硼和氟掺杂ZSM-5分子筛的制备及其甲醇制丙烯反应性能
冯 锐 胡晓燕 闫新龙 武建军
(中国矿业大学化工学院,徐州 221116)
Propylene,as one of the most important intermediates in petrochemical industry,is traditionally produced by the steam cracking of naphtha or ethane,as well as the fluid catalytic cracking(FCC)process.To meet the ever-increasing demand of propylene and reduce its dependence on crude oil resource,new strat-egies for propylene production from cheaper and/or renewable feedstocks have attracted an increasing interest in recent decades.Among the newly-developed technologies,methanol to olefins(MTO)and methanol to propylene(MTP)technologies have been paid much attention due to the easy availability of methanol and high selectivity to light olefins(C2=~C4=).Specifically,the MTP process can optimize the propylene yield over ZSM-5 catalyst due to its unique microporous structure and surface acidity.However,the propylene selectivity and catalytic lifetime need to be enhanced.Many studies have shown that the physiochemical properties of ZSM-5 zeolite such as surface acidity,pore structure,and crystal sizes,play important roles in improving propylene selectivity and catalyst lifetime.For example,the acid sites in ZSM-5 zeolites are the active sites not only for methanol conversion and propylene formation,but also the undesired secondary reactions such as hydrogen transfer,cyclization and aromatization[1].Generally,high-density acid sites induce the rapid deactivation of catalysts due to coke deposition;and the intrinsic micropores of ZSM-5 zeolites increase the diffusion resistance of propylene,the secondary reactions of propylene decrease its selectivity[2-3].Therefore,in the last decade,efforts have been devoted to the ZSM-5 zeolites:(a)synthesizing the hierarchical or nanosized ZSM-5 to increase the diffusion of propylene[3-4];(b)modifying the surface acid strength and acid density to decrease the adsorption and hydrogen transfer of propylene on acid sites[5,7].For example,Liu et al.[8]modified high-silicon ZSM-5 with 0.1% phosphorus to reduce the acid strength of Brønsted acid sites,which increased the propylene selectivity by 10% in the MTP reaction.In our previous study,the Lewis acid sites were found to be important sites for coke formation in hydrocarbon cracking reactions[9-11].However,the effect of acid sites type of ZSM-5 zeolite in the methanol conversion process has not been fully considered yet.In this study,to elucidate the role of Lewis and Brønsted acid sites on the activity and selectivity in the MTP reaction,ZSM-5 zeolites with varied acid properties were synthesized with boron and/or fluorine doping and tested in the MTP reaction.
1 Experimental
1.1 Raw materials
Ammonium fluoroborate(NH4BF4,97.00%(w/w))was purchased from Shanghai SSS Reagent Co.,Ltd.Ammonium fluoride(NH4F),boric acid(H3BO3),tetraethyl orthosilicate(TEOS),aluminium isopropoxide,and sodium hydroxide(NaOH)with analytical purity werepurchasedfrom Sinopharm GroupChemical Reagent Co.,Ltd.Tetrapropyl ammonium hydroxide(TPAOH,25%(w/w)aqueous solution)was purchased from Sinopharm Group Chemical Reagent Co.,Ltd.
1.2 Sample preparation
By varying the synthesis temperature during the crystallization,the nucleation and growth process should be controlled to obtain ZSM-5 zeolites with small and uniform crystals[12].Here,boron and/or fluorine doped ZSM-5 zeolites were synthesized using a two-stage crystallization method.Appropriate amounts of TEOS,TPAOH,NaOH,and aluminum isopropoxide were mixed with deionized water and stirred at room temperature for 6 h.Then,NH4BF4,NH4F,or H3BO3was added into the above mixture as modifiers and stirred for another 12 h.The final gel compositions of synthesizing systemnadditive)were 50∶1.0∶2:8∶3 000∶x.Here,three samples were marked as Z5-BF1,Z5-BF2,Z5-BF3 when NH4BF4was used as additive withx=3,6,and 9,in turn;two samples were marked as Z5-F1 and Z5-F2 when NH4F was used as additive withx=12 and 36,respectively;two samples were marked as Z5-B1 and Z5-B2 when H3BO3was used as additive withx=3 and 9,respectively.In comparison,the sample was marked as Z5 whenx=0.The above gel was then transferred into an autoclave for pre-crystallization at 110℃for 3 h and further crystallization at 170℃for 48 h.Thereafter,the as-synthesized solid products were filtered,washed with deionized water,dried overnight,and calcined at 550℃for 4 h in air to remove the organic templates.The H-form ZSM-5 zeolites were finally obtained after twice ion-exchanges with 1.0 mol·L-1of NH4Cl solution and once calcination at 550℃for 4 h in air.
1.3 Characterization
The crystalline phases were measured by X-ray diffraction(XRD)on a Bruker D8 Advance Diffractometer,using a CuKαradiation(λ=0.154 06 nm)operating at 40 kV and 30 mA,and the scanning angle of 5°~50°.The surface area and pore volume of zeolites were derived from the nitrogen sorption curves on a Micrometrics Tristar 3000 analyzer.Solid-state magic angle rotation nuclear magnetic resonance(MAS NMR)spectra were obtained from a Bruker AdvanceⅢHD 600 MHz instrument.The29Si NMR spectra were used to analyze the framework silica-to-alumina ratios(nSiO2/nAl2O3)of zeolites.Acid sites of all samples were measured on a Thermo Nicolet iS5 Fourier transform infrared(FT-IR)spectrometer,using pyridine as a probe molecule.Acid strength distributions were measured by NH3temperature-programmed desorption(NH3-TPD)on a Quanta chrome ChemStarTMinstrument.About 100 mg of samples were activated in flowing Ar at 350℃for 1 h,then cooled to room temperature and exposed to flow of 6.5%(V/V)of NH3/Ar gas.Physically adsorbed NH3was removed in Ar flow at 100℃for 2 h before collecting data at temperature ramped up to 650℃ at a rate of 10°C·min-1in helium flow of 30 mL·min-1.Field emission scanning electron microscopy(SEM)images were collected by a Quanta 250 instrument with an acceleration voltage of 15 kV.
1.4 Evaluation of MTP performance
The methanol to propylene reaction tests were performed in a fixed bed of a quartz tubular reactor with inner diameter of 6 mm.ZSM-5 catalysts with particle sizes of 40~60 mesh were prepared by pressing,crushing and sieving process.100 mg of catalyst was placed in the constant temperature zone of the reactor and pretreated at 550℃for 1 h under Ar gas before the reaction.Methanol was injected into the reactor by a constant flux pump using a 50 mL·min-1of Ar as carrier gas.The reaction temperature was set to 450℃and the weight hourly space velocity(WHSV)of methanol was 4.0 h-1.The reaction products were analyzed by an on-line gas chromatograph(GC-2014C,Shimadzu GC),which is equipped with a TG-BONG Q column,a thermal conductivity detector(TCD)and a flame ionization detector(FID).The maximum absolute error(<4%)was measured by triplicate experiments.The methanol conversion was defined as usual:XMeOH=(mMeOH,in-mMeOH,out)/mMeOH,in,while selectivity toiproduct was defined as follows:Si=mi/∑mi,wheremiis the mass of compoundi,and∑miis the total mass of all products.
2 Results and discussion
The X-ray diffraction(XRD)pattern in Fig.1 shows the characteristic peaks of MFI structure of ZSM-5 zeolite,corresponding to the(101),(020),(501),(151)and(303)crystal faces,respectively(PDF No.01-085-1208)[13-14].It shows that all samples exhibited a typical MFI phase and no peaks of crystallized impurity appeared.Fig.1b shows the shifts of two diffraction peaks to higher 2θangles with increasing the NH4BF4usages,indicative of the increase of framework SiO2/Al2O3ratios(nSiO2/nAl2O3).The diffraction peak shift of H3BO3modified samples such as Z5-B2 was more obvious than NH4F modified ones,indicating that H3BO3does more to increase the framework SiO2/Al2O3ratio.It might be attributed to the substitution of Al atoms by B atoms,since an increased B content was detected with increasing the boron usages[15-16].The relative crystallinities(RC)were calculated by comparing the peak areas of the characteristic peaks at 22°~25°and listed in Table 1.It shows that the crystallinity of ZSM-5 zeolites changed a little with heteroatom doping,and the relative crystallinity values of ZSM-5 zeolites decreased with increasing the NH4BF4usages.
The29Si NMR spectra in Fig.2 exhibits a strong resonance at about-113(chemical shift,the same below),which corresponds to the Si(OSi)4species in the zeolites framework.A weak shoulder resonance at about-106 was ascribed to the Si(OSi)3(AlO)species.A weak resonance at about-102 was ascribed to the(SiO)3SiOH species.No resonance was observed below-100,indicating that there were no other silicate species[17-18].The framework SiO2/Al2O3ratios were calculated from the deconvoluted profileusing a Gaussian-Lorentzian mixed function and listed in Table 1.It shows that the variation of framework SiO2/Al2O3ratios was in consistent with that derived from XRD pattern.The doping of B and/or F increased the framework SiO2/Al2O3ratios compared with Z5 sample.The27Al NMR spectra of four selected ZSM-5 zeolites in Fig.3a exhibits two distinct resonances at 52 and-3,corresponding to a tetra-coordinated framework and a hexa-coordinated extra-framework of the Al species,respectively.No shoulderresonance ofa pentacoordinated Al species was observed at approximately 35.The results show that combining F and B for Z5-BF2 remarkably decreases the percentage of the extraframework Al species,but the percentages of extraframework Al species with B and F alone changed small.
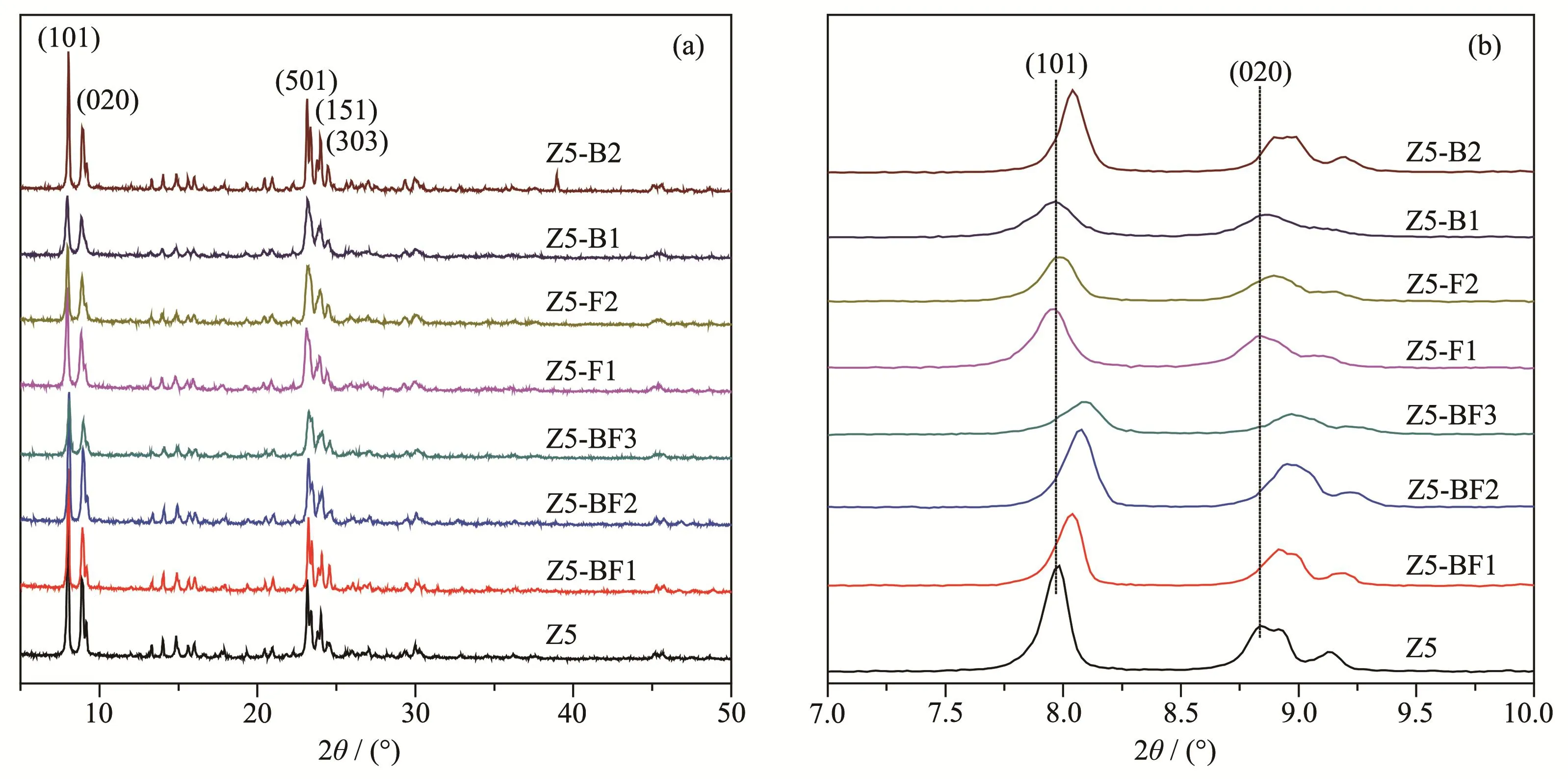
Fig.1 XRD patterns of as-synthesized ZSM-5 zeolites
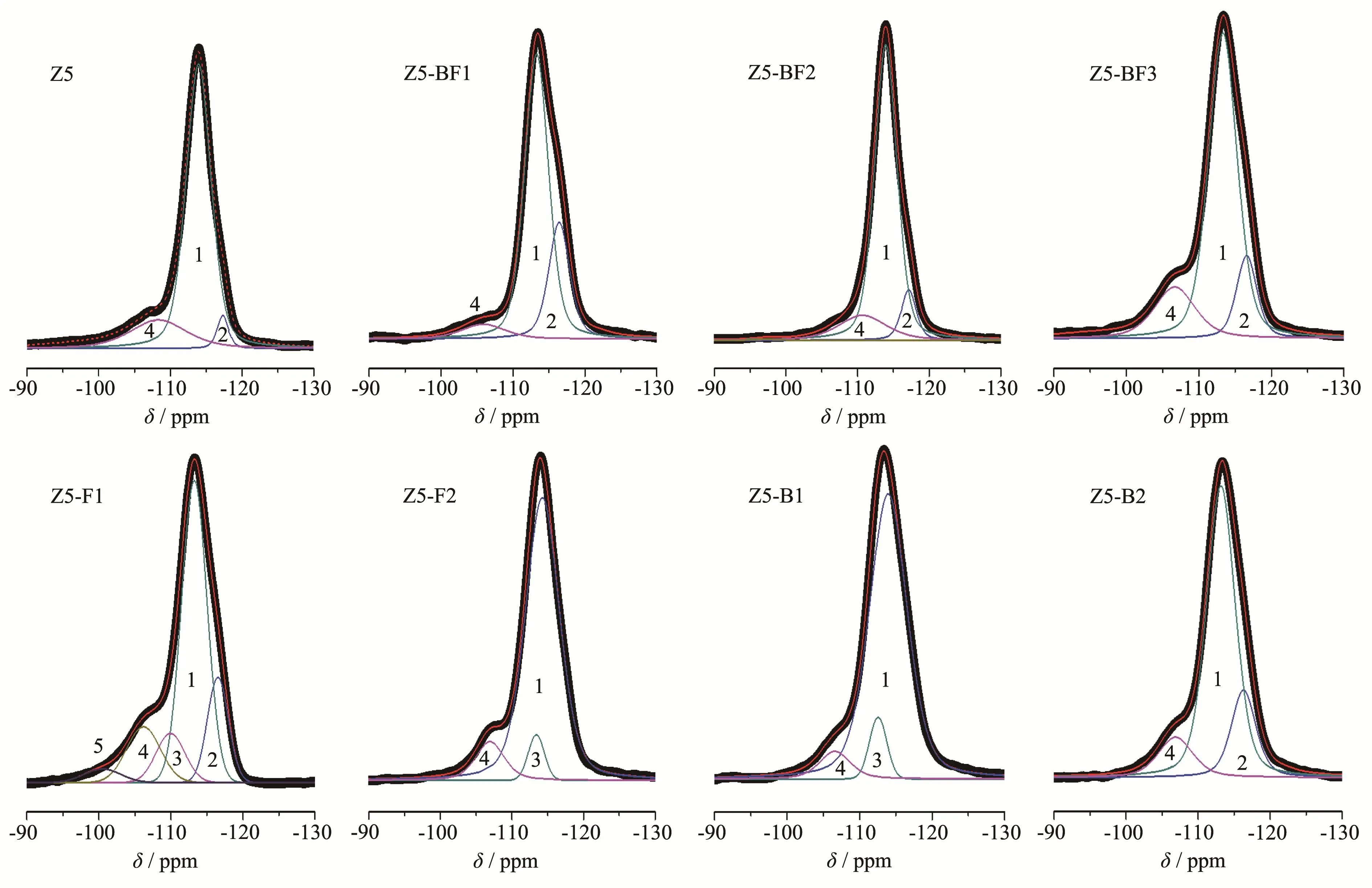
Fig.229Si NMR spectra of as-synthesized ZSM-5 zeolites with revolved resonances
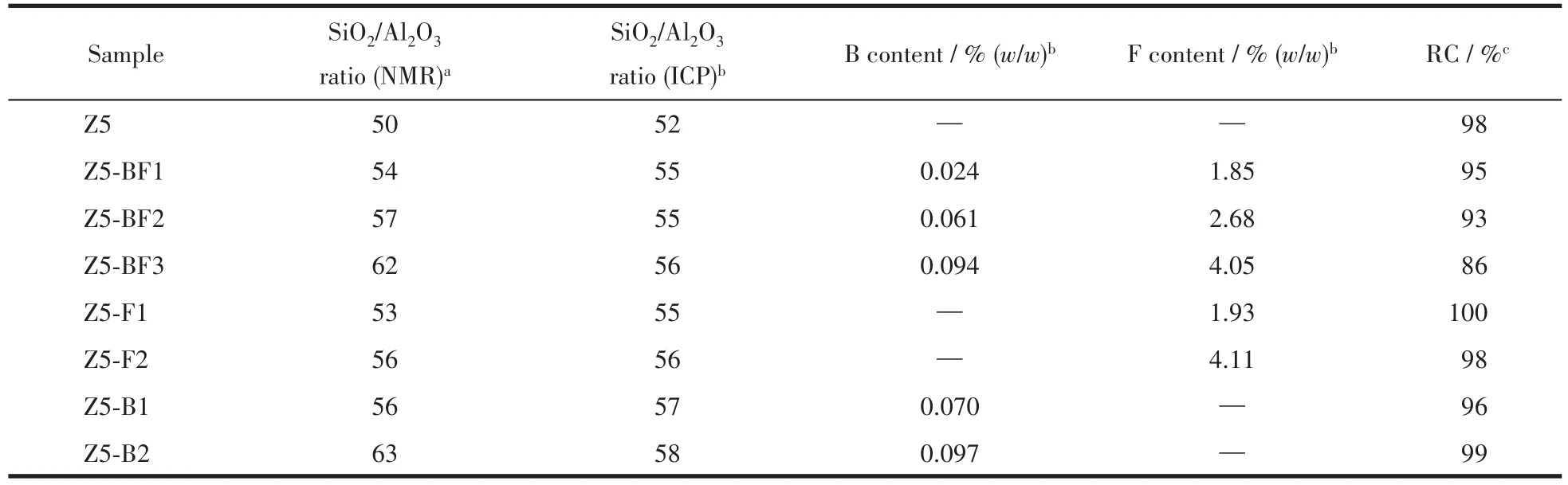
Table 1 Physiochemical properties of as-synthesized ZSM-5 zeolites
The11B NMR spectra in Fig.3b displays two main peaks at-3.9 and 13.8.The former resonance is attributed to the tetrahedral coordinated framework boron,and the latter broad band is assigned to the trigonal coordinated extraframework boron species[15,19].The small shoulder between these two bands is ascribed to the second-order quadrupole broadening of the trigonal coordinated framework boron,which is easily derived from the dehydration of tetrahedral coordinated framework boron species[16].This result confirms that the majority of B species is incorporated into the framework of zeolite.The19F NMR spectra in Fig.3c shows that there are five peaks for zeolites Z5-BF2 and Z5-F1.The peaks from-66 to-87 are attributed to F-anions located in the cages of silica zeolites[20-21].The peak at around-123 is assigned to the presence of the F-ion in zeolite channels as counter ion of balanced cations such as H+,Na+or NH4+[22-23].The peaks at-141 and-142 are associated with the extraframework Al species such as AlF63-.The peaks at-158 and-161 are related to Si-F groups which are caused by the replacement of hydroxyl group in the silanols by fluo-rine atom,or a terminal fluorine atom at the surface,indicative of the fluorination in the form of(SiO3)Si-F groups[9,23].The peaks at-177 and-180 are assigned to the partially hydrated AlF3species[24-25].The NMR data indicate that the boron and fluorine exist in the form of framework incorporation and chemical bonding with extraframework Al species.

Fig.3 27Al NMR spectra(a),11B NMR spectra(b),and19F NMR spectra(c)of selected as-synthesized ZSM-5 zeolites
Fig.4 shows the N2sorption isotherms and pore size distributions of the ZSM-5 zeolites.The isotherms are identified as typeⅣ,which is characteristic of micropore and mesopore mixed structure of ZSM-5 materials.The Brunauer-Emmet-Teller(BET)specific surface areas of the samples were calculated from N2isotherms and listed in Table 2.It shows that with increasing the NH4BF4usages,microporous surface areas of ZSM-5 zeolites increased,but their mesoporous surface areas decreased obviously.As a result,the total surface areas gradually decreased,from 322 m2·g-1for Z5 to 272 m2·g-1for Z5-BF3.The B and F doping contributes to the formation of microspore but impaired the mesopore,being in consistent with that of H3BO3and NH4F modified samples.The same tendency has also been observed for the pore volumes,i.e.,the micropore volumes of ZSM-5 zeolites containing B and F were higher than that of Z5.The pore size distribution obtained from the desorption branch indicates the reduction of mesoporous structure for B and F doped samples,particularly for the F doped samples.
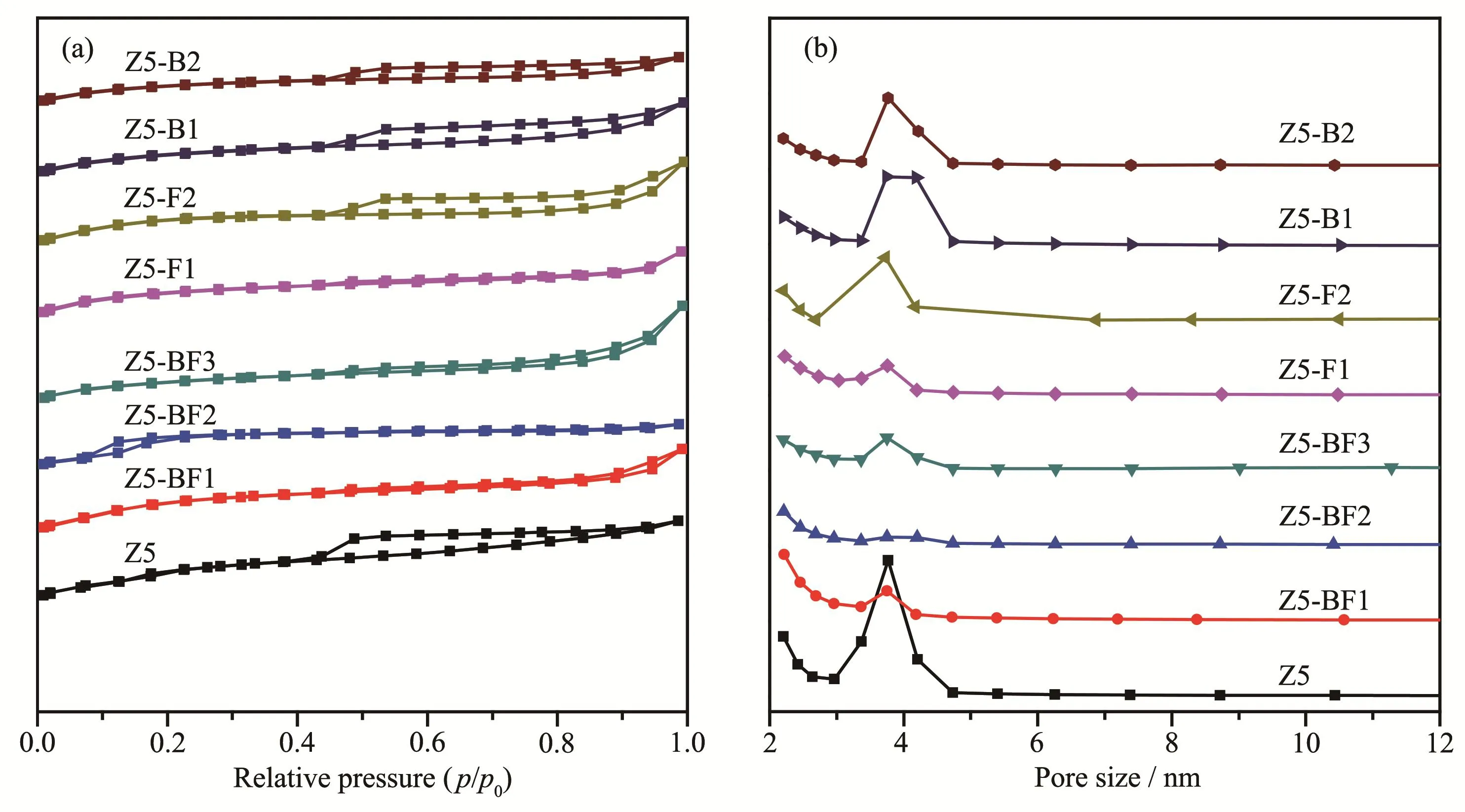
Fig.4 N2sorption isotherms(a)and pore size distributions(b)of as-synthesized ZSM-5 zeolites
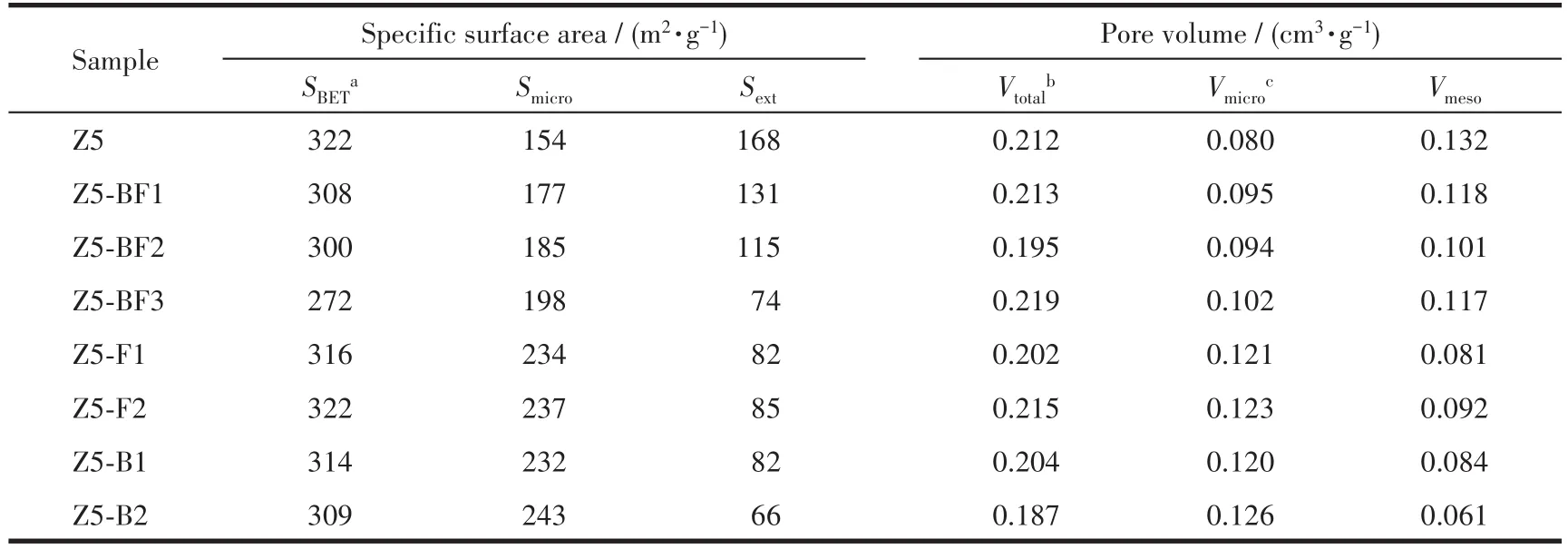
Table 2 Textural properties of as-synthesized ZSM-5 zeolites
FT-IR analysis was carried out to characterize the acid amounts of ZSM-5 zeolites.As highlighted in Fig.5a,it is generally recognized that the bands at 1 447 and 1 546 cm-1are ascribed to the pyridine molecules coordinated to Lewis acid sites and pyridinium ions formed by protonation of pyridine on Brønsted acid sites,respectively[10,26-27].The band at 1 490 cm-1is assigned to pyridine associated with both the two kinds of acid sites.The band at 1 597 cm-1is ascribed to the hydrogen bonded pyridine.The acid amount was calculated using a semi-quantitative method proposed by Emeis et al[28].The results in Table 3 show that with the increase of NH4BF4usages,the amounts of Brønsted acid sites of Z5-BFxobviously increased;in contrast,the amounts of Lewis acid sites remarkably decreased.It is caused by the doping of boron and fluorine atoms,which could effectively increase the amount of Brønsted acid sites but decrease the amount of Lewis acid sites,in view of the results of H3BO3and NH4F used alone.
The FTIR spectra of OH stretching region in Fig.5b is used to characterize the effect of B and F doping on OH groups.The band at 3 742 cm-1is ascribed to the OH stretching vibration(nOH)of free silanols which is thought to be located at the external surface of zeolite,namely SiOHs[26,29].It shows that combing B and F increases the amount of external surface SiOHs which is indicative of the external surface dealumination,being in consistent with the results of27Al NMR analysis in Fig.3.The bands at 3 691 and 3 604 cm-1are assigned to the vibration of extraframework Al-OH and bridging Si-OH-Al groups,respectively[30-31].Obviously,B and/or F doping could decrease the amounts of extraframework Al-OH and bridging Si-OH-Al species.Those species play important roles in constituting the acid sites,especially Brønsted acid sites,which is consistent with the variation tendency of Brønsted acid amounts with increasing the heteroatom doping(Table3).
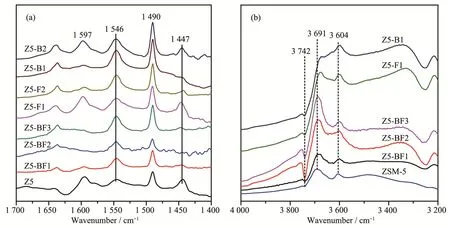
Fig.5 FT-IR spectra of pyridine absorbed by as-synthesized ZSM-5 zeolites
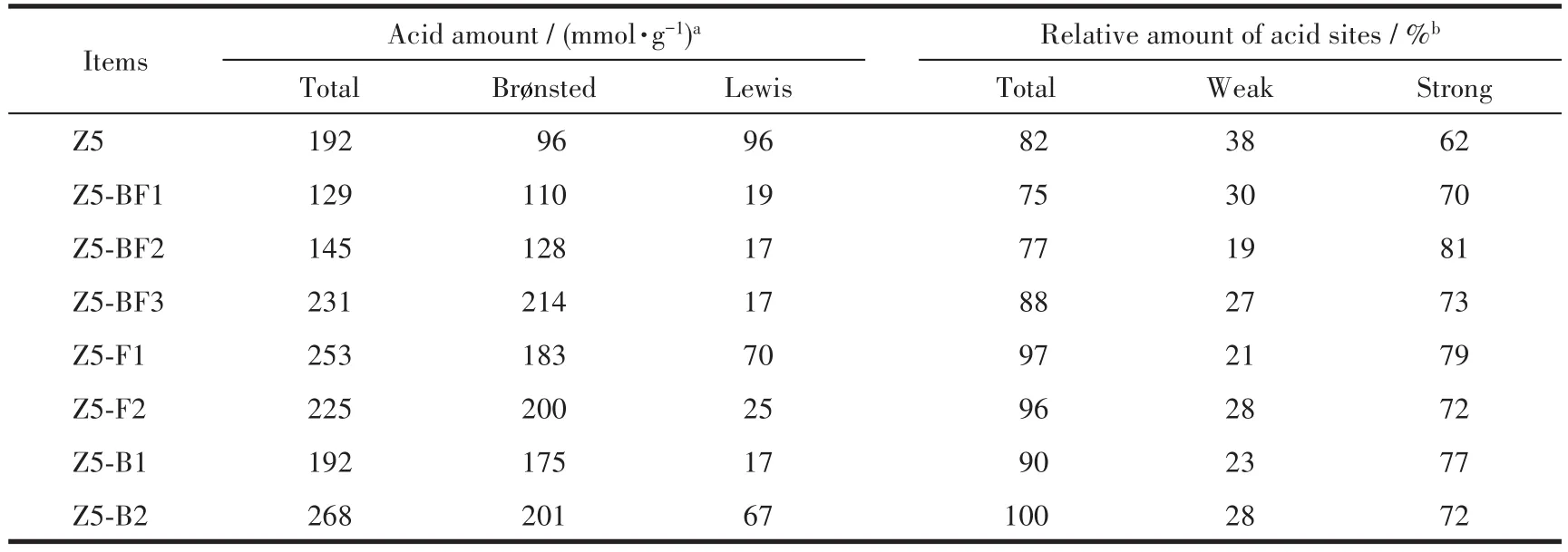
Table 3 Surface acidity of as-synthesized ZSM-5 zeolites

Fig.6 NH3-TPD profiles of as-synthesized ZSM-5 zeolites
NH3-TPD tests were also performed to characterize the acid strength distributions of ZSM-5 zeolites.As shown in Fig.6,all samples exhibited two resolved desorption peaks:the low-temperature around 250℃and high-temperature peak higher than 300℃,corresponding to the weak and strong acid sites,respectively.It is obvious that the peak of strong acid sites on B and/or F doped samples shifted to higher temperatures compared to Z5.The relative amounts of acid sites derived from the resolved overlapped curves are listed in Table 3.It can be seen that the weak acid sites of B and/or F doped samples decreased compared with Z5 sample,which is consistent with the change of Lewis acid amount;however,the change of strong acid amount is in keeping with the Brønsted acid amount(Table 3).The decrease of Lewis acid sites is probably attributed to the isomorphous substitution of Al atom by B atom or the bond combination of B and Al atoms;in contrast,the strong electronegativity of F atom reduces the electron cloud on Si-OH-Al group,enhancing the strength of Brønsted acid sites[9-10,32].

Fig.7 SEM images of as-synthesized ZSM-5 zeolites
SEM images in Fig.7 show that all samples possessed relatively uniform particles.The pre-crystallization at low temperature contributes to the formation of crystal nucleus and further crystallization at high temperature are beneficial to the synthesis of small sized ZSM-5 zeolite with good uniformity in a short time.Sample Z5 had well dispersed spherical shape parti-clesca.2 μm.With increasing the NH4BF4usages,the particle sizes of Z5-BFxdecreased gradually.Z5-BF1 had cubic and round cornered particles with diameter ofca.500 nm,while Z5-BF3 had the smallest particles with diameter of approximately 200 nm.Compared to NH4BF4,H3BO3and NH4F used alone had the reverse effect on the particle size of ZSM-5 zeolites.At low usage of modifiers,the particle sizes of Z5-B1 and Z5-F1 were smaller than sample Z5,while they were much larger than Z5 at higher usage.Notably,high concentration of fluorine alone could not only accelerate the crystallization rate of zeolite but also etch the crystal surface(Fig.7g),as previously reported[33-34].In contrast,boron alone made the ZSM-5 particle surface coarser than Z5(Figs.7h and 7i).
TEM images of typical Z5 and Z5-BF2 samples is shown in Fig.8.Both samples possessed relatively uniform crystals with smooth surface,being in consistent with that measured by SEM images.Some cavities are observed on the border and inside the crystals,indicating that the mesoporous structures detected in the N2sorption isotherms are mainly intra-crystal mesopores.
The products distributions of all ZSM-5 catalysts in MTP reaction are listed in Table 4.All ZSM-5 catalysts exhibited nearly full methanol conversion during the initial reaction period,indicative of their high initial activity.By defining 85% of methanol conversion as the point of catalyst deactivation,the boron and/or fluorine doped catalysts showed longer catalytic lifetime than Z5.With the increases of NH4BF4usages,the lifetimes of Z5-BFxincreased first and then decreased.Z5-BF2 possessed the longest lifetime of 23 h.By contrast,catalysts with boron used alone had better performance in improving the catalyst lifetime compared with that used fluorine alone.As for product selectivity,the Z5-BF2 had the highest propylene selectivity up to 41.5%.In contrast,the propylene selectivity on catalysts with fluorine alone decreased greatly compared with Z5 catalyst.
Methanol conversion and selectivity to light olefins(C2=,C3=and C4=)as a function of time on stream(TOS)over three selected catalysts are displayed in Fig.9.It shows that the selectivity of light olefins(C2=~C4=)on ZSM-5 catalysts was high at the initial period,and then decreased to a stable stage with slight fluctuation.Of all catalysts,Z5-BF2 possessed the highest average selectivity to propylene and light olefins,up to 41.5% and 63.6%,respectively.In the methanol conversion on zeolites,aromatic-based cycle and olefinbased cycle simultaneously occur,and ethylene mainly comes from the former while propylene and other heavi-er olefins mainly come from the latter[35].Taking Z5 and Z5-BF2 for examples,decreasing acid site density and the amount of Lewis acid sites could reduce the rates of aromatization and hydrogen transfer,resulting in lower ethylene selectivity but higher propylene selectivity for Z5-BF2.In contrast,the higher ethylene selectivity of Z5-BF3 catalyst is attributed to its improved amount of Brønsted acid sites,compared to Z5 catalyst.Similarly,high acid site density and high amount of Lewis acid site could increase the selectivity of C5+products(mainly aromatic species),due to the aromatization reactions[18,36].

Fig.8 TEM images of Z5(a,b)and Z5-BF2(c,d)
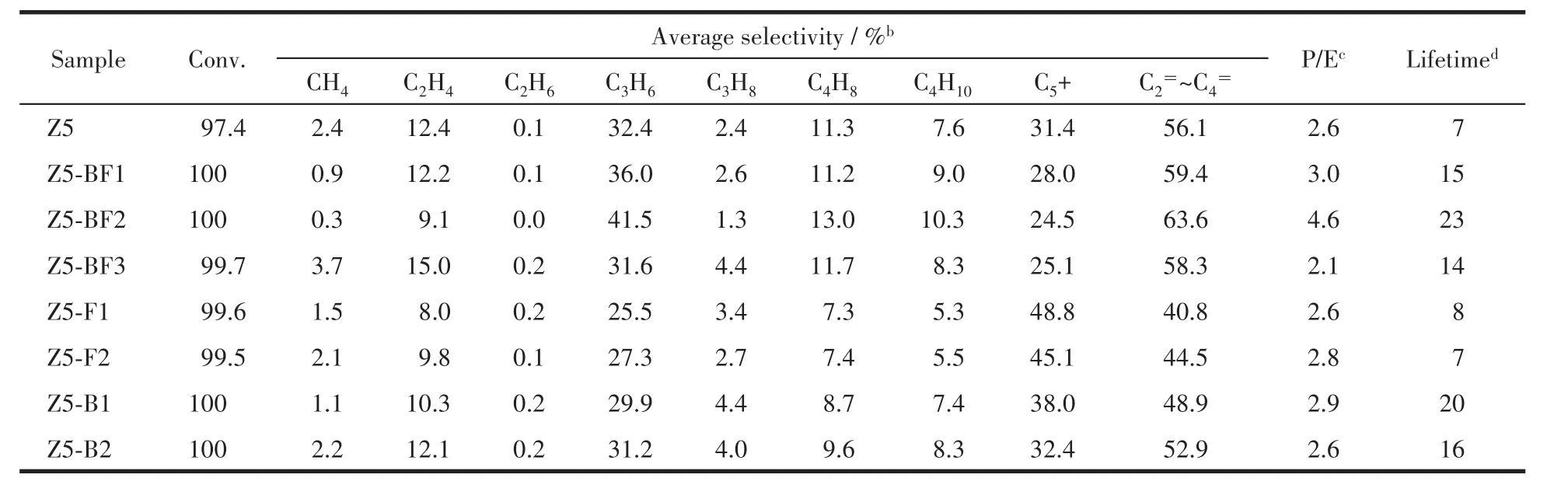
Table 4 MTP reaction results on H-form ZSM-5 catalysts with boron and/or fluorine dopinga
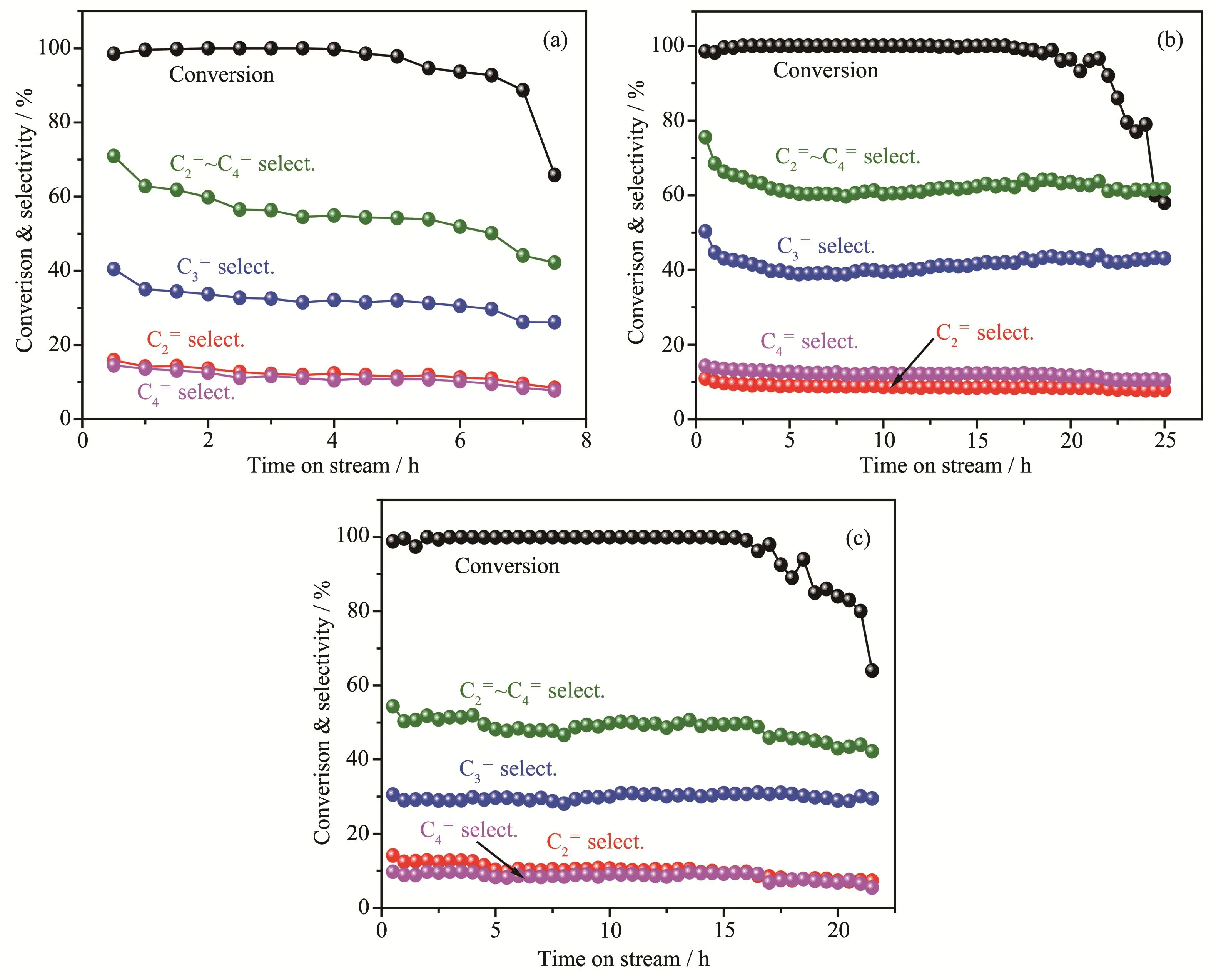
Fig.9 Conversion and product selectivity for the MTP reaction vs time-on-stream(TOS)over catalysts:(a)Z5;(b)Z5-BF2;(c)Z5-B1
From the foregoing,boron alone could improve the lifetime of ZSM-5 catalyst while fluorine alone had limited effect on prolonging the catalyst lifetime.The combination of boron and fluorine on Z5-BFxcatalysts improved both catalyst lifetime and selectivity of light olefins,propylene in particular.Generally,the deactivation of MTP catalysts is caused by the accumulation of coke species on the surface of ZSM-5 zeolites,which cover the surface acid sites or plug pores and suppress the access of reactants to acid sites[7,37-38].However,it seems that mesopore did not do much in reducing the coke deactivation,considering that the decrease of mesoporous volumes of Z5-BFxdid not affect their lifetimes compared with Z5 catalyst.
The crystal size is important in improving propylene selectivity and catalyst lifetime[39-42].Generally,nanosized ZSM-5 zeolites have been reported to show superior catalytic stability in the MTP reaction due to containing short diffusion paths.Despite prolonging the catalytic lifetime for nanosized ZSM-5 zeolites in most cases,their exposed external surface acid sites are more likely to absorb light olefins and coke precursors and contribute little to improving propylene selectivity because of their weakened shape selectivity.In this study,with increasing the NH4BF4usages,the particle sizes of Z5-BFxdecreased gradually.However,propylene selectivity and catalyst lifetime did not monotonically increase or decrease with the variation of crystal sizes.The superior catalytic performance of Z5-BF2 more reasonably results from the optimization of acid sites in view of the significant changes of surface acidity compared to Z5 in Table 3.
By contrast,acid sites,especially the strong acid sites,are thought to be the predominant reason for coke deposition[38,43-46].The strong adsorption of light olefins on strong acid sites(the Brønsted acid sites in this work)and further polymerization with other light olefins should be responsible for the catalyst deactivation[2,39].In view of the acid properties of catalysts in Table 3,the decrease of Lewis acid amount of Z5-BF1 and Z5-BF2 obviously improved the catalyst lifetimes compared with Z5 catalyst;while the sharp increase of Brønsted acid amount of Z5-BF3 reduced the catalyst lifetime compared with Z5-BF2.Therefore,Lewis acid sites and strong acid sites,especially strong Brønsted acid sites,are unfavorable due to the fast coking deactivation,as previously reported[11,47-48].The amount of strong acid sites on ZSM-5 catalyst should be controlled to a relatively low level.
Some references related with boron or fluorine modified ZSM-5 and their catalytic performances in the MTP reactions are listed in Table 5.In the previous studies,most of the B or F modified ZSM-5 catalysts possessed higher propylene selectivity and longer catalyst lifetimes than unmodified ZSM-5 catalysts.As shown in Table 5,all catalysts had relatively high propylene selectivity more than 40%,however,their catalyst lifetimes differed widely.The big differences of cat-alyst lifetimes are caused by many factors such as the acidity,pore structure,and crystal sizes.It is noted that the reaction conditions such as catalyst loading amount,water addition into methanol feed,and even inner diameter of the reactor are also important factor influencing the results.In our two studies[36,49],the commercial MTP catalysts which was tested under two different reaction conditions exhibited totally different catalytic lifetimes(25 h vs>400 h).In this study,we used the prepared ZSM-5 catalyst in the same reactor with that used in our previous studies[3,36].23 h of catalyst lifetime was comparable to that of the commercial MTP catalyst.More importantly,we believe that it is more meaningful to compare the catalytic performances of catalysts in the same reactor and same reaction conditions.
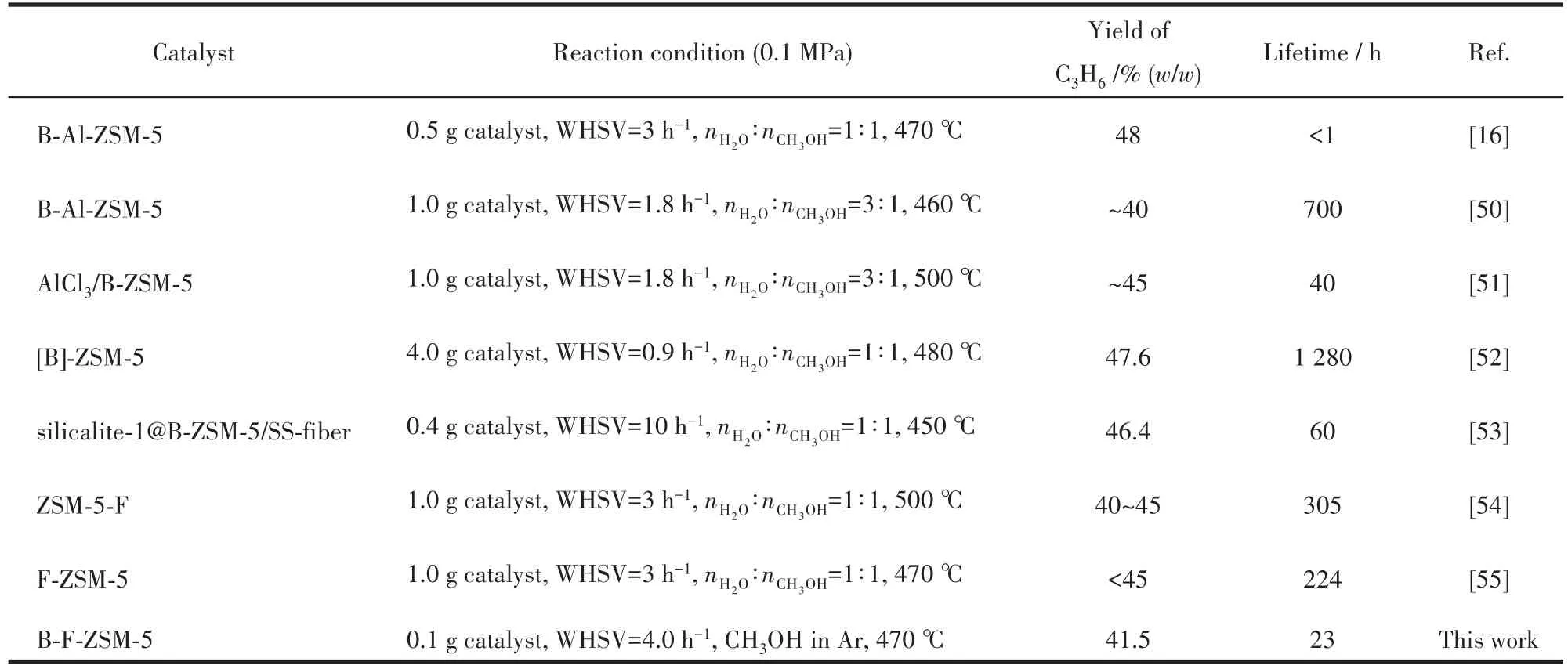
Table 5 Comparison of MTP performance on B or F modified ZSM-5 catalysts
3 Conclusions
A series of ZSM-5 catalysts were successfully synthesized in the presence of boron and/or fluorine containing reagents.The structural and morphological characterization results reveal that the appropriate amount of NH4BF4increases the micropores but reduces the Lewis acid sites without obvious increase of Brønsted acid sites.Compared to the conventional Z5 catalyst,Z5-BF2 exhibits a superior catalytic performance in the methanol to propylene reaction with higher propylene selectivity and longer lifetime.Moreover,reducing Lewis acid sites and controlling the acid strength of Brønsted acid sites is expected to be suitable for improving the performance of MTP reaction.
Acknowledgements:This work was financially supported by the Fundamental Research Funds for the Central Universities(Grant No.2018QNB04).

