化学防控玉米蛀穗害虫对减轻拟轮枝镰孢穗腐病及伏马毒素的作用
李琴珵,石洁,何康来,王振营
化学防控玉米蛀穗害虫对减轻拟轮枝镰孢穗腐病及伏马毒素的作用
李琴珵1,石洁2,何康来1,王振营1
1中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室,北京 100193;2河北省农林科学院植物保护研究所,河北保定 071030
拟轮枝镰孢()引起的穗腐病严重影响玉米产量和品质,其产生的伏马毒素威胁食品安全。亚洲玉米螟()和桃蛀螟()等穗期害虫危害可导致玉米严重减产,并加重玉米穗腐病的发生。【】评估2种杀虫剂(甲维盐和氯虫苯甲酰胺)、3种杀菌剂(氰烯菌酯、戊唑醇和苯醚甲环唑)对玉米穗期病虫害的防治效果以及对玉米产量和籽粒中伏马毒素含量的影响;明确施用杀虫剂后接菌对穗腐病发病的影响,探究防治玉米穗期病虫害的有效方案,为玉米安全生产提供技术支撑。以郑单958为供试玉米,在2019年春、夏两季于河北廊坊进行田间试验。玉米大量吐丝后5 d和20 d两次施药,接菌处理于吐丝后7 d进行。在玉米完熟期调查虫害级别、穗腐病发病率和病情指数、果穗穗长、行粒数、穗重和百粒重,计算防治效果和增产情况,并用高效液相色谱-串联质谱法分析籽粒中伏马毒素B1、B2的含量。与对照相比,施用甲维盐和氯虫苯甲酰胺均可显著降低平均虫害级别、穗腐病发病率、病情指数、伏马毒素含量。与单独施用杀虫剂相比,施用杀虫剂与杀菌剂的混剂未显著降低平均虫害级别、穗腐病发病率、病情指数、伏马毒素含量,也未显著提高产量和对上述病虫害的防治效果。与仅接菌的处理相比,施用氯虫苯甲酰胺后接菌处理在穗腐病发病率、病情指数和伏马毒素含量方面均显著下降。在春玉米和夏玉米试验中,25 g·hm-2氯虫苯甲酰胺及其与杀菌剂的混剂对穗部螟虫的防治效果分别达82.1%—92.7%、94.2%—95.0%,而30 g·hm-2甲维盐及其与杀菌剂的混剂对穗部螟虫的防治效果显著低于25 g·hm-2氯虫苯甲酰胺,仅为57.8%—78.0%、83.1%—89.9%。2种杀虫剂对穗腐病的防治效果无显著差异,春玉米防治效果均>60%,夏玉米防治效果均>88%。对于产量而言,各处理均对果穗穗长和行粒数无显著影响,药剂处理后的果穗穗重均显著高于对照,且各处理间无显著差异。施用杀虫剂后接菌对产量无显著影响。分别施用2种杀虫剂单剂或其与杀菌剂的混剂后,春玉米可增产5.49%—13.49%,夏玉米可增产9.20%—13.95%,玉米籽粒中伏马毒素含量均低于500 μg·kg-1,而对照玉米中伏马毒素含量达2 817 μg·kg-1。喷雾接菌处理的玉米中伏马毒素含量高达8 710 μg·kg-1,而接菌前施用杀虫剂可将伏马毒素含量降至1 500 μg·kg-1以下。施用25 g·hm-2氯虫苯甲酰胺和30 g·hm-2甲维盐均可通过显著降低玉米蛀穗害虫的危害,从而减轻穗腐病的发生并降低籽粒中伏马毒素含量,提高玉米产量和品质,而杀虫剂/杀菌剂混用与杀虫剂单用对虫害防控效果差异不显著;穗期害虫对果穗的伤害在穗腐病的发生过程中起决定性作用。综合各方面因素,25 g·hm-2的氯虫苯甲酰胺是防治穗期玉米害虫、减轻穗腐病较为理想的药剂。
化学防治;虫害;拟轮枝镰孢;玉米穗腐病;产量;伏马毒素
0 引言
【研究意义】玉米是我国重要的粮食作物、饲料作物和工业原料。穗腐病又称穗粒腐病,是我国玉米生产上的重要病害,近年来呈加重趋势。至今已鉴定出引起穗腐病病原菌的种类有30余种。玉米受镰孢菌侵染后,造成单粒或成片籽粒腐烂,降低产量和品质。更为严重的是,镰孢菌侵染玉米后,常会在籽粒中产生有毒的次生代谢物,如拟轮枝镰孢()可产生伏马毒素[1]。食用含有毒素的玉米能够导致马脑蛋白软化症[2-3]、猪肺水肿[4-5],也威胁人类健康。食道癌的高发病率已被证实与含伏马毒素食物的大量摄入有关[6-9]。因此,防治玉米穗腐病并减少玉米产品中伏马毒素的含量,是保障食用和饲用安全的重要措施。【前人研究进展】据报道,穗期害虫危害与玉米穗腐病发生和籽粒伏马毒素含量水平有密切联系。亚洲玉米螟()、欧洲玉米螟()、桃蛀螟()、玉米楷夜蛾()、非洲大螟()等穗期害虫危害能明显加重穗腐病的发生[10-14]。玉米果穗受到虫害后,伏马毒素含量显著升高,严重时甚至超过限量标准数十至数百倍[15-17]。因此,防治玉米穗腐病不能仅从致病菌入手,还要综合考虑虫害的影响。国外研究表明,单独使用杀菌剂防治穗腐病的效果不佳[18-19],而防控穗期害虫可有效减少穗腐病的发生和籽粒中伏马毒素含量。DARVAS等[20]以转基因抗虫品系MON810为材料,发现不受虫害的果穗无一发病;BOWERS等[21-22]研究发现,转基因抗虫玉米显著降低了虫害,并降低了穗腐病发病和伏马毒素含量;HAMMOND等[23]在美国107个地点进行的试验表明,在不进行任何防治时,玉米中伏马毒素的含量往往超出美国的限量标准,转基因抗虫玉米减少了虫害和穗腐病的发生,从而减少了伏马毒素含量;ALMA等[24]研究发现,与完好果穗相比,受虫害的果穗籽粒中伏马毒素含量高出40倍;BLANDINO等[25]研究表明,在合适的时期施用杀虫剂高效氯氟氰菊酯能够显著降低玉米穗腐病发生和籽粒中伏马毒素含量。对于杀虫/杀菌剂混用防治穗腐病的效果存在一定争议,MAZZONI等[19]研究表明杀虫剂能够显著降低穗腐病发生和伏马毒素含量,用杀菌剂与其复配增效甚微;而DE CURTIS等[26]研究表明杀虫剂与杀菌剂混用相对于单用杀虫剂能够显著降低穗腐病发生和伏马毒素含量。由于伏马毒素的致癌性和其他危害,欧盟、国际食品法典委员会(CAC)、美国先后出台了玉米中伏马毒素的限量标准。各国对玉米中伏马毒素的限量不尽相同,但均规定未加工的玉米中伏马毒素最大限量不得超过4 000 μg·kg-1。而对于直接供人食用的玉米及玉米制品,伏马毒素的含量限度在1 000—2 000 μg·kg-1[27]。迄今为止,我国尚未规定玉米中伏马毒素的限量标准。【本研究切入点】我国对玉米穗期病虫害的防治以化学防治为主。随着药剂的更新换代,防治玉米病虫害的最佳方案需要进一步完善。我国对穗期害虫的化学防治通常以降低虫害而非降低穗腐病发病为目的,且防治穗期害虫与玉米中伏马毒素含量的关系尚不清楚。由于杀虫/杀菌剂混用防治穗腐病、降低玉米中伏马毒素含量的效果存在争议,在我国,玉米穗期病虫害化学防治的方案还需进一步明确。【拟解决的关键问题】探讨防治玉米穗期害虫和拟轮枝镰孢穗腐病的有效方案,在保障防治效果和食品安全的同时尽可能减少化学农药的使用,降低生产成本和对生态的干扰。
1 材料与方法
1.1 材料
1.1.1 供试玉米 供试玉米品种为郑单958。春玉米于2019年5月13日播种,种植于中国农业科学院农业高新技术产业园(河北省廊坊市);夏玉米于2019年6月29日播种,种植于中国农业科学院植物保护研究所廊坊中试基地。所有处理均为人工播种,双粒点播,出苗后间苗,留生长一致的苗用作田间试验。耕作、施肥、杂草管理和灌溉均按常规做法进行。
1.1.2 供试药剂 试验选用2种杀虫剂(甲维盐和氯虫苯甲酰胺)、3种杀菌剂(氰烯菌酯、戊唑醇和苯醚甲环唑),研究防治玉米蛀穗害虫和拟轮枝镰孢穗腐病的最佳方案(表1)。
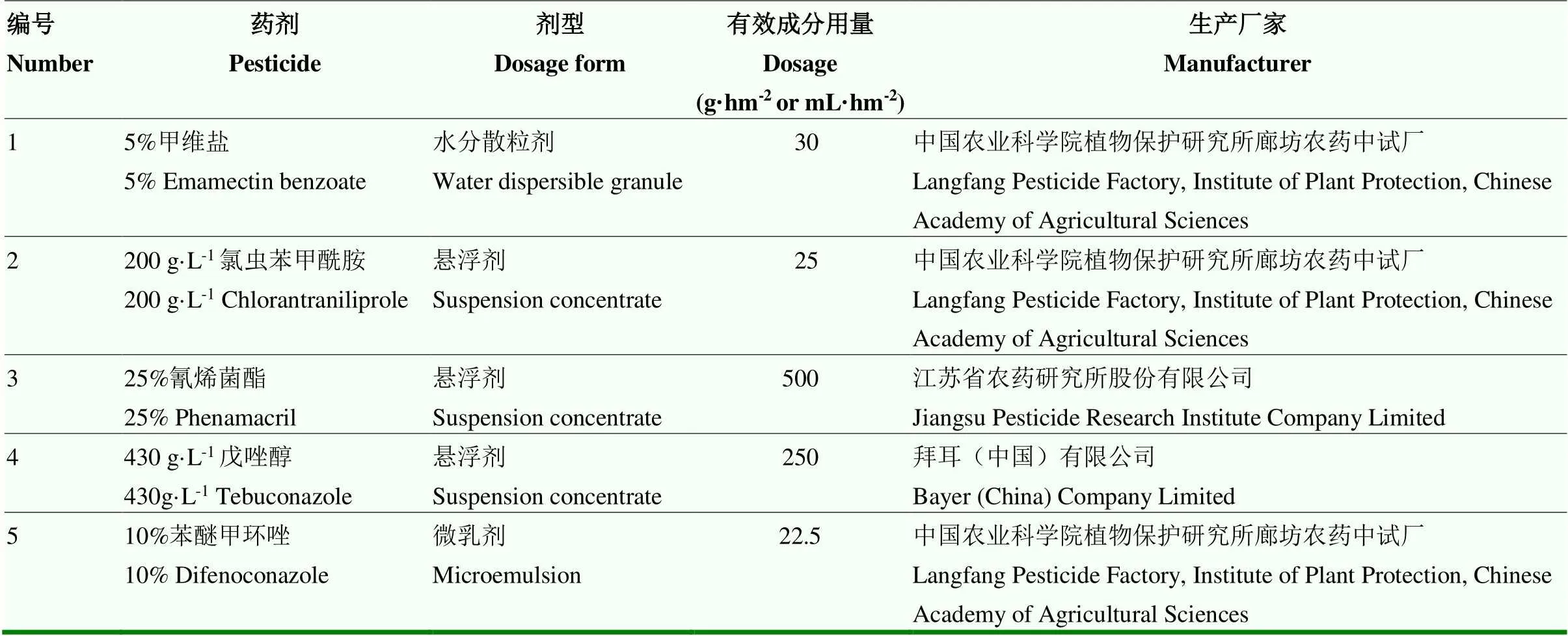
表1 试验所用化学药剂
1.1.3 供试菌种 拟轮枝镰孢由河北省农林科学院植物保护研究所玉米综防课题组提供,由田间典型穗腐病果穗分离,经纯化鉴定后用于试验,菌种采用镰孢菌产孢培养基培养。
1.2 方法
1.2.1 田间试验设计 采用随机区组设计,每处理6次重复,每重复选取30株生长发育一致的植株进行试验,用吊牌和红色塑料绳作为标记。每小区13.5 m2(4.5 m×3 m),行距60 cm,株距30 cm,每区6行,每行15株,各小区间隔1.5 m,边行作为保护行。玉米生长期不使用供试药剂以外的任何化学药剂。所有喷雾均于傍晚、无风条件下进行。喷药或接菌时,各处理之间以2 m高的塑料布相隔,避免交叉污染。所有试验均只处理和调查玉米第一果穗。在玉米吐丝后5 d和吐丝后20 d进行两次施药,空白对照喷施清水。试验除空白对照外,设甲维盐单剂、甲维盐与戊唑醇混剂、甲维盐与苯醚甲环唑混剂、甲维盐与氰烯菌酯混剂、氯虫苯甲酰胺单剂、氯虫苯甲酰胺与戊唑醇混剂、氯虫苯甲酰胺与苯醚甲环唑混剂、氯虫苯甲酰胺与氰烯菌酯混剂8种药剂处理,以及仅接菌、施用甲维盐后接菌、施用氯虫苯甲酰胺后接菌3个处理。接菌处理于第一次施药后2 d(玉米吐丝后7 d)进行。
1.2.2 调查方法 在玉米完熟期,每个小区从标记的植株中随机收获30个玉米果穗,记录玉米果穗的虫害级别、穗腐病发病率和发病级别。
玉米果穗受害虫危害级别按照Windham等[28]提出的方法划分:0-果穗完好;1-仅果穗顶部受损;2-果穗顶部受损+少量穗顶部以下轻度受损;3-果穗顶部受损+约1/4果穗顶部以下轻度受损到中度受损;4-果穗顶部受损+约1/2果穗顶部以下中度受损到严重受损;5-果穗顶部受损+约3/4果穗顶部以下中度受损到严重受损;6-果穗顶部受损+全部果穗顶部以下中度受损到严重受损。
玉米穗腐病发病级别划分为0、1、3、5、7、9共6个级别,具体参照转基因玉米环境安全检测技术规范[29]。
果穗发病率(%)=(发病穗数/总穗数)×100;病情指数=[Σ(各级病情穗数×相应级别)/(总穗数×最高级别)]×100;防治效果(%)=[对照区病情指数(平均虫害级别)-处理区病情指数(平均虫害级别)] /对照区病情指数(平均虫害级别)×100。
1.2.3 产量测定 不同处理玉米果穗在统计病虫害级别后分别装于尼龙网袋中自然风干。风干后测量处理果穗穗长、行粒数、单穗重、百粒重。测单穗重时,按质量平均法选取10株果穗,将每株果穗的籽粒手工搓下,剔除发霉变色和有虫伤的籽粒后称重;测百粒重时,将剩余果穗的籽粒混合均匀,随机数出100粒,记录完好籽粒的质量。随后计算籽粒损失率(%)=(1-完好粒数/总粒数)×100和单穗增产率(%)=(处理穗重/对照穗重-1)×100。
1.2.4 伏马毒素测定 分析所用仪器为高效液相色谱Agilent ultivo triple Quad LC/MS1290-6465。
样品采集与前处理:将各小区测产剩下的籽粒混合均匀,随机取100 g籽粒加入粉碎机研磨,并使磨碎的玉米粉通过40目网筛,编号,分装备用。
样品提取:准确称取25 g样品至烧杯中,加入50 mL甲醇﹕水溶液(3﹕1)。均质充分后静置10 min,取上清,1 800 r/min离心5 min,取上清液过滤,收集5 mL至离心管中,用1 mol·L-1NaOH溶液调节pH至5.8—6.5。
样品净化:采用Bond Elut SAX固相萃取柱,规格500 mg,3 mL。用5 mL甲醇淋洗固相萃取柱,再以5 mL甲醇-水溶液(3﹕1)淋洗。移取过滤液2 mL,以<2 mL·min-1的速度过柱上样。以5 mL甲醇-水(3﹕1)淋洗一遍,然后3 mL甲醇淋洗一次。以9 mL 1%甲酸甲醇溶液为洗脱液,以<1 mL·min-1的速度上柱洗脱,洗脱液收集于离心管中,氮吹浓缩,用0.5 mL乙腈-水(1﹕1)溶液复溶,涡旋30 s,过0.22 μm滤膜至进样瓶中。
高效液相色谱分析条件参考KUSHIRO等[30]的方法,略有改动。
1.3 数据统计与分析
采用统计软件SAS 9.4对试验数据进行ANOVA单因素方差分析,在分析之前,将病情指数、平均虫害级别、发病率、籽粒损失率开方后进行反三角函数转化;将伏马毒素B1、B2浓度按照’=ln(+1)进行数据转化。处理间差异显著性以Student-Newman- Keuls法检验。表中所有数据均为转化前原始数据。
2 结果
2.1 杀虫/杀菌剂混用对虫害、穗腐病发生、防控和伏马毒素含量的影响
两次试验的对照蛀穗率较高,均超过70%,但多数玉米仅顶部5行籽粒受到虫害。玉米穗腐病的主要发病部位为受虫害的籽粒及其相邻籽粒,而未受虫害的果穗发病率极低。在春玉米和夏玉米试验中,各施药处理蛀穗率、平均虫害级别均显著低于对照,氯虫苯甲酰胺及其与杀菌剂的混剂各处理的蛀穗率、虫害级别均低于甲维盐及其相对应的混剂,但仅有个别处理达到了显著水平(<0.05);与对照相比,各施药处理均可显著降低穗腐病发病率、病情指数和伏马毒素含量,两种杀虫剂间以及对应的混剂间的穗腐病发病率、病情指数和伏马毒素含量均无显著差异。两种杀虫剂与杀菌剂混用的处理与仅施用杀虫剂相比,蛀穗率、平均虫害级别、穗腐病发病率、病情指数和伏马毒素含量均无显著差异。各药剂处理在两次试验中的伏马毒素总含量均低于500 μg·kg-1,而春玉米试验的对照伏马毒素总含量可达2 817 μg·kg-1,接近于各国规定的毒素限量(表2)。
在春玉米试验中,25 g·hm-2氯虫苯甲酰胺及其与杀菌剂的混剂对穗期虫害的防治效果均显著好于30 g·hm-2甲维盐及相应的混剂;而在夏玉米试验中杀虫剂及相应混剂对穗期虫害的防治效果无显著差异。两次试验中杀虫剂单剂与混剂对穗期虫害和穗腐病的防治效果均无显著差异。春玉米试验中,各药剂对穗腐病的防治效果>60%,夏玉米试验中,各药剂对穗腐病的防治效果>88%(表2)。

表2 杀虫/杀菌剂混用对虫害和拟轮枝镰孢穗腐病发生、防控的影响
2.2 接菌前施杀虫剂对虫害、穗腐病发生和伏马毒素含量的影响
接菌处理的蛀穗率在两次试验中均超过60%,与对照无显著差异。未受虫害的果穗发病率极低。接菌处理的穗腐病发病率、病情指数和伏马毒素含量均高于对照,在春玉米试验中两者差异达到显著水平。与接菌处理相比,接菌前施用杀虫剂均能显著降低蛀穗率、平均虫害级别、穗腐病发病率、病情指数和伏马毒素含量。春玉米接菌后,玉米中伏马毒素含量可达8 710 μg·kg-1,超过限定标准的两倍,而接菌前施药的伏马毒素含量<1 500 μg·kg-1(表3)。
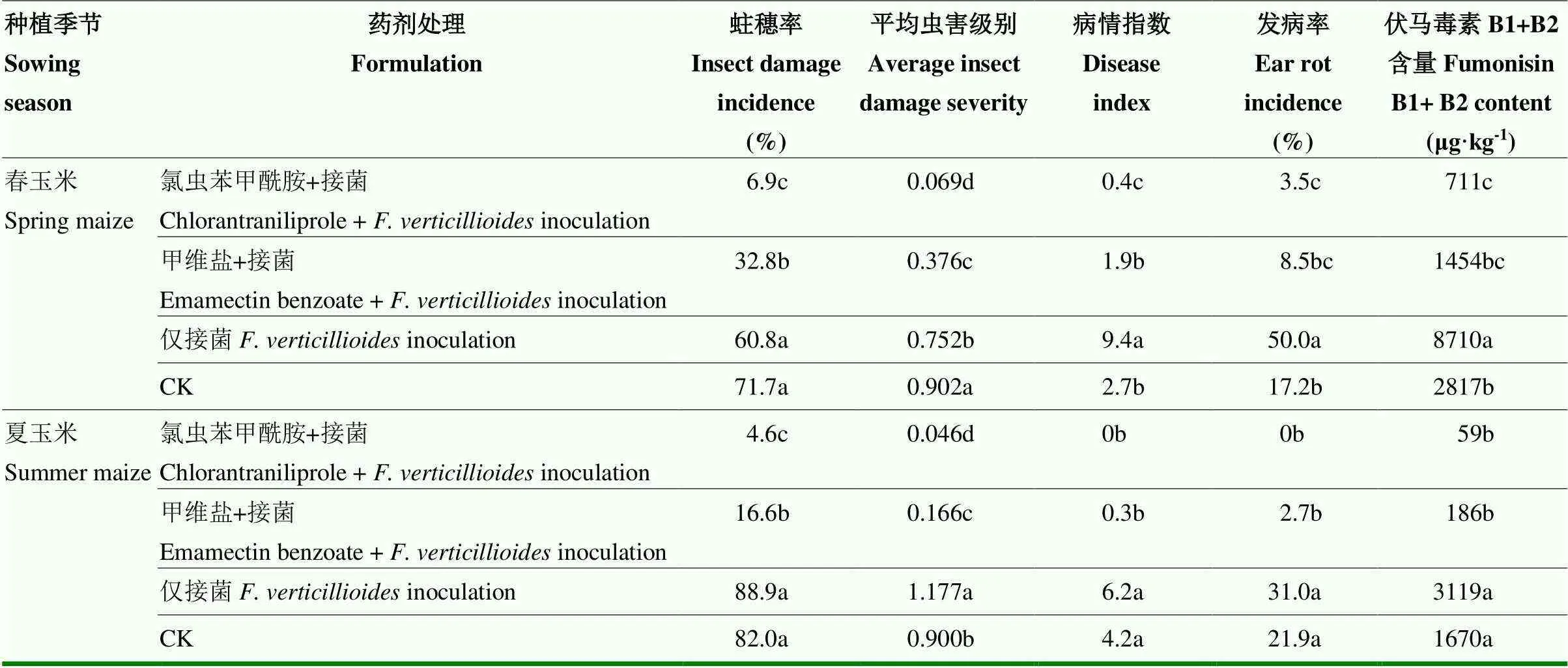
表3 接菌前施药对虫害和拟轮枝镰孢穗腐病发生的影响
2.3 化学防治对玉米产量的影响
综合春、夏玉米两次试验可见,施用杀虫剂和杀菌剂对穗长和行粒数无显著影响。春玉米各施药处理均能显著减少损失,增加百粒重和穗重,增产率为5.49%—13.49%。从夏玉米来看,与对照相比,玉米各施药处理均能显著减少损失,增加穗重,增产率为9.20%—13.95%;施用甲维盐与苯醚甲环唑混剂、甲维盐与氰烯菌酯的混剂显著增加百粒重,而其余处理增加百粒重的效果不显著。施用杀虫剂后,接菌对产量无显著影响(表4)。
3 讨论
国外对于防治玉米穗期害虫对穗腐病发生的影响有着较为详尽的报道,也存在着一定争议。多数学者认为,用适宜的方法防治穗期害虫,包括物理隔离[31]、施用杀虫剂[32-34]、种植转Bt基因抗虫玉米[35-36],不仅能减少产量损失,促进丰产,还能降低穗腐病的发生和籽粒伏马毒素含量,提高品质。然而,也有研究得到了相反的结论,即与对照相比,转基因抗虫玉米[37]和高效氯氟氰菊酯[38]的应用并未显著降低伏马毒素含量。另有文献报道,防控穗期害虫能否降低伏马毒素含量受到环境的影响[39-40]。国内在这方面的研究,特别是化学防治对伏马毒素含量影响的研究相对较少。
本研究中,氯虫苯甲酰胺和甲维盐各处理下蛀穗率、平均虫害级别均显著低于对照;且施用量25 g·hm-2的氯虫苯甲酰胺的防治效果显著好于施用量30 g·hm-2的甲维盐,是控制穗期害虫较为理想的杀虫剂。两次试验中,施用杀虫剂的处理与对照相比均能降低穗腐病发病率、病情指数和伏马毒素含量,这与前人研究的结论相一致[32,34]。穗腐病发病减轻的原因可能是杀虫剂减少了害虫对拟轮枝镰孢的传播和拟轮枝镰孢通过害虫造成的籽粒伤口侵染玉米的机会。施用杀虫/杀菌剂混剂与单独使用杀虫剂相比,对病虫害的防治效果不能显著提升,也不能显著降低籽粒中伏马毒素含量。这意味着相比单用杀虫剂,使用杀虫/杀菌剂混剂防治穗腐病并无优势,MAZZONI等[19]经过6年的田间试验,认为通过控制欧洲玉米螟可以减少穗腐病的发生,进而减轻籽粒中伏马毒素B1的污染,而针对拟轮枝镰孢添加的杀菌剂成分作用不明显。本研究结论与此研究相一致,进一步验证了穗期害虫危害对拟轮枝镰孢穗腐病诱发的重要作用,也在一定程度上解释了田间单独施用杀菌剂不能有效控制拟轮枝镰孢穗腐病的原因是存在大量蛀穗害虫[18,41]。
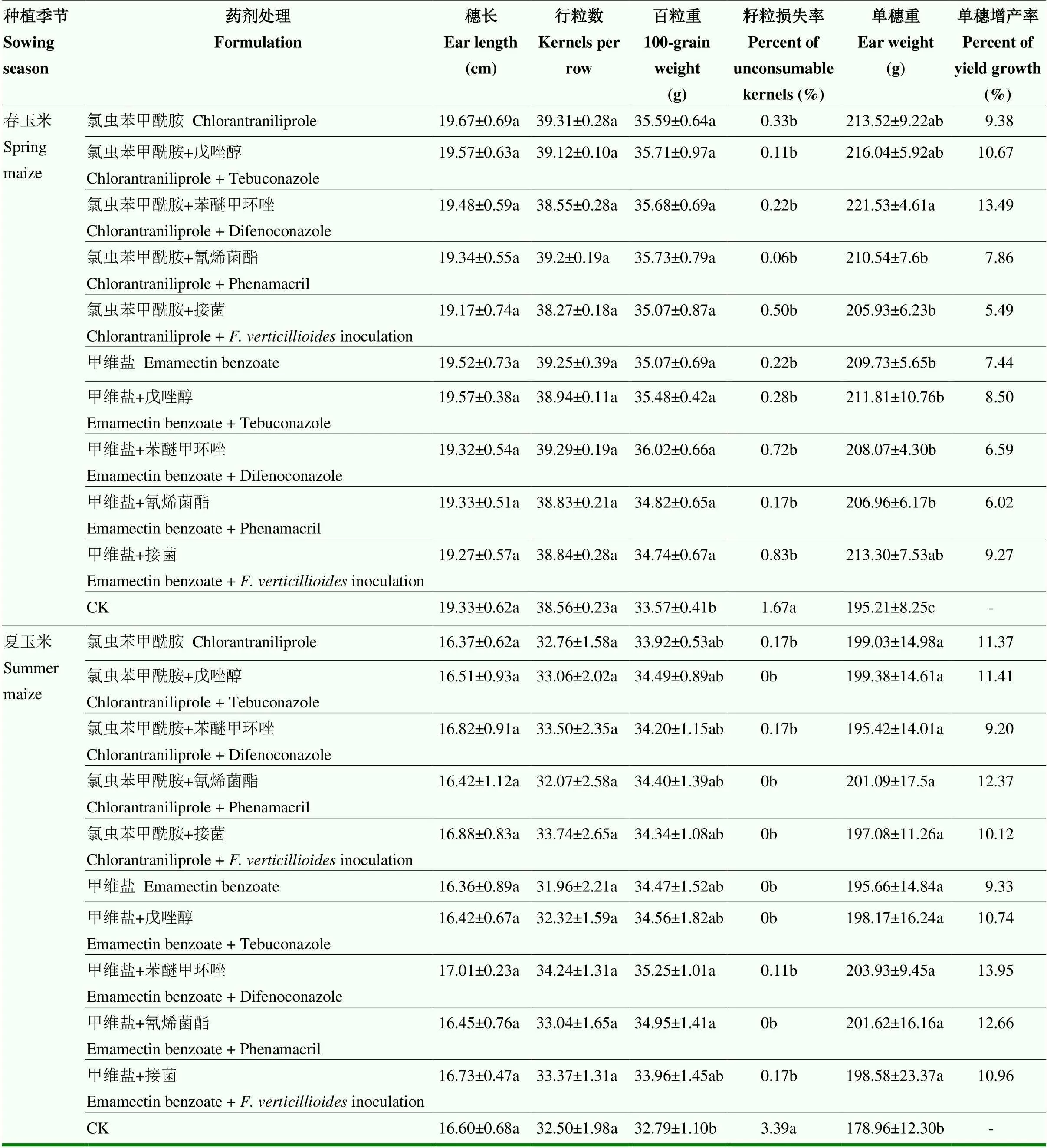
表4 化学防治对玉米产量的影响
接菌后在大量病原菌存在的情况下,单独施用杀虫剂的两个处理发病率、病情指数和伏马毒素含量与对照达到显著差异,在夏玉米试验中,施用氯虫苯甲酰胺后接菌的拟轮枝镰孢穗腐病发病率为0。上述结果表明在大田生产中虫害在拟轮枝镰孢引起发病的过程中具有极其重要的作用。拟轮枝镰孢侵染籽粒引起穗腐病的途径主要有两种:伤口或者花粉管途径[42-43]。在病害流行年份,气候适宜拟轮枝镰孢侵染时,拟轮枝镰孢可以通过开放的花粉管侵染易感品种的籽粒[44],这种侵染不依赖害虫造成的伤口,因此控制虫害对于穗腐病发病的影响可能不显著。而在大多数年份,如本研究所处阶段,即使有大量病原菌存在(人为接菌),气候不适宜发病,拟轮枝镰孢也很难直接通过花丝侵染引起穗腐病的发生。而蛀穗害虫造成的伤口,给拟轮枝镰孢的侵染提供了捷径,促进并加重了穗腐病的发生。施用杀虫剂减轻了穗期害虫的危害,从而减少对拟轮枝镰孢的传播和拟轮枝镰孢利用伤口侵染的机会,拟轮枝镰孢穗腐病的发病率显著下降。因此,防控穗期害虫在降低拟轮枝镰孢穗腐病的发生中具有至关重要的作用。
由于穗期害虫在拟轮枝镰孢侵染果穗过程中起着关键作用,在鉴定玉米品种抗性时,应将玉米品种对穗腐病的抗性和穗期的抗虫性相结合,综合考虑病虫害和环境关系,制订更完善的抗性鉴定方法,筛选出抗拟轮枝镰孢穗腐病的优良品种,提高玉米产量和品质,保障食品安全。
本研究中施用杀虫剂或其混剂后,玉米籽粒中伏马毒素的含量远低于各国对伏马毒素的限量;这意味着在适当的化学保护方案下,玉米籽粒的伏马毒素不会危及人、畜健康。而春玉米试验中,对照玉米籽粒中伏马毒素的含量已接近伏马毒素的最大限量,超过了食用限量;可见在不进行防治的情况下,籽粒的伏马毒素存在潜在风险,可能不适于食用。春玉米接菌处理的玉米,籽粒中伏马毒素含量高达8 710 μg·kg-1,超过最大限量两倍以上;因此当环境中菌量较大时,若不进行防治,玉米籽粒中伏马毒素可能对食品安全造成严重的威胁。接菌前施用杀虫剂则可将玉米中的伏马毒素含量降低至最大限度的一半以下。由此可见,即使环境中菌源量较大,施用杀虫剂也可通过保护玉米籽粒不受虫害而将籽粒中伏马毒素降低至安全范围内。
结合春玉米和夏玉米的两次试验结果,总体来看,各药剂处理对产量的影响有明显的规律性。与对照相比,各施药处理的穗长和行粒数均无显著差异,但均可显著降低籽粒损失率、增加穗重和单穗产量,减少籽粒中伏马毒素的含量。这意味着杀虫/杀菌剂混用和单用杀虫剂均可显著增产,提高籽粒品质,且效果无显著差异。因此从促进丰产、保障食品安全,同时保护环境、节约成本的角度来看,无需在杀虫剂中添加杀菌剂成分。当然,在保证防治效果的同时提高收益,需全面考虑各种相关因素,建立完善的病虫害综合防治制度或体系。由于导致玉米穗腐病的病原菌种类较多,在不是以拟轮枝镰孢为优势致病菌的地区,防治穗期害虫和穗腐病是否需要增加杀菌剂则还需要深入研究。
4 结论
综合各药剂处理后玉米病虫害、产量和伏马毒素含量情况,同时考虑成本、生态和食品安全因素,笔者认为在当地玉米穗期害虫产卵高峰期前后两次施用25 g·hm-2氯虫苯甲酰胺为防治拟轮枝镰孢穗腐病的最佳方案。
[1] PRESELLO D A, BOTTA G, IGLESIAS J, EYHÉRABIDE G H. Effect of disease severity on yield and grain fumonisin concentration of maize hybrids inoculated with. Crop Protection, 2008, 27(3/5): 572-576.
[2] PIENAAR J G, KELLERMAN T S, MARASAS W F. Field outbreaks of leukoencephalomalacia in horses consuming maize infected by(=) in South Africa. Journal of the South African Veterinary Association, 1981, 52(1): 21-24.
[3] ROSS P F, NELSON P E, RICHARD J L, OSWEILER G D, RICE L G, PLATTNER R D, WILSON T M. Production of fumonisins byandisolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Applied and Environmental Microbiology, 1990, 56(10): 3225-3226.
[4] WILSON T M, ROSS P F, RICE L G, OSWEILER G D, NELSON H A, OWENS D L, PLATTNER R D, REGGIARDO C, NOON T H, PICKRELL J W. Fumonisin B1 levels associated with an epizootic of equine leukoencephalomalacia. Journal of Veterinary Diagnostic Investigation, 1990, 2(3): 213-216.
[5] MARIN S, RAMOS A J, CANO-SANCHO G, SANCHIS V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food and Chemical Toxicology, 2013, 60: 218-237.
[6] FRANCESCHI S, BIDOLI E, BARÓN A E, LA VECCHIA C. Maize and risk of cancers of the oral cavity, pharynx, and esophagus in northeastern Italy. Journal of the National Cancer Institute, 1990, 82(17): 1407-1411.
[7] CHU F S, LI G Y. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Applied and Environmental Microbiology, 1994, 60(3): 847-852.
[8] THIEL P G, MARASAS W F, SYDENHAM E W, SHEPHARD G S, GELDERBLOM W C. The implications of naturally occurring levels of fumonisins in corn for human and animal health. Mycopathologia, 1992, 117(1/2): 3-9.
[9] ALIZADEH A M, ROHANDEL G, ROUDBARMOHAMMADI S, ROUDBARY M,Sohanaki H, Ghiasian S A, Taherkhani A, Semnani S,AGHASI M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pacific journal of cancer prevention, 2012, 13(6): 2625-2628.
[10] 刘玥, 李荣荣, 何康来, 白树雄, 张天涛, 丛斌, 王振营. 桃蛀螟为害对春玉米镰孢穗腐病发生及产量损失的影响. 昆虫学报, 2017, 60(5): 576-581.
LIU Y, LI R R, HE K L, BAI S X, ZHANG T T, CONG B, WANG Z Y. Effects of(Lepidopteran: Crambidae) infestation on the occurrence of Fusarium ear rot and yield loss of spring corn. Acta Entomologica Sinica, 2017, 60(5): 576-581. (in Chinese)
[11] 宋立秋, 石洁, 王振营, 何康来, 丛斌. 亚洲玉米螟为害对玉米镰孢穗腐病发生程度的影响. 植物保护, 2012, 38(6): 50-53, 58.
SONG L Q, SHI J, WANG Z Y, HE K L, CONG B. Effects of the Asian corn borer injury on the incidence of Fusarium ear rot caused byat different developmental stages of corn ear. Plant Protection, 2012, 38(6): 50-53, 58. (in Chinese)
[12] NCUBE E, FLETT B C, VAN DEN BERG J, ERASMUS A, VILJOEN A. The effect ofinfestation, fungal inoculation and mechanical wounding on Fusarium ear rot development and fumonisin production in maize. Crop Protection, 2017, 99: 177-183.
[13] SCHULTHESS F, CARDWELL K F, GOUNOU S. The effect of endophyticon infestation of two maize varieties by lepidopterous stemborers and coleopteran grain feeders. Phytopathology, 2002, 92(2): 120-128.
[14] CHRISTENSEN J J, SCHNEIDER C L. European corn borer (Hbn.) in relation to shank, stalk and ear rots of corn. Phytopathology, 1950, 40(3): 284-291.
[15] AVANTAGGIATO G, QUARANTA F, DESIDERIO E, VISCONTI A. Fumonisin contamination of maize hybrids visibly damaged by. Journal of the Science of Food and Agriculture, 2003, 83(1): 13-18.
[16] BLANDINO M, SCARPINO V, VANARA F, SULYOK M, KRSKA R, REYNERI A. Role of the European corn borer () on contamination of maize with 13 Fusarium mycotoxins. Food Additives and Contaminants Part A: Chemistry, Analysis, Control Exposure and Risk Assessment, 2015, 32(4): 533-543.
[17] MADEGE R R, LANDSCHOOT S, KIMANYA M, TIISEKWA B, DE MEULENAER B, BEKAERT B, AUDENAERT K, HAESAERT G. Early sowing and harvesting as effective measures to reduce stalk borer injury,incidence and associated fumonisin production in maize. Tropical Plant Pathology, 2019, 44(2): 151-161.
[18] FOLCHER L, JARRY M, WEISSENBERGER A, GÉRAULT F, EYCHENNE N, DELOS M, REGNAULT-ROGER C. Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers andmycoflora in maize (L.) fields. Crop Protection, 2009, 28(4): 302-308.
[19] MAZZONI E, SCANDOLARA A, GIORNI P, PIETRI A, BATTILANI P. Field control of Fusarium ear rot,(Hübner), and fumonisins in maize kernels. Pest Management Science, 2011, 67(4): 458-465.
[20] DARVAS B, BÁNÁTI H, TAKÁCS E, LAUBER É, SZÉCSI Á, SZÉKÁCS A. Relationships of,andon MON 810 maize. Insects, 2011, 2(1): 1-11.
[21] BOWERS E, HELLMICH R, MUNKVOLD G. Comparison of fumonisin contamination using HPLC and ELISA methods in bt and near-isogenic maize hybrids infested with European corn borer or western bean cutworm. Journal of Agricultural and Food Chemistry, 2014, 62(27): 6463-6472.
[22] BOWERS E, HELLMICH R, MUNKVOLD G. Vip3Aa and Cry1Ab proteins in maize reduce Fusariumear rot and fumonisins by deterring kernel injury from multiple Lepidopteran pests. World Mycotoxin Journal, 2013, 6(2): 127-135.
[23] HAMMOND B G, CAMPBELL K W, PILCHER C D, DEGOOYER T A, ROBINSON A E, MCMILLEN B L, SPANGLER S M, RIORDAN S G, RICE L G, RICHARD J L. Lower fumonisin mycotoxin levels in the grain of Bt corn grown in the United States in 2000-2002. Journal of Agricultural and Food Chemistry, 2004, 52(5): 1390-1397.
[24] ALMA A, LESSIO F, REYNERI A, BLANDINO M. Relationships between(Lepidoptera: Crambidae) feeding activity, crop technique and mycotoxin contamination of corn kernel in northwestern Italy. International Journal of Pest Management, 2005, 51(3): 165-173.
[25] BLANDINO M, REYNERI A, VANARA F, PASCALE M, HAIDUKOWSKI M, CAMPAGNA C. Managing fumonisin contamination in maize kernels through the timing of insecticide application against European corn borerHübner. Food Additives and Contaminants Part A: Chemistry, Analysis, Control Exposure and Risk Assessment, 2009, 26(11): 1501-1514.
[26] DE CURTIS F, DE CICCO V, HAIDUKOWSKI M, PASCALE M, SOMMA S, MORETTI A. Effects of agrochemical treatments on the occurrence of Fusarium ear rot and fumonisin contamination of maize in Southern Italy. Field Crops Research, 2011, 123(2): 161-169.
[27] 尚艳娥, 杨卫民. CAC、欧盟、美国与中国粮食中真菌毒素限量标准的差异分析. 食品科学技术学报, 2019, 37(1): 10-15.
SHANG Y E, YANG W M. Variation analysis of cereals mycotoxin limit standards of CAC, EU, USA, and China. Journal of Food Science and Technology, 2019, 37(1): 10-15. (in Chinese)
[28] WINDHAM G L, WILLIAMS W P, DAVIS F M. Effects of the southwestern corn borer onkernel infection and aflatoxin accumulation in maize hybrids. Plant disease, 1999, 83(6): 535-540.
[29] 中华人民共和国农业农村部. 转基因玉米环境安全检测技术规范: NY/T720.1-720.3, 2003.
Ministry of Agriculture and Rural Affairs,The People’s Republic of ChinaEnvironmental impact testing of genetically modified maize: NY/T720.1-720.3, 2003. (in Chinese)
[30] KUSHIRO M, NAGATA R, NAKAGAWA H, NAGASHIMA H. Liquid chromatographic detection of fumonisins in rice seed. Report of National Food Research Institute, 2008, 72: 37-44.
[31] SCARPINO V, REYNERI A, VANARA F, SCOPEL C, CAUSIN R, BLANDINO M. Relationship between European corn borer injury,andinfection and moniliformin contamination in maize. Field Crops Research, 2015, 183: 69-78.
[32] BLANDINO M, PEILA A, REYNERI A. Timing clorpirifos + cypermethrin and indoxacarb applications to control European corn borer damage and fumonisin contamination in maize kernels. Journal of the Science of Food and Agriculture, 2010, 90(3): 521-529.
[33] PARSONS M W, MUNKVOLD G P. Associations of planting date, drought stress, and insects with Fusarium ear rot and fumonisin B1 contamination in California maize. Food Additives and Contaminants Part A: Chemistry, Analysis, Control, Exposure and Risk Assessment, 2010, 27(5): 591-607.
[34] BLANDINO M, REYNERI A, VANARA F, PASCALE M, HAIDUKOWSKI M, SAPORITI M. Effect of sowing date and insecticide application against European corn borer (Lepidoptera: Crambidae) on fumonisin contamination in maize kernels. Crop Protection, 2008, 27(11): 1432-1436.
[35] PAPST C, UTZ H F, MELCHINGER A E, EDER J, MAGG T, KLEIN D, BOHN M. Mycotoxins produced byspp. in isogenic Bt vs. non-Bt maize hybrids under European corn borer pressure. Agronomy Journal, 2005, 97(1): 219-224.
[36] BAKAN B, MELCION D, RICHARD-MOLARD D, CAHAGNIER B. Fungal growth andmycotoxin content in isogenic traditional maize and genetically modified maize grown in France and Spain. Journal of Agricultural and Food Chemistry, 2002, 50(4): 728-731.
[37] MAGG T, MELCHINGER A E, KLEIN D, BOHN M. Relationship between European corn borer resistance and concentration of mycotoxins produced byspp. in grains of transgenic Bt maize hybrids, their isogenic counterparts, and commercial varieties. Plant Breeding, 2002, 121(2): 146-154.
[38] NCUBE E, FLETT B C, VAN DEN BERG J, ERASMUS A, VILJOEN A. Fusarium ear rot and fumonisins in maize kernels when comparing a Bt hybrid with its non-Bt isohybrid and under conventional insecticide control ofinfestations. Crop Protection, 2018, 110: 183-190.
[39] SANTIAGO R, CAO A, MALVAR R A, BUTRON A. Is it possible to control fumonisin contamination in maize kernels by using genotypes resistant to the Mediterranean corn borer?. Journal of economic entomology, 2013, 106(5): 2241-2246.
[40] CLEMENTS M J, CAMPBELL K W, MARAGOS C M, PILCHER C, HEADRICK J M, PATAKY J K, WHITE D G. Influence of Cry1Ab protein and hybrid genotype on fumonisin contamination and Fusarium ear rot of corn. Crop Science, 2003, 43(4): 1283-1293.
[41] PARKER N S, ANDERSON N R, RICHMOND D S, LONG E Y, WISE K A, KRUPKE C H. Larval western bean cutworm feeding damage encourages the development of Gibberella ear rot on field corn. Pest management science, 2017, 73(3): 546-553.
[42] MUNKVOLD G P. Epidemiology ofdiseases and their mycotoxins in maize ears. European Journal of Plant Pathology, 2003, 109(7): 705-713.
[43] MUNKVOLD G P, MCGEE D C, CARLTON W M. Importance of different pathways for maize kernel infection by. Phytopathology, 1997, 87(2): 209-217.
[44] DUNCAN K E, HOWARD R J. Biology of maize kernel infection by. Molecular Plant-Microbe Interactions, 2010, 23(1): 6-16.
Effects of Chemical Control of Ear Borers on ReducingEar Rot and Fumonisin Level
LI QinCheng1, SHI Jie2, HE KangLai1, WANG ZhenYing1
1State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193;2Plant Protection Institute, Hebei Academy of Agricultural and Forestry Sciences, Baoding 071030, Hebei
【】is responsible for ear rot occurrence and quality degradation in maize. It gives rise to the production of fumonisin and poses a threat on food security. Ear borers inculdingandcan cause severe yield loss and lead to an increase in ear rot occurrence.【】The objective of this study is to evaluate the efficacy of two insecticides (emamectin benzoate and chlorantraniliprole) combined with three fungicides (phenamacril, tebuconazole and difenoconazole) in promoting yield, reducing ear rot severity and fumonisin kernel contamination under natural conditions, clarify the effect of artificial inoculation ofon insecticide efficacy, define an effective schedule for the control of maize pests and provide a theoretical basis for the pesticide field application.【】Hybrid Zhengdan 958 typically cultivated in China was selected in this study. Field experiments were conducted in Langfang, Hebei Province. Pesticide treatments were conducted 5 d and 20 d after silking, and artificial inoculation ofwas conducted 7 d after silking. Insect damage, ear rot occurrence, ear length, kernels per row, 100-grain weight and ear weight were investigated and recorded at harvest phenological stage. Fumonisins B1 and B2 level in kernels was analyzed by LC-MS/MS.【】Compared with controls,emamectin benzoate and chlorantraniliproleapplication could significantly reduce borer damage, ear rot occurrence and fumonisins level. While insecticides have been shown to give advantages in their application, adding a fungicide didn’t lead to a significant lower insect damage, ear rot occurrence or fumonisins level. Additional fungicide didn’t lead a significant higher control effect or yield. After inoculating, chlorantraniliprole application led to a significant decrease in fumonisins level, ear rot incidence and severity. Control effect of 25 g·hm-2chlorantraniliprole and its combinations on insect damage was 82.1%-92.7% and 94.2%-95.0% in spring and summer maize, respectively. Control effect of 30 g·hm-2emamectin benzoate was significantly lower, at the level of 57.8%-78.0% in spring maize and 83.1%-89.9% in summer maize. Control effect on ear rot occurrence was >60% in spring maize and >88% in summer maize. No significant difference was found among pesticide treatments. Regarding yield, insecticide application had no significant effect on ear length or kernels per row, while significantly promoted ear weight compared with controls. No significant difference was found among insecticide treatments and mixture treatments. Artificial inoculation ofhad no significant impact on yield after insecticide application. The yield of spring and summer maize increased by 5.49%-13.49% and 9.20%-13.95% after applying insecticides or mixture of insecticides with fungicides, respectively. Kernel fumonisins level was lower than 500 μg·kg-1after insecticide or mixture of insecticides with fungicides application, while the level in controls was 2 817 μg·kg-1. Kernel fumonisins level afterinoculatingreached up to 8 710 μg·kg-1, while the number could be reduced to 1 500 μg·kg-1after insecticide application.【】These results indicated that 25 g·hm-2chlorantraniliprole and 30 g·hm-2emamectin benzoate application can reduce ear rot occurrence and fumonisin level, improve maize yield and quality by controlling insect damage. No significant difference was found in insecticide treatments and mixture of insecticide with fungicide treatments. Insect infestation plays a decisive role in theinfection. Taking all aspects into consideration, 25 g·hm-2chlorantraniliprole is relative ideal in maize pest control.
chemical control; pest;; maize ear rot; yield; fumonisin

10.3864/j.issn.0578-1752.2021.17.012
2020-12-05;
2020-12-28
国家现代农业产业技术体系(CARS-02)、中国农业科学院重大科研任务(CAAS-ZDRW202004)
李琴珵,E-mail:ashofmournhold@163.com。通信作者王振营,E-mail:zywang@ippcaas.cn
(责任编辑 岳梅)

