Effect of herb-partitioned moxibustion in improving tight junctions of intestinal epithelium in Crohn disease mediated by TNF-α-NF-κB-MLCK pathway
Gao Yan-ling (高艳玲), Wang Yu-ning (王雨宁), Guo Ya-jing (郭娅静), Sun Yi (孙怡), Wang Yi-ran (王逸然), Zhou Jing (周竞), Zhao Ji-meng (赵继梦),3, Wu Huan-gan (吴焕淦),3, Shi Yin (施茵),3
1 Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
2 Shanghai TCM-integrated Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200082, China
3 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
Abstract
Keywords: Indirect Moxibustion; Medicinal Cake-partitioned Moxibustion; Crohn Disease; Tumor Necrosis Factor-alpha; NF-kappa B; Myosin-Light-Chain Kinase; Tight Junctions; Rats
Crohn disease (CD) is a chronic non-specific granulomatous inflammatory bowel disease, characterized by various symptoms, recurrent attacks and resulting in complications such as intestinal stenosis, fistulas and abscesses in a long period of attack[1-2]. Many studies have shown that sustained remission of intestinal mucosal inflammation reduces CD recurrence and the risk of intestinal cancer[3-4]. Intestinal epithelial barrier is the first defense to maintain the normal function of intestines, with tight junctions (TJs) between intestinal epithelium as its important structural basis, and loss of TJs expression or abnormal distribution can lead to intestinal epithelial barrier injury[5].
TJs are composed of transmembrane proteins such as occludin and claudin and intracellular proteins such as zonula occludens protein 1 (ZO-1), which connect intracellular cytoskeleton to extracellular connexin[6]. Studies have shown that tumor necrosis factor-α (TNF-α)-induced activation of nuclear factor kappa B (NF-κB) can initiate multiple pathways affecting intestinal epithelial barrier permeability[7-9]. Among those pathways, TNF-α-NF-κB-myosin-light-chain kinase (MLCK) pathway which mediates abnormal or absent expression of intestinal epithelial TJs is one of the main pathways and the most widely studied[10]. Zinc finger protein A20 (A20), as a newly discovered ubiquitin- modifying enzyme, can negatively regulate TNF-α- NF-κB-MLCK pathway to affect TJs.
Our previous studies have found that moxibustion [herb-partitioned moxibustion (HPM) and mild moxibustion] may promote the expressions of tight junction proteins and thus heal or protect the epithelial barrier injury through reducing the abnormally increased TNF-α in CD rats[11-13]. In this study, we treated CD rat models with HPM and mesalazine (MESA), respectively. The colonic tissues were separated, purified and cultured to establish the intestinal epithelial barrier of CDin vitroto observe whether HPM can protect the intestinal epithelial barrier from injury by affecting intestinal epithelial TJs through TNF-α- NF-κB-MLCK pathway.
1 Materials and Methods
1.1 Experimental animals
Forty-eight Sprague-Dawley rats [male, specific pathogen free grade, weighing (150±20) g] were purchased from Shanghai University of Traditional Chinese Medicine (License No.: SYXK 2014-0008). All animals were housed at the Animal Care Center of Shanghai University of Traditional Chinese Medicine, and provided with humane care in a temperature- controlled room [temperature (22±1) ℃ and humidity 50%-70%] under a 12 h/12 h light/dark cycle with free access to food and water in the home cage. All animal experiments in this study were performed according to guidelines set forth by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (Approval No.: 2013025).
1.2 CD model establishment
Forty-eight male SD rats were randomly divided into a normal control (NC) group, a model control (MC) group, an HPM group and a MESA group, with 12 rats in each group. All the groups were administered with 2,4,6 trinitrobenzene sulfonic acid (TNBS) to establish experimental CD models except the NC group.
The establishment of CD model in rats was as follows: the rats were fasted but had free access to water for 24 h. The rats were weighed and intraperitoneally injected with 2% sodium pentobarbital at 30 mg/kg and then TNBS was applied. The TNBS enema solution was prepared with 5% TNBS and 50% alcohol mixed at 2:1 and the 50% alcohol was mixed by double distilled water and absolute alcohol at 1:1. All the rats received TNBS solution enema by a rubber tube at 3 mL/(kg·bw) except that those in the NC group received physiological saline enema in the same way also at 3 mL/(kg·bw). The rubber tube was put into the anus by 6-8 cm. Following enema, each rat was put upside down for about 1 min to prevent loss of the injected solution. The enema was repeated every 7 d for 4 weeks.
1.3 The identification of CD models
When the CD modeling process finished, two rats were randomly selected from each group for pathological observation to check if the model was a success. The following experiment was carried out only when the model was confirmed a success.
1.4 Treatment methods
After the experimental CD rat models were successfully established, the rats were exposed to different treatments. The rats in the MC and NC groups did not receive any treatments.
In the HPM group, moxa cones made of refined mugwort floss were placed on the herbal cake at Tianshu (ST 25) and Qihai (CV 6) and ignited. The herbal powder formula was as follows:Fu Zi(Radix Aconiti Lateralis Praeparata) 10 g,Rou Gui(Cortex Cinnamomi) 2 g,Dan Shen(Radix et Rhizoma Salviae Miltiorrhizae) 3 g,Hong Hua(Flos Carthami) 3 g andMu Xiang(Radix Aucklandiae) 2 g. For use, 2.5 g herbal powder was fully mixed with 3 g Shaoxing rice wine into a thick paste, and then shaped into herbal cakes (1 cm in diameter and 0.5 cm high) using a mold specifically for the experimental rats. Two moxa cones were used for each point each rat in a session, once a day for 10 d.
In the MESA group, rats were fed with MESA (Specification No.: 01B06515LA, DrFalk, Germany) via intragastric administration at 1:56 to the dose for human adults (body weight 70 kg, 4 g/d)[14], 2 mL per day, twice a day for 10 d.
1.5 Establishment of an intestinal epithelial barrier model in vitro and TNF-α induction
Colon tissues collected from all the groups were digested with type IV collagenase and trypsin. After supernatants were centrifuged, the colonic epithelial cells were purified and seeded into culture dishes with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum. The culture medium was changed every other day in an incubator at 37 ℃ and 5% CO2. Colonic epithelial cells were purified, digested with trypsin, and resuspended in complete medium before counting. Cells (1×105) were taken and placed in 48-well plates for adherence and observation. Wells with high proportions of epithelial cells were expanded in culture. The above operations were repeated until most of the epithelial cells were visible under a Wetzlar light microscope (Leica Microsystems GmbH, Germany). Cytokeratin-19 (CK-19) monoclonal antibody was detected by SP immunohistochemical staining, and the positive reaction was that the cytoplasm was stained yellowish brown. The culture lasted for 2 weeks until thein vitrointestinal epithelial barrier was formed. TNF-α was added (100 ng/mL) in the culture medium and maintained for 24 h to establish an increased epitheilal permeability model. At the same time, same amount of cells were taken from the NC group and not incubated with TNF-α, which was considered as the negative control.
1.6 Immunohistochemical assay
The sections were exposed to 0.01 mol/L citrate buffer (pH 6.0), microwaved at 30% power for 20 min for heat fixation, and then cooled to room temperature. The sections were washed 3 times with phosphate buffered saline (PBS) for 3 min and exposed to 0.3% H2O2for 20 min at room temperature to inhibit endogenous peroxidases. Following a final PBS wash (3 min × 3 times), the samples were exposed to 20% normal goat serum and incubated for 30 min. Antibodies were added CK-19 dropwise (1:50, Bioworld, USA), and the sections were incubated at 37 ℃ for 2 h. The sections were washed with PBS 3 times for 3 min, incubated in horseradish peroxidase/rabbit reagent at 37 ℃ for 30 min, and again washed with PBS (3 min × 3 times). The sections were then incubated in 3,3-diaminobenzidine (DAB) chromogenic reagent for 8-12 min and dyed with hematoxylin-eosin (HE) in the presence of hot water. After drying, the sections were sealed with neutral gum for further observation under a light microscope. A semi-quantitative analysis of the staining was performed using the MIQAS medical image quantitative analysis system (Shanghai Qiuwei Biomedical Technology Co., Ltd., China). Positive results were indicated by the presence of brown or tan particles in the stained colon tissue cells.
1.7 Measurement of colonic TEER
Cultured colonic cells from each group were washed once with PBS and digested with 0.25% trypsin. After culture medium was added and the digestion was terminated, the cells were counted and adjusted to a density of 2.5×105cells/mL. Transwell chambers (Millicell-ERS, USA) for measuring transmembrane resistance were placed in 24-well plates. The complete medium (600 μL) was added into the lower chamber, while a cell suspension (200 μL) was into the upper one, then cultured at 37 ℃ with 5% CO2. After 24 h, TNF-α was added into different groups and cultured for another 48 h for TEER measurement. The medium in the upper and lower transwell chambers was replaced with 200 μL and 600 μL medium, respectively. A small chamber with medium only was set as a negative control. After an electrode was plugged vertically into the chamber, the values were taken when they were stabilized for 3 times in different areas.
1.8 Western blot
Rat colon samples with TNF-α for 24 h were homogenized with a Dounce homogeniser in lysis buffer (added 200 μL for each 20 mg samples, 12 000 r/min for 15 min at 4 ℃), and 100 μL protein extracted from the isolated intestinal epithelial tissue samples was separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. Blots were blocked for 1 h in 5% BSA, and then incubated with primary antibodies overnight at 4 ℃. Primary anti- bodies were directed against NF-κB (CST, USA; 1:1 000), MLCK (Epitmics, USA; 1:1 000), myosin light chain (MLC, CST, USA; 1:1 000), tumor necrosis factor receptor- associated factor 6 (TRAF6, Epitmics, USA; 1:1 000), receptor interaction protein-1 (RIP1, Abcam, UK; 1:500), A20 (CST, USA; 1:1 000) and GAPDH (CST, USA; 1:1 500). The films were visualized using an enhanced chemiluminescence system (Pierce Company, USA).
1.9 Immunofluorescence staining
The single cell suspension was dripped onto a cover slip in a 24-well culture plate and cultured at 37 ℃ in a 5% CO2culture case for 24-48 h. The sections were washed with 0.01 mol/L PBS (pH 7.4) for 15 min, left to cool down at room temperature, and then washed twice in 0.01 mol/L PBS for 5 min. The sections were incubated at 4 ℃ overnight. The next day, sections were washed twice with PBS for 5 min, left to cool down at room temperature and then washed twice with distilled water, followed by rinsing with PBS (5 min × 3 times), and incubated with 1:1 000 occludin (Cat. No.: ab31721, Abcam, USA), claudin-1 (Cat. No.: ab15098, Abcam, USA), ZO-1 (Cat. No.: sc-10804, Santa Cruz, USA), F-actin (Cat. No.: ab205, Abcam, USA) at 4 ℃ overnight. The next day, the sections were washed twice with 0.01 mol/L PBS for 5 min each time and then with 0.01 mol/L PBS for 3 times, 5 min each. The samples were finally incubated with DAPI staining solution for 10 min. Images were obtained under fluorescence microscope (Nikon, Japan).
1.10 Statistical analyses
The experimental data were analyzed with SPSS version 21.0 software and Graphpad Prism 5 (GraphPad Software Inc., USA). All the measurement data were presented as mean ± standard deviation (±s). Inter- group comparisons were analyzed using a one-way analysis of variance.P<0.05 indicated statistical significance.
2 Results
2.1 Establishment of colonic epithelial barrier model
In the NC group, CK-19 was more significantly expressed in the colonic epithelial cells (Figure 1-A); in the MC group, CK-19 was less expressed in the colonic epithelial cells (Figure 1-B); in the HPM group, CK-19 was less expressed in the colonic epithelial cells (Figure 1-C); in the MESA group, CK-19 was less expressed in the colonic epithelial cells (Figure 1-D).
2.2 Effects of HPM on the function of intestinal epithelial barrier in CD rats
Compared with the NC group, the TEER values were significantly decreased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.001); compared with the NC+TNF-α group, the TEER values were significantly decreased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (P<0.001 orP<0.01); compared with the MC+TNF-α group, the TEER values were significantly increased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.001); the TEER value in the HPM+TNF-α group was higher than that in the MESA+TNF-α group (P<0.05), (Figure 2).
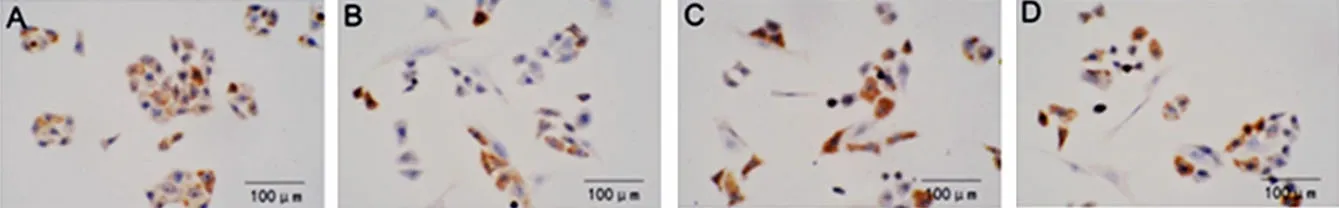
Figure 1. The expression of CK-19 in the colonic epithelial cells in each group

Figure 2. The TEER value in colonic epithelial cells
2.3 Effects of HPM on the related protein expressions in intestinal epithelium in CD rats
2.3.1 Expression level of NF-κBp65
Compared with the NC group, the expression levels of NF-κBp65 were significantly increased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the NC+TNF-α group, the expression level of NF-κBp65 was significantly increased in the MC+TNF-α group (P<0.01); compared with the MC+TNF-α group, the expression levels of NF-κBp65 were significantly decreased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01); there was no significant difference between the HPM+TNF-α group and the MESA+TNF-α group (P>0.05), (Figure 3).
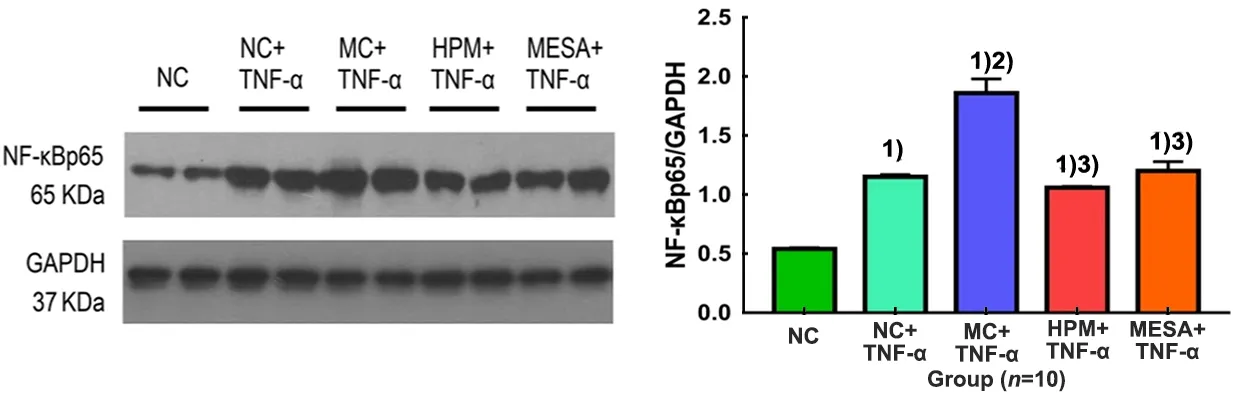
Figure 3. The expression level of NF-κBp65 in colonic epithelial cells in each group
2.3.2 Expression level of MLCK
Compared with the NC group, the expression levels of MLCK were significantly increased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the NC+TNF-α group, the expression level of MLCK was significantly increased in the MC+TNF-α group (P<0.01); compared with the MC+TNF-α group, the expression levels of MLCK were significantly decreased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01); there was no significant difference between the HPM+TNF-α group and the MESA+TNF-α group (P>0.05), (Figure 4).
2.3.3 Expression level of MLC
Compared with the NC group and the NC+TNF-α group, the expression levels of MLC were significantly increased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the MC+TNF-α group, the expression levels of MLC were significantly decreased in the HPM+ TNF-α group and MESA+TNF-α group (bothP<0.01); the expression level of MLC in the HPM+TNF-α group was higher than that in the MESA+TNF-α group (P<0.05). There was no significant difference between the NC group and the NC+TNF-α group (P>0.05), (Figure 5). 2.3.4 Expression level of TRAF6
Compared with the NC group, the expression level of TRAF6 was significantly decreased in the NC+TNF-α group (P<0.05), and it was significantly increased in the MC+TNF-α group (P<0.01); compared with the NC+TNF-α group, the expression levels of TRAF6 were significantly increased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (P<0.01 orP<0.05); compared with the MC+TNF-α group, the expression levels of TRAF6 were significantly decreased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01); there was no significant difference between the HPM+TNF-α group and the MESA+TNF-α group (P>0.05), (Figure 6).

Figure 4. The expression level of MLCK in colonic epithelial cells in each group

Figure 5. The expression level of MLC in colonic epithelial cells in each group
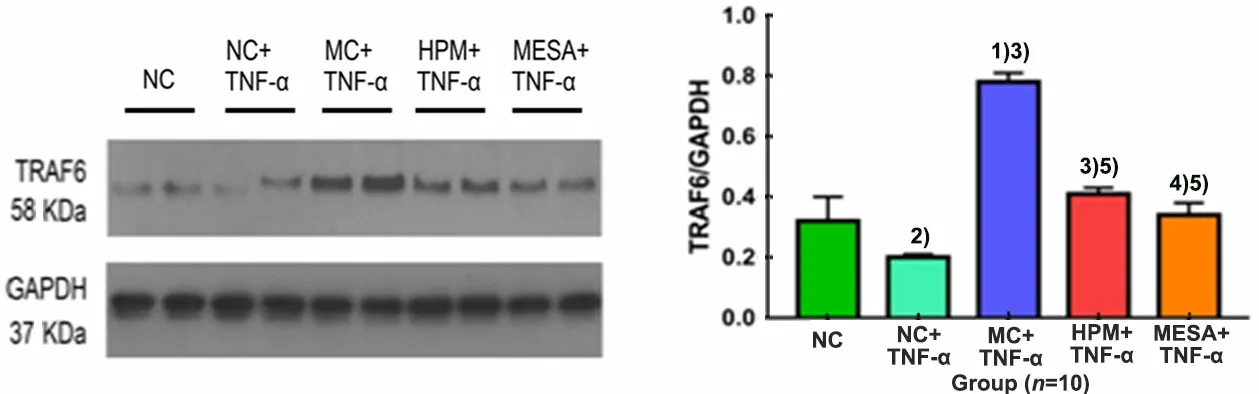
Figure 6. The expression level of TRAF6 in colonic epithelial cells in each group
2.3.5 Expression level of RIP1
Compared with the NC group and NC+TNF-α group, the expression levels of RIP1 were significantly increased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (P<0.01 orP<0.05); compared with the MC+TNF-α group, the expression levels of RIP1 were significantly decreased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.05); there was no significant difference between the NC group and the NC+TNF-α group, or between the HPM+TNF-α group and the MESA+TNF-α group (bothP>0.05), (Figure 7).
2.3.6 Expression level of A20
Compared with the NC group, the expression levels of A20 were significantly decreased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (P<0.01 orP<0.05); compared with the NC+TNF-α group, the expression levels of A20 were significantly decreased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (P<0.01 orP<0.05); compared with the MC+TNF-α group, the expression levels of A20 were significantly increased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01), and there was no significant difference between the HPM+TNF-α group and the MESA+TNF-α group (P>0.05), (Figure 8).
2.4 Effects of HPM on the expression levels of tight junction proteins occludin, claudin-1, ZO-1 and F-actin in intestinal epithelium in CD rats
2.4.1 Expression level of occludin
Compared with the NC group, the expression levels of occludin were significantly decreased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the NC+TNF-α group, the expression levels of occludin were significantly decreased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the MC+TNF-α group, the expression levels of occludin were significantly increased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01), and the expression level of occludin in the HPM+TNF-α group was markedly higher than that in the MESA+TNF-α group (P<0.01), (Figure 9).

Figure 7. The expression level of RIP1 of colonic epithelial cells in each group

Figure 8. The expression level of A20 in colonic epithelial cells in each group
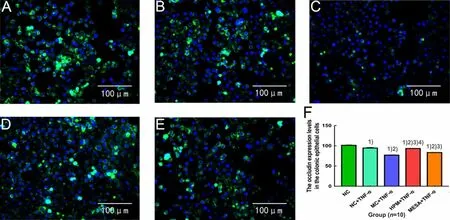
Figure 9. The expression level of occludin in colonic epithelial cells in each group
2.4.2 Expression level of claudin-1
Compared with the NC group, the expression levels of claudin-1 were significantly decreased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the NC+TNF-α group, the expression levels of claudin-1 were significantly decreased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the MC+TNF-α group, the expression levels of claudin-1 were significantly increased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01), and the expression level of claudin-1 in the HPM+TNF-α group was markedly higher than that in the MESA+TNF-α group (P<0.01), (Figure 10).

Figure 10. The expression level of claudin-1 in colonic epithelial cells in each group
2.4.3 Expression level of ZO-1
Compared with the NC group, the expression levels of ZO-1 were significantly decreased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the NC+TNF-α group, the expression levels of ZO-1 were significantly decreased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the MC+TNF-α group, the expression levels of ZO-1 were significantly increased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01), and the expression level of ZO-1 in the HPM+TNF-α group was significantly higher than that in the MESA+TNF-α group (P<0.01), (Figure 11).
2.4.4 Expression level of F-actin
Compared with the NC group, the expression levels of F-actin were significantly decreased in the NC+TNF-α group, MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (P<0.01 orP<0.05); compared with the NC+TNF-α group, the expression levels of F-actin were significantly decreased in the MC+TNF-α group, HPM+TNF-α group and MESA+TNF-α group (allP<0.01); compared with the MC+TNF-α group, the expression levels of F-actin were significantly increased in the HPM+TNF-α group and MESA+TNF-α group (bothP<0.01), and the expression level of F-actin in the HPM+TNF-α group was markedly higher than that in the MESA+TNF-α group (P<0.05), (Figure 12).
3 Discussion
With multiple factors like environment, infection and heredity involved in, intestinal immune system is initiated, which leads to immune cascade reaction and persistent intestinal inflammation, thereby resulting in CD. Intestinal epithelial barrier is not only a simple physical barrier, but also a highly dynamic tissue that can respond to a large number of signals and is subject to strict immune regulation to reduce abnormal damage[5,15]. The permeability of the intestinal epithelial barrier depends on both the transepithelial pathway and the paracellular pathway. The latter one is controlled by TJs which mainly decide if the macromolecular substances (bacteria and endotoxins, etc.) can pass through the intestinal epithelial barrier[16].

Figure 11. The expression level of ZO-1 in colonic epithelial cells in each group

Figure 12. The expression level of F-actin of colonic epithelial cells in each group
TNF-α, an important initiator of intestinal epithelial barrier injury in CD, directly or indirectly affects the TJs of intestinal epithelia. TNF-α affects the expressions of ZO-1 and occludin, regulates their distribution and thus influences the permeability of intestinal epithelium[17-18]. Clinically, in CD active and non-active phases, the expressions of occludin, claudin-1, ZO-1 and their mRNAs are significantly decreased, and the mucosal permeability is increased. In addition, epithelial barrier injury is present[19-20]. Animal experiments have also confirmed that, if tight junction proteins in intestinal epithelial cells are knocked out, animals with congenital gene defect will present pathological changes similar to those in CD[21]. In this study, we observed that the TEER values in colonic epithelial cells in both the NC+TNF-α group and MC+TNF-α group were significantly lower than the value in the NC group, after the epithelial cells were cultured and incubated with TNF-α. It is suggested that TNF-α remarkably increases the permeability of epithelial barrier in normal rats and CD rats. In addition, being induced by TNF-α, occludin, claudin-1, ZO-1 and F-actin in four groups showed varying decreases, which indicated that TNF-α can increase the permeability of colonic epithelial barrier by affecting the expressions of tight junction and actin proteins. However, after treated with HPM or MESA, the expressions of these proteins increased, suggesting that HPM and MESA can repair the permeability of epithelial barrier by up-regulating the expressions of these proteins.
Apart from directly destroying tight junction proteins, TNF-α can also affect TJs and heal epithelial barrier function by increasing the phosphorylation of MLCK[22]. When binding to TNFR1 on the surface of epithelial cells, TNF-α activates NF-κB through a series of signal transduction molecules, such as RIP1 and TRAF6. Activated NF-κB then binds to the downstream NF-κB binding site in the MLCK promoter region to initiate its transcription[23-24]. After activated by calmodulin, MLCK, a special substrate of myosin light chain (MLC), can phosphorylate MLC. Phosphorylated MLC activates the ATPase at the head of myosin heavy chain, which produces the energy that loosens the actin filaments of cytoskeleton. As a result, TJs have loosened and intercellular space has formed, causing increased intestinal epithelial permeability and inducing the occurrence and development of CD[25-26]. In this study, TNF-α induced increased expressions of NF-κBp65, MLCK and TRAF6 in the intestinal epithelial cells in normal rats, while the expressions of NF-κBp65, MLCK, MLC, TRAF6 and RIP1 increased significantly in the MC+ TNF-α group, suggesting that the NF-κB-MLCK pathway induced by TNF-α may be one of the pathways causing CD intestinal epithelial barrier damage. However, in the HPM group and MESA group, although induced by TNF-α, the expressions of NF-κBp65, MLCK, MLC, TRAF6 and RIP1 were significantly decreased, suggesting that HPM can reduce the expressions of TNF-α-NF-κB- MLCK-pathway-related proteins in the intestinal epithelia of CD rats.
It has been recently found that zinc finger protein A20 induced by TNF-α can act as a negative feedback loop to regulate NF-κB which is activated by TNF-α[27]. Clinical evidence has revealed that not only TNF-α and TNFR1, but also NF-κBp65 is significantly over- expressed in the colonic epithelial tissues of CD patients[28-29]. Over-activated NF-κB plays a central role in the pathogenesis of CD[30]. A20 is the most critical negative regulator in the NF-κB pathway and plays an important role in regulating immunity and preventing the inflammatory response from getting out of control[31]. In this study, we found that the expression of A20 in the epithelial cells was reduced to different degrees after being induced by TNF-α in both normal rats and CD rats, especially in CD rats. The expression of A20 was significantly increased in the HPM group and MESA group despite being induced by TNF-α.
In paracellular transport pathway of the intestinal epithelial barrier, TNF-α can mediate NF-κB-MLCK pathway, which results in the rearrangement or absence of TJs and its surrounding actin, leading to epithelial barrier damage and increased permeability of the intestinal epithelium. After treated with HPM or MESA, the expression of A20 in intestinal epithelium was promoted to regulate the abnormal activation of NF-κB-MLCK pathway induced by TNF-α. Therefore, the abnormality or absence of TJs was improved, thereby protecting the intestinal epithelial barrier or healing the damage induced by TNF-α. The improvements of MLC, occludin, claudin-1, ZO-1 and F-actin in the HPM+TNF-α group were slightly better than those in the MESA+TNF-α group. In improving the intestinal epithelial barrier function in CD, HPM can not only reduce the expressions of pro-inflammatory factors, but also improve the curative effect by increasing the secretions of anti-inflammatory factors and improving the internal environment of intestinal tract. Therefore, the improvements of some indicators were better in HPM+TNF-α group than in the MESA+TNF-α group.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China (国家自然科学基金项目, No. 81674069 and No. 81473757); National Basic Research Program of China (973 Program, 国家重点基础研究发展计划项目, No. 2015CB554500)
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria.
Received: 20 December 2019/Accepted: 26 March 2020
 Journal of Acupuncture and Tuina Science2021年1期
Journal of Acupuncture and Tuina Science2021年1期
- Journal of Acupuncture and Tuina Science的其它文章
- Effect of electroacupuncture on calcium-activated chloride channel currents in interstitial cells of Cajal in rats with diabetic gastroparesis
- Effect of electroacupuncture pretreatment on the protein expression of c-fos in fastigial nucleus and lateral hypothalamus area in rats with acute myocardial ischemia-reperfusion injury
- Ginger-partitioned moxibustion plus pediatric massage for treating infantile diarrhea due to spleen deficiency: a randomized controlled clinical trial
- Efficacy observation of long-retaining scalp acupuncture plus interactive training for upper-extremity dysfunction after cerebral stroke
- Observation on therapeutic efficacy of thunder-fire moxibustion for hypomenorrhea after induced abortion
- Clinical observation on the time-effect relationship of moxibustion for primary dysmenorrhea due to stagnation and congelation of cold-damp
