Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
Zhang Dan; Yang Ping; Zhang Youhua; Duan Linhai; Meng Xiuhong
(School of Chemical Engineering, Guangdong University of Petrochemical Technology, Maoming 525000)
Abstract: An approach for dominating the channel form of the flexible MIL‐53(Fe) was developed by using a surfactant‐assisted modified method.The surfactant tetraethylammonium hydroxide (TEAOH) can control the channel form of MIL‐53(Fe) to be a “closed‐pore” form, whereas the surfactant polyoxyethylene polyoxypropylene polyoxyethylene (P123)or polyvinyl alcohol (PVA) can dominate the channel form of MIL‐53(Fe) for a “large‐pore” form.The photocatalytic performance of MIL‐53(Fe) with different channel forms was investigated through the degradation of rhodamine B (Rh B) in water under violet light irradiation.The results showed that MIL‐53(Fe) with a “large‐pore” form exhibited higher photocatalytic activity than that of MIL‐53(Fe) with a “closed‐pore” form.MIL‐53(Fe) modified with PVA exhibit the best photocatalytic activity for degrading almost 100% Rh B in 90 minutes.
Key words: MIL‐53(Fe); surfactant; photocatalysis
1 Introduction
MIL‐53(Fe) is a porous metal‐organic framework material with a one‐dimensional pore structure, which is connected by bis‐bidendate of terephthalate using FeO4(OH)2octahedron as the node.The material has the advantages of simple preparation, good stability, low price, and non‐toxicity, and it has been widely used in photocatalysis[1‐4], biosensors[5], and drug delivery[6].The skeleton structure of MIL‐53(Fe) is flexible (Figure 1),and this effect, also called “breathing”, can produce a dramatic increase or decrease in cell volume without a loss of crystallinity or bond breaking[7‐11].The flexible MOFs can be applied in the gas separation process due to its suitable pore confinement and strong binding affinity[12‐14].From the literature studies, it is found that even if the same synthesis method and post‐treatment method are used, the skeleton structure of MIL‐53(Fe)exhibits different forms[15‐17].Therefore, it is difficult to control the pore structure of MIL‐53(Fe).At present,there are two solutions to address this difficulty, namely,adjusting the temperature and using guest molecules[18‐19].Franck has found that variable‐temperature powder X‐ray diffraction shows that MIL‐53(Fe) exhibits large pore at 423 K, and exhibits narrow pore at 298 K.Moreover, the transformation process is reversible and reproducible[8].MIL‐53(Fe) can adapt their pore opening to accommodate guest species, and it shows narrow pore form, intermediate pore form, and large pore form as the CH4pressure rises[10].However, these methods are of little practical significance.Adjusting the temperature can control the degree of expansion of the MIL‐53(Fe)skeleton structure; however, the application temperature of MIL‐53(Fe) may be inconsistent with the temperature used for controlling the degree of expansion of the channel of the material.The fact denoting that guest molecules can control the MIL‐53(Fe) skeleton structure is even more meaningless, because filling the channels of MIL‐53(Fe) with guest molecules is unfavorable to its applications.However, the degree of expansion of the MIL‐53(Fe) channels has a great influence on its application in photocatalysis, adsorption, and other fields.Therefore, it is very important to find a simple method to control the skeleton structure of MIL‐53(Fe).

Figure 1 Schematic diagram of flexible MIL-53(Fe) framework structure
To some extent, surfactants can control the particle size,appearance, and pore structure of MOFs materials, and even change the crystal phase structure of MOFs[20‐22].On the one hand, the surfactant can form microemulsion in the solution to become a reactor, which can accommodate the central metal ions and organic ligands to take part in reaction.By adjusting the amount of surfactant and regulating the spatial shape of the reactor, the material with different morphology and particle size can be obtained[23].On the other hand, surfactants can be used as inhibitors or steric arresters to control the crystal growth rate, affect the crystal growth direction of MOFs, and control the morphology of the products[24].However,the effect of surfactant on the structure and properties of MIL‐53(Fe) is less studied.
In this study, we have found a simple method to control the skeleton structure of MIL‐53 (Fe).The influence of the surfactants including tetraethylammonium hydroxide (TEAOH), polyoxyethylene polyoxypropylene polyoxyethylene (P123), and polyvinyl alcohol (PVA)on the skeleton structure of MIL‐53 (Fe) is studied.A photocatalytic degradation experiment with rhodamine B (Rh B) is carried out to investigate the photocatalytic performance of MIL‐53 (Fe)‐surfactant.
2 Experimental
2.1 Materials
All chemical reactants were of analytically pure grade and were used without any further purification.Iron (III) chloride hexahydrate, ethanol, rhodamine B, andN,N‐dimethylformamide (DMF) were purchased from the Sinopharm Chemical Reagent Co.Ltd., 1,4‐benzenedicarboxylic acid (BDC) and tetraethylammonium hydroxide aqueous solution(TEAOH, 25% mass fraction in water) were obtained from the Aladdin Reagent Co.Ltd., polyoxyethylene polyoxypropylene polyoxyethylene (P123,Mn≈5880)was provided by the Sigma‐Aldrich Reagent Co.Ltd.,polyvinyl alcohol (PVA, with a polymerization degree of 1750±50) was purchased from the Tianjin Second Chemical Reagent Factory.
2.2 Synthesis of the materials
General consideration: MIL‐53(Fe) was prepared according to the previous report of Férey[25].MIL‐53(Fe)‐surfactant was synthesized by using different surfactants.MIL‐53(Fe) modified with the surfactant TEAOH was named MIL‐53(Fe)‐TEAOH; MIL‐53(Fe) modified with the surfactant P123 was named MIL‐53(Fe)‐P123; MIL‐53(Fe) modified with the surfactant PVA was named MIL‐53(Fe)‐PVA.A mixture of FeCl3·6H2O (1.35 g, 5 mmol), H2BDC (0.83 g, 5 mmol), DMF (112 mL) and an appropriate amount of surfactant was put into a 200‐mL Teflon‐lined stainless‐steel autoclave, the mixture was then heated at 150℃ for 15 h.It was found that MIL‐53(Fe) modified with 1.2 g of TEAOH, 0.05 g of P123,0.25 g of PVA, respectively, showed good crystallization phase structure.The specific synthesis steps are as follows.
2.2.1 Synthesis of MIL-53(Fe)-TEAOH
FeCl3·6H2O (1.35 g, 5 mmol), H2BDC (0.83 g, 5 mmol),TEAOH (1.2 g, 2 mmol) and DMF (112 mL) were placed in a 200‐mL beaker and stirred for 120 min at room temperature until it became clear.Then, the mixture was placed in an ultrasonic generator for 120 min.After that,the mixture was transferred into a Teflon liner and a metallic Paar bomb; the mixture was then heated for 15 hours at 150℃.The activation was performed as follows:the crude product was mixed with 200 mL of C2H5OH and was agitated ultrasonically for 2 hours, and then the solid was dried under vacuum at 150℃ for 15 hours.
2.2.2 Synthesis of MIL-53(Fe)-P123
0.05 g (0.009 mmol) of P123 was used instead of TEAOH.The rest procedures of preparation and post‐treatment were the same as those used for MIL‐53(Fe)‐TEAOH.
2.2.3 Synthesis of MIL-53(Fe)-PVA
0.25 g (0.003 mmol) of PVA was used instead of TEAOH.The rest procedures of preparation and post‐treatment were the same as those used for MIL‐53(Fe)‐TEAOH.
2.3 Characterizations
The powder X‐ray diffraction (XRD) patterns were obtained on a Rigaku D/Max‐2500 diffractometer with CuKαradiation at 20 mA and 30 kV.The data were recorded in a 2θ range of 5°‒40°.The pore structure of samples was determined by nitrogen adsorption‐desorption isotherms in an ASAP 2020 surface area analyzer (Micromeritics, USA), the original MIL‐53(Fe)‐surfactant was preliminary degassed under vacuum overnight at 150℃.The UV‒Vis diffuse reflectance spectra were obtained by a Lamda 650 spectrophotometer.The photocurrent measurements were conducted with a CHI660 workstation (Shanghai Chenhua Co.) and 0.1 mol/L of Na2SO4solution was used as the electrolyte.
2.4 Photocatalytic activity test
The photocatalytic activity of MIL‐53(Fe)‐surfactant was evaluated by the photodegradation of rhodamine B dye under a high‐pressure Hg lamp (ML250W, Philips) at room temperature.In the photocatalytic activity test, 20 mg of MIL‐53(Fe)‐surfactant and 100 mL of rhodamine B aqueous solution (20 mg/L) were put into a 250‐mL beaker.Prior to irradiation, the reaction mixture was stirred in the dark for 60 min to reach the adsorption/desorption equilibrium.After that, the mixture solution was uninterruptedly stirred during the photodegradation reaction.4 mL of the solution were withdrawn at given time intervals and immediately centrifuged to remove the catalyst.The rhodamine B concentration was evaluated at a maximum absorbance wavelength of 553 nm with a UV‐Vis spectrophotometer (721 spectrometer, Shanghai Jinghua Instruments Co., Ltd).
3 Results and Discussion
3.1 Characterization results
The different skeleton structures of MIL‐53 (Fe) could drastically affect the X‐ray diffraction (XRD) patterns.Figure 2 shows the XRD patterns of MIL‐53 (Fe)prepared by using the surfactants.As for MIL‐53 (Fe)‐TEAOH, its main diffraction peaks are identified at 9.22ºand 12.46º which are consistent with the simulated pattern of MIL‐53 (Fe) with narrow pores[7].In contrast, the main diffraction peaks of MIL‐53 (Fe)‐P123 and MIL‐53 (Fe)‐PVA are displayed at 9.32º and 18.68º, which are in good agreement with the simulated one of MIL‐53 (Fe) with large pores used in reference[10].The results showed that the surfactants could control the degree of expansion of the MIL‐53 (Fe) channels.The surfactant TEAOH could promote the preparation of narrow‐pore MIL‐53 (Fe),whereas the surfactant P123 or PVA was conducive to the formation of large‐pore MIL‐53 (Fe).

Figure 2 XRD patterns of MIL-53(Fe)-surfactant
Figure 3 shows the representative scanning electron microscopic (SEM) images of MIL‐53(Fe) and the synthesized MIL‐53(Fe) products prepared with different surfactants (TEAOH, P123, and PVA).As shown in Figure 3a, when no surfactant was added, the microsized crystals of MIL‐53(Fe) were produced with a morphology of bipyramidal hexagonal prism, 2.7 μm in length and 1.5 μm in width.Under the same synthetic conditions, however, in the presence of TEAOH, P123, and PVA, the morphology of MIL‐53(Fe) crystals was changed.Figure 3b showsthat MIL‐53(Fe)‐TEAOH has rod shaped crystals.MIL‐53(Fe)‐P123 presents hexagonal bipyramids with no apexes and octahedral shape (Figure 3c).When surfactant PVA was added to the synthesis solution, the morphology had showed block shaped crystals.These results indicate that the surfactant plays some role in the preparation of MIL‐53(Fe).The transformation in crystals morphology might be due to reactor shape generated by surfactant.
The degree of pore expansion in MIL‐53 (Fe) can also be verified by detecting the pore structure of the product.Figure 4 shows the N2adsorption‐desorption isotherms of MIL‐53(Fe), MIL‐53(Fe)‐TEAOH, MIL‐53(Fe)‐P123, and MIL‐53(Fe)‐PVA.As shown in Figure 4, MIL‐53(Fe), MIL‐53(Fe)‐P123, and MIL‐53(Fe)‐PVA are porous materials containing a mesoporous structure.In contrast, MIL‐53(Fe)‐TEAOH is a non‐porous material.Table 1 shows the specific surface area and pore volume data of the materials.The specific surface area of MIL‐53(Fe), MIL‐53(Fe)‐P123, and MIL‐53(Fe)‐PVA is 44.99 m2/g, 110.11 m2/g, and 146.96 m2/g, respectively; in contrast, the specific surface area of MIL‐53(Fe)‐TEAOH is only 4.4 m2/g.MIL‐53 (Fe)‐P123,and MIL‐53 (Fe)‐PVA had a larger specific surface area owing to their larger degree of channel expansion.The nonionic surfactants (P123 and PVA) are block copolymers,macromolecules, which have constructed large pores of MIL‐53 (Fe) as structure directing agents[26].However,MIL‐53 (Fe)‐TEAOH has been confirmed as a nonporous material, which indicates that its channels are closed.

Table 1 Pore characteristics of samples

Figure 4 N2 adsorption-desorption isotherm of MIL-53(Fe)-surfactant
The optical absorption properties of MIL‐53(Fe)‐surfactant were measured by an UV‐Vis diffuse reflectance spectrometer, with the results shown in Figure 5.An enhanced absorbance is found in the ultraviolet and visible light regions for MIL‐53(Fe)‐P123 and MIL‐53(Fe)‐PVA.For MIL‐53(Fe)‐TEAOH, its UV‐Vis diffuse reflectance spectrum corresponds to reduced absorbance.MIL‐53(Fe)‐PVA displays a highest absorption intensity in the UV region.When absorbance in ultraviolet and visible light regions increases, the light energy can be utilized more effectively.Consequently, we can speculate that MIL‐53(Fe)‐PVA can exhibit a best photocatalytic activity.

Figure 3 SEM images of MIL-53(Fe)-surfactant
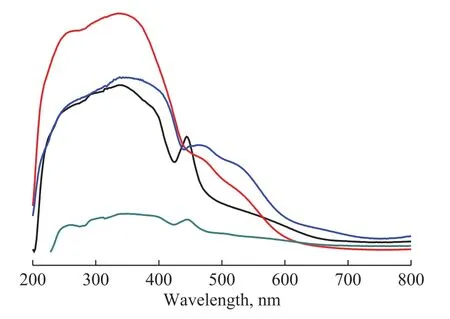
Figure 5 UV-Vis diffuse reflectance spectra of MIL-53(Fe)-surfactant
To further understand the photocatalytic activity of MIL‐53(Fe)‐surfactant, the photocurrent time curves for the MIL‐53(Fe)‐surfactant are displayed in Figure 6.Compared with other samples, MIL‐53(Fe)‐PVA exhibits a highest photocurrent density.Therefore, MIL‐53(Fe)‐PVA possesses a longer lifetime of photogenerated electro-hole pairs which is beneficial to the photocatalytic reaction.

Figure 6 Transient photocurrent responses of MIL-53(Fe)-surfactant
3.2 Photocatalysis properties
The photocatalytic activity of MIL‐53(Fe)‐surfactant was investigated by performing a photocatalytic degradation experiment with rhodamine B.Figure 7 shows the photocatalytic degradation of rhodamine B on the pure and surfactant‐assisted prepared MIL‐53(Fe).As presented in Figure 7, the pure MIL‐53 (Fe) could degrade 56.8%of rhodamine B after 90 min of high‐pressure Hg light irradiation, whereas MIL‐53 (Fe)‐P123, and MIL‐53 (Fe)‐PVA with a large pore exhibited higher efficiency in the photocatalytic degradation of rhodamine B, which reached 98.1% and 100% in 90 min, respectively.In contrast,the photocatalytic degradation of rhodamine B on MIL‐53 (Fe)‐TEAOH with a narrow pore was lower, since only 11.7% of Rh B was degraded in 90 min.The results illustrated that MIL‐53 (Fe)‐P123 and MIL‐53 (Fe)‐PVA had stronger photocatalytic activity than pure MIL‐53(Fe).This outcome was mainly due to the fact that MIL‐53 (Fe)‐P123 and MIL‐53 (Fe)‐PVA with a large pore can adsorb more rhodamine B molecules, which is favorable to the photocatalytic degradation of rhodamine B.The light absorbance ability of MIL‐53 (Fe)‐PVA is better than MIL‐53 (Fe)‐P123 (Figure 5) and MIL‐53(Fe)‐PVA possesses a longer lifetime of photogenerated electro-hole pairs than MIL‐53 (Fe)‐P123 (Figure 6), so MIL‐53(Fe)‐PVA shows better photocatalytic activity.However, the introduction of TEAOH into MIL‐53(Fe) closed the pores of MIL‐53(Fe),which could lead to a lower photocatalytic activity.

Figure 7 Photocatalytic degradation of rhodamine B on the pure and surfactant-assisted MIL-53(Fe)
4 Conclusions
In summary, MIL‐53 (Fe) was synthesized by using different surfactants, and the degree of expansion of its channel was controlled.The cationic surfactant TEAOH, as a small molecule, can control the pore form of MIL‐53 (Fe) as a closed form.The surfactants P123 and PVA, as macromolecules, can control the pore form of MIL‐53 (Fe) as a fully expanded form.Meanwhile, we found that the photocatalytic performance of large‐pore MIL‐53 (Fe) was better than that of closed‐pore MIL‐53 (Fe).Our work provides a simple method for controlling the channel form of materials with a flexible skeleton.
Acknowledgement:This research was supported by the Natural Science Foundation of Guangdong Province (No.2018A030307058), and the Projects of Talents Recruitment of GDUPT (72100003177).
- 中国炼油与石油化工的其它文章
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Synthesis of Bimodal Mesoporous TiO2 -PTA/BMMS and Its Enhanced Performance in the Photocatalytic Oxidative Desulfurization
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts
- Preparation of Ultra-High Molecular Weight Polypropylene Using Ziegler-Natta Catalyst via Combining Internal Electron Donor and Cocatalyst Loading
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation

