by Hanet al[15]. Some studies also demonstrated that the efficacy of marked washout in differentiating ICC from HCC can only be slightly improved even by postponing the onset time of marked washout to 3 min[15,18]. Zhenget al[21]found 142 out of 153 LR-M nodules showing early washout within 60 s and without punch-out before 5 min were HCCs. The authors re-categorized lesions showing the aforementioned washout patterns into LR-5, and the specificity and PPV of LR-M as a predictor of non-HCC malignancy were remarkably improved from 88% to 96% and 36% to 58%, respectively (P< 0.001). In our study, marked washout within 2 min did not show up alone in both entities. Thus, this feature in LR-M criteria may need to be refined for better practical application.
There are several limitations of our study. First, due to the limited number of ICC cases, CEUS LI-RADS was applied in patients without risk factors for HCC. In clinical practice, chronic hepatitis or cirrhosis would not present in the majority of ICC patients. However, the LR-M features enabled the differentiation of ICC from LR-M HCC in our study, as also validated by Liet al[18]. Second, the scope of the study focused only on ICC and LR-M HCC. Other hepatic malignancies, such as combined hepatocellular-cholangiocarcinoma and metastasis, which also frequently present as LR-M tumors, were not enrolled in our study. Further studies are needed to validate the findings demonstrated in our study and determine, for example, how much referential value marked washout offers the LR-M category in the absence of arterial phase rim APHE and early washout and whether the onset time of early washout and marked washout should be adjusted to reduce the number of HCCs classified as LR-M tumors.
In conclusion, rim APHE is a key predictor for differentiating ICC from LR-M HCC. Rim APHE plus elevated CA 19-9 and normal AFP is a strong predictor of ICC rather than LR-M HCC. Early washout and marked washout have limited value for the differentiation between the two entities.
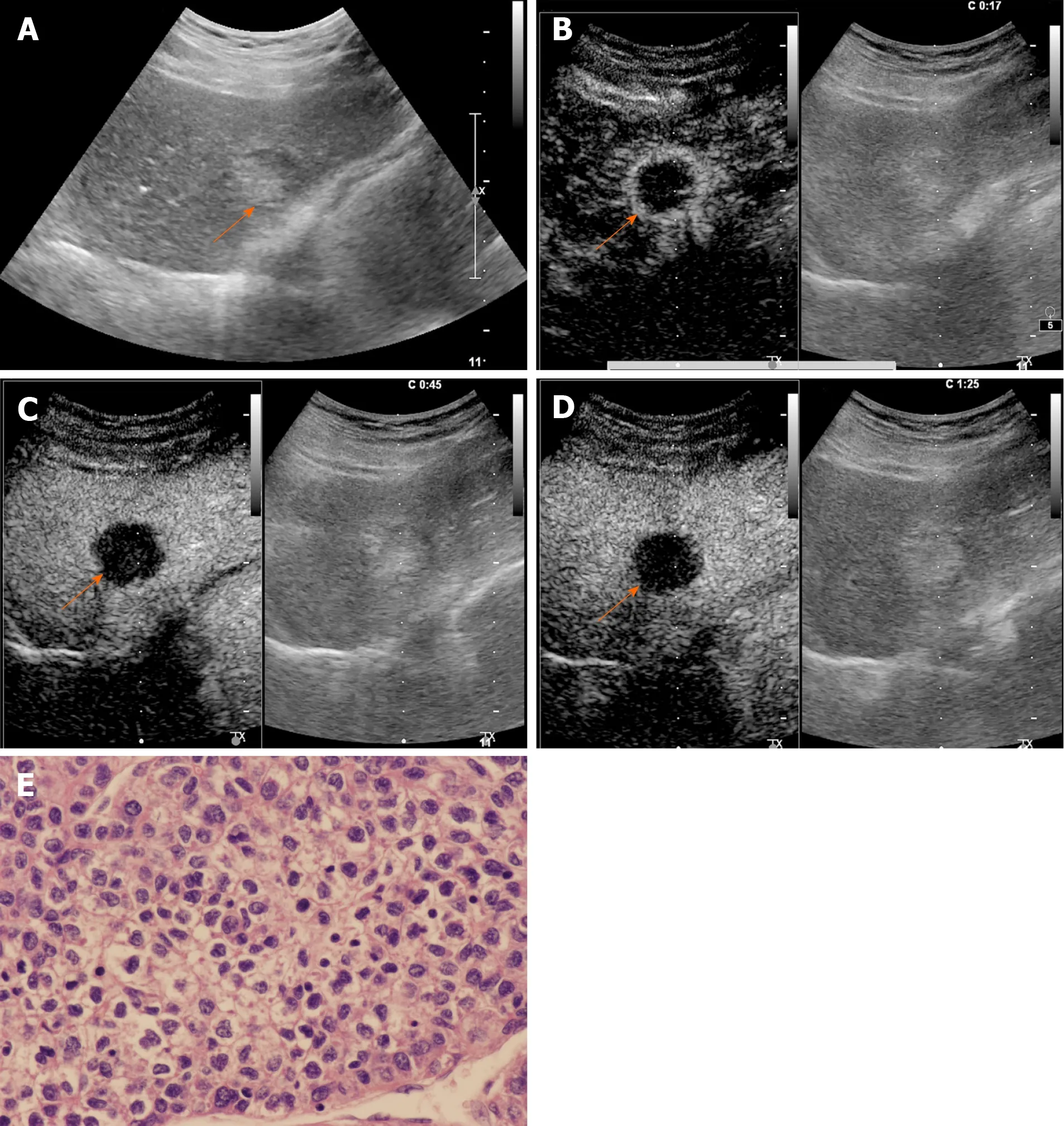
Figure 4 Contrast-enhanced ultrasound examination of a 68-year-old male patient with chronic hepatitis B infection. A: Conventional grayscale ultrasound demonstrated a mixed echo nodule (arrow) measuring 3.0 cm in diameter in the left lobe of the liver; B: Contrast-enhanced ultrasound illustrated rim arterial phase hyperenhancement (arrow) in the arterial phase; C: Early washout of the contrast agent within 60 s was observed (arrow); D: Late-phase imaging demonstrated marked contrast washout (arrow) within 120 s. The lesion was classified as LR-M due to the aforementioned features; E: The nodule was revealed to be poorly differentiated hepatocellular carcinoma by histopathology (hematoxylin and eosin staining, × 400).

Figure 5 A 69-year-old female patient with an LR-M lesion. A: A hypoechoic nodule measuring 3.2 cm in diameter (arrow) was observed by conventional grayscale ultrasound in the left lobe of the liver; B: Contrast-enhanced ultrasound illustrated homogeneous hyperenhancement (arrow) in the arterial phase; C: Early washout was demonstrated (arrow) at 60 s after SonoVue injection; D: No marked washout (arrow) was observed by 120 s; E: This lesion was classified as LR-M, and moderately differentiated intrahepatic cholangiocarcinoma was confirmed by histopathology (hematoxylin and eosin staining, × 200).
ARTICLE HIGHLIGHTS
Research background
Liver cancer is the sixth most common cancer worldwide and the fourth leading cause of cancer-related death. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) account for the majority of all primary liver cancers and differ in treatment and prognosis.
Research motivation
Contrast-enhanced ultrasound (CEUS) has been recommended and widely used for the characterization of focal liver lesions. However, the value of CEUS in differentiating between ICC and HCC remains controversial. The CEUS liver imaging reporting and data system (LI-RADS) released by the American College of Radiology has been developed for standardizing CEUS criteria for the diagnosis of focal liver lesions. In the criteria, the LR-M category represents malignancies but is not specific to HCC. Of note, the presence of a substantial number of HCCs in this category elevates the difficulty in the differential diagnosis between ICC and HCC, and the efficacy of LR-M features for the differentiation between them has not yet been fully evaluated.
Research objectives
The purpose of this study was to investigate the possibility and efficacy of differentiating ICC from HCC classified in the LR-M category according to the CEUS LI-RADS.
Research methods
Patients with complete CEUS records together with pathologically confirmed ICC and LR-M HCC (HCC classified in the CEUS LI-RADS LR-M category) between January 2015 and October 2018 were included in this retrospective study. Each ICC was assigned a category as per the CEUS LI-RADS. The enhancement pattern, washout timing, and washout degree between the ICC and LR-M HCC were compared using theχ2test. Logistic regression analysis was used for prediction of ICC. Receiver operating characteristic curve analysis was used to investigate the possibility of LR-M criteria and serum tumor markers in differentiating ICC from LR-M HCC.
Research results
A total of 228 nodules (99 ICCs and 129 LR-M HCCs) in 228 patients were included. The mean sizes of ICC and LR-M HCC were 6.3 ± 2.8 cm and 5.5 ± 3.5 cm, respectively (P= 0.03). Peripheral rim-like arterial phase hyperenhancement (rim APHE) was detected in 50.5% (50/99) of ICCsvs16.3% (21/129) of LR-M HCCs (P< 0.001). Early washout was found in 93.4% (93/99) of ICCsvs96.1% (124/129) of LR-M HCCs (P> 0.05). Marked washout was observed in 23.2% (23/99) of ICCs and 7.8% (10/129) of LR-M HCCs(P= 0.002), while this feature did not show up alone either in ICC or LRM HCC. Homogeneous hyperenhancement was detected in 15.2% (15/99) of ICCs and 37.2% (48/129) of LR-M HCCs (P< 0.001). The logistic regression showed that rim APHE, carbohydrate antigen 19-9 (CA 19-9), and alpha fetoprotein (AFP) exhibited significant correlations with ICC (r= 1.251, 3.074, and -2.767, respectively;P< 0.01). Rim APHE presented the best enhancement pattern for diagnosing ICC, with an area under the receiver operating characteristic curve (AUC) of 0.70, sensitivity of 70.4%, and specificity of 68.8%. When rim hyperenhancement was coupled with elevated CA 19-9 and normal AFP, the AUC and sensitivity improved to 0.82 and 100%, respectively, with specificity decreasing to 63.9%.
Research conclusions
This study illustrated that rim APHE is a key predictor for differentiating ICC from LR-M HCC. Rim APHE plus elevated CA 19-9 and normal AFP is a predictor of ICC rather than LR-M HCC. Early washout and marked washout have limited value for the differentiation between the two entities.
Research perspectives
Rim APHE is a key predictor for differentiating ICC from LR-M HCC, and rim APHE plus elevated CA 19-9 and normal AFP is a predictor of ICC rather than LR-M HCC. The reference values of early washout (< 60 s) and marked washout within 120 s in the LR-M category are needed to further refine the CEUS LI-RADS criteria to avoid unnecessary biopsy.
 World Journal of Gastroenterology2020年27期
World Journal of Gastroenterology2020年27期
- World Journal of Gastroenterology的其它文章
- Histopathological landscape of rare oesophageal neoplasms
- Details determining the success in establishing a mouse orthotopic liver transplantation model
- Modified percutaneous transhepatic papillary balloon dilation for patients with refractory hepatolithiasis
- Serum ceruloplasmin can predict liver fibrosis in hepatitis B virusinfected patients
- Acceptance on colorectal cancer screening upper age limit in South Korea
- Transarterial chemoembolization with hepatic arterial infusion chemotherapy plus S-1 for hepatocellular carcinoma
