Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation
Hou Kaige; Qin Bo; Han Junjie; Du Yanze; Ma Jinghong; Li Ruifeng
(1.College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024;2.SINOPEC Dalian Research Institute of Petroleum and Petrochemicals, Dalian)
Abstract: The catalytic transformation of methylcyclohexane as an accepted probe reaction to evaluate zeolitic acidity(concentration, strength, and accessibility) is employed to study the acidity and the reactivity of three commercial dealuminated Y zeolites (DAY) with different Si/Al ratios and meso/microporosities, with their properties analyzed by N2 adsorption/desorption, pyridine‐IR, and hydroxyl‐IR spectroscopy technologies.The global activity (conversion) is largely dependent on the concentration of the acid sites, and the activity of the protonic sites in terms of turnover frequency (TOF)reflects the accessibility of acid sites.The products of aromatics and isomers, and the yield of cracking products increase with the increase of concentration of strong protonic sites in zeolite micropores.Moreover, the decrease of aromatics with the reduction of the concentration of acid sites and the diffusion length within DAY zeolites are observed due to the decrease of the secondary reaction.For the same reason, it results in the increasing of C7 products and alkenes/alkanes ratios in the cracking products.The high i‐C4 product selectivity is a unique reflection of the high percentage of very strong acid sites,which is characterized by the hydroxyl‐IR band at 3600 cm‐1.
Key words: methylcyclohexane catalytic transformation; industrially modified Y zeolite; zeolitic acidity; strong protonic sites; spectroscopic technology
1 Introduction
Zeolite Y, a large‐pore zeolite (with a pore diameter of 0.74 nm) with FAU topological structure and three‐dimensional (3‐D) cavities, has been used as solid‐acid catalyst for fluid catalytic cracking (FCC) and hydrocracking process of crude oil into fuels and chemicals[1‐3].However,the as‐synthesized zeolite Y usually does not meet the stability requirements of industrial catalysis because of its low Si/Al ratio.To make high‐silica zeolite as an active and stable catalyst, the primordial Y zeolite is modified usually by steaming and/or acid dealumination.The dealumination of the as‐synthesized Y zeolite has a large influence on its structure and surface acidity that control the catalytic activity and reactivity of the zeolite catalyst.Zeolite acidity, especially the Brønsted acid, is an extremely key parameter in the catalytic process of oil and biomass refining, since many processes involve protolytic C‐C (or C‐H) bond cleavage and the generation of carbocations.It is well known that the so-called dealuminated zeolite Y(DAY) has become the best choice as catalyst or support for processing heavy oil fraction due to the manipulation of acidity and the generation of mesoporosity[4].
Typical FCC feedstocks are heavy vacuum gas oil(VGO), which is principally composed of naphthenes,aromatics and alkanes.The transformation of alkanes and aromatics have been deeply concerned, however,only few studies on the conversion of naphthenes over zeolites have been reported[5].Naphthenes are primarily distributed between C6‒C8in FCC gasoline fraction.The transformation of these naphthenic compounds into other more valuable ones, such as olefins, gasoline, or diesel,not only can improve fuel quality, but also can minimize naphthenic compounds in the refinery cuts, because they could undergo dehydrogenation, generating aromatic compounds that should meet rigorous environmental regulations[6].Methylcyclohexane (MCH), as a typicalrepresentative of naphthenes, has certain similarities with polycyclic cycloalkanes[7].MCH transformation as a model reaction can provide imperative reference value to further understand the conversion regularity of polycyclic cycloalkanes during the catalytic cracking process.And most of all, the MCH transformation includes all the reaction types in the FCC process, such as cracking,ring‐opening, isomerization, and those accompanied by bimolecular hydrogen transfer reaction and coke formation[8], which can help to understand the FCC process.Since the discovery and application of zeolite as catalyst,the correlation between the acidity and catalytic property of zeolite has received considerable attention.As an example,the conversion and selectivity ofn‐heptane cracking at 723 K were investigated over HBEA zeolite catalysts prepared by steaming, hydrochloric acid solution and ammonium hexafluorosilicate treatments, to build a link betweenn‐heptane conversion and acid concentration in zeolite.It is showed that a quasi‐linear correlation between the activity and the square of the protonic site concentration was observed.The higher the concentration of the protonic sites (Brønsted acid), the greater the activity, and the faster the deactivation.Moreover, the Lewis acid sites in the zeolite play a direct or indirect role for catalytic cracking ofn-heptane[9].In addition, the porosity of zeolites has a pronounced influence on the selectivity of products.In MCH transformation, the yield of isomers was much favored over H‐FAU zeolite than over H‐MFI zeolite[10‐11].
MCH transformation is a complex network, and is extensively used as a powerful tool for characterizing the acidity and porosity of zeolites as a model reaction.However, relatively few studies have addressed the chemistry of cycloalkane cracking on FAU zeolite,notwithstanding cycloalkane that is an important component of commercial FCC feedstock.Therefore, in this work,the MCH transformation combined with IR spectrometry technology are employed to evaluate the impact of acidity and porous structure on the catalytic performance of DAY zeolites.This research is aimed at establishing the relation between the reactivity (activity and selectivity) and acidity of different DAY zeolites via MCH transformation.
2 Experimental
2.1 Samples and characterizations
Three samples (DAY‐1, DAY‐2, DAY‐3), commercial DAY zeolites from Dalian/Fushun Research Institute of Petroleum & Petrochemicals, were studied in the present study.These samples were obtained by dealumination through hydrothermal treatment and acid treatment of NH4Y zeolite (with a Si/Al atomic ratio of 2.2).
All the samples were characterized by powder X‐ray diffraction (Shimadzu XRD‐6000, 40 kV, 30 mA, Cu‐Kα radiation with a scanning step of 0.02(°)/min and a 2θangle scanning range of 5°‒35°) and gas adsorption analyzer (Quantachrome QUANRASORB SI).The porous parameters of the samples were obtained by using N2adsorption and desorption at 77 K.Prior to the adsorption, all samples were degassed at 573 K for 3 h in vacuum.Specific surface areas were determined from the BET equation in ap/p0range of 0.01‒0.15.The micropore volume (Vmic) and mesopore volume(Vmeso) were calculated by the NLDFT method.The29Si MAS‐NMR spectra were taken on a Bruker Avance III 500 MHz spectrometer.The global Si/Al ratios of the samples were obtained by elemental analysis using ICP‐AES (inductively coupled plasma‒atomic emission spectroscopy) on an Optima 4300 DV (Perkin‐Elmer).
2.2 FTIR measurement
The in‐situ FTIR spectra of OH groups and pyridine adsorption were carried out on a Shimadzu IR‐8400 spectrometer with a vacuum cell.The wafers (about 10 mg/cm2) were initially pretreated at 673 K in vacuum with a pressure of lower than 10−4Pa for 4 h and then were cooled down to 423K.After the FTIR spectra were collected, pyridine was introduced into the system.The physisorbed pyridine was removed by degassing for 1 h at the same temperature, and the stepwise desorption was carried out at 423 K, 523 K, and 623 K, respectively, to evaluate the amount and strength of Brønsted and Lewis acid sites by integrating the areas of adsorption bands at 1 542 cm−1and 1454 cm−1with the extinction coefficients determined previously[12].
2.3 Catalytic test
The catalytic test of methylcyclohexane (MCH with a purity of >99%, made by Aladdin) transformation was carried out in a fixed‐bed microreactor containing the zeolite catalyst sample at 623 K under atmospheric pressure, in which the catalyst has a particle size of 20‒40mesh.First of all, the zeolite catalyst was pretreated at 723 K for 8 h under nitrogen flow (60 mL/min) and then was cooled to reaction temperature, while the reactant was injected at a constant rate (under conditions covering aPMCHof 14 kPa, and a N2/MCH molar ratio of 7.2).The products in different reaction time were collected with a 10‐position high temperature (200◦C) valve (provided by VICI) and were analyzed online with an Agilent 7890B gas chromatograph equipped with a HP‐PLOT Al2O3S capillary column (50 m in length and 0.53 mm in diameter) and a flame ionization detector.
3 Results and Discussion
3.1 Physicochemical characteristics of DAY samples
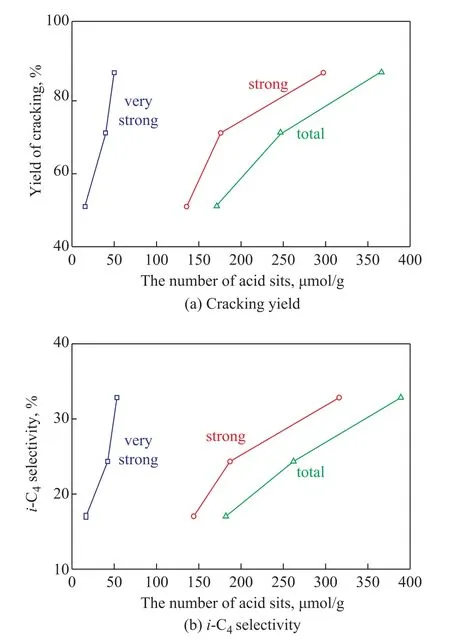
The XRD patterns of three DAY zeolite samples depicted that the diffraction patterns corresponded to those of parent NH4Y sample and exhibited more crystallinity.The main physicochemical characteristics of the zeolite samples are presented in Table 1.Total surface areas (SBET), micropore volume (Vmic), and mesopore volume (Vext) of these three samples increased in the following order: DAY‐1< DAY‐2 Table 1 Physico-chemical properties of the DAY samples The interaction of hydroxyl group (possible acid site) with pyridine probe molecule can be detected and characterized by studying the vibration modes through FT‐IR analysis.Herein the IR bands from the accessible acid sites (hydroxyls) and nonacidic and/or inaccessible hydroxyls are distinguished by adsorbed pyridine molecules on the zeolite samples.By comparing with the spectra of DAY zeolites alone, it is possible to identify and reveal the bands corresponding to the acidic groups.The FTIR differential spectra before and after pyridine adsorption on DAY‐1, DAY‐2, and DAY‐3 samples are depicted in Figure 1.Several ‐OH bands at 3 500‒3 700 cm‐1can be identified in the FTIR spectra of the three DAY zeolitic samples.The extra‐framework aluminum species HO‐Al or framework Al‐OH‐Si groups in defects appear at 3 670 cm‐1, while the bridged hydroxyl Al‐OH‐Si groups in the supercage and the sodalite cage appear at 3 625 cm‐1and 3 560 cm‐1.The bands at 3 600 cm‐1and 3 525 cm‐1correspond to the red shift of the bands at 3 625 cm‐1and 3 560 cm‐1due to the interaction of bridged hydroxyl Al‐OH‐Si groups in both cages with extra‐framework aluminum species after undergoing delocalization.The hydroxyl groups at 3600 cm‐1are the most acidic sites among all the hydroxyl groups[13].It is quite obvious that the distribution of spectra for DAY‐1, DAY‐2, and DAY‐3 are different and show a decrease of the overall intensity in the hydroxyl region, reflecting their different strength of the Brønsted acid sites and the decreasing number.Specially, the band area at 3 600 cm‐1with extremely high acid strength reduces in the following order: DAY‐1>DAY‐2> DAY‐3,accompanied by a decrease of the band at 3 670 cm‐1.The amount of acid sites determined by the area of hydroxyl group vibration bands at 3 600 cm‐1and the extinction coefficients reported[14]are 53.2 μmol/g, 42.2 μmol/g, and 16.5 μmol/g for DAY‐1, DAY‐2, and DAY‐3, respectively. Figure 1 FTIR spectra of -OH groups over DAY samples The Brønsted and the Lewis acid sites of the three zeolite samples were quantified using the pyridine‐adsorbed FTIR bands appearing at 1 545 cm‐1(PyH+) and 1 454 cm‐1(PyL) at different desorption temperature of 423 K, 523 K, and 623 K.The acid sites in zeolites, which are able to retain pyridine at a temperature higher than 623 K,can be generally considered as strong acid sites.As listed in Table 2, both total amounts of Brønsted and Lewis acid sites of the three samples at different desorption temperature of pyridine are reduced sequentially with the increase of Si/Al ratios.It is interesting and important to know that the so‐called stronger Brønsted and Lewis acid amounts characterized by pyridine‐desorption at 623 K are also decreased sequentially. Table 2 Py-IR spectra of DAY samples MCH transformation over the zeolite catalysts took place with a weight hourly space velocity of 3.16 h‐1at 623 K.Figure 2 shows the evolution of MCH conversion with reaction time over three DAY zeolite samples.It can be seen that a fast deactivation occurred at the early stage of the reaction, followed by a quasi‐plateau of activity for three DAY samples, possibly due to the formation of coke that overlapped the stronger acid sites in zeolitic microporous structure[5].The characterization of porosity based on N2adsorption for the three spent DAY zeolite catalysts displayed that the micropore volume decreased from 0.29, 0.31 and 0.34 cm3/g to 0.15, 0.21 and 0.26 cm3/g after the reaction, respectively, for DAY‐1, DAY‐2 and DAY‐3.The results confirmed again the formation and retention of coke in the zeolite micropores during the reaction, indicating a reduction of the protonic active sites concentration and/or their accessibility by pore blockage.It is also validated by the results that the MCH conversion on the three samples decreased in the following order:DAY‐1 > DAY‐2 > DAY‐3 within the examined period of reaction time.MCH transformation is a typical acid‐catalyzed reaction, therefore, the differences of their conversion can be originated mainly from the different numbers of acid sites in the three catalysts.Indeed, the conversion achieved over the three catalysts corresponded to their numbers of acid sites, but the turnover frequency(TOF) calculated according to the total acid sites or Brønsted sites from pyridine‐desorption at 423 K, 523 K and 623 K, respectively, showed the opposite trend,denoting that the indicator of these zeolites increased in the following order: DAY‐1 < DAY‐2 < DAY‐3.This case showed that not all the acid sites could participate in and contribute to the reaction, but the availability of acid sites inside the micropores of Y zeolite was increased[15‐16].Furthermore, the mesoporous structure in the zeolites improved the diffusion of the reactant and product molecules due to the increase of mesoporosity in DAY‐1, DAY‐2, and DAY‐3 zeolites.Therefore, the deep dealumination seems to exert two antagonistic effects on the MCH transformation, resulting in shortening the diffusion path and improving the utilization of active sites despite a decreased number of acid sites. Figure 2 Conversion of MCH transformation reaction on DAY samples Table 3 presents the detailed distribution of products obtained over the three catalysts after 5 and 45 minutes of reaction,respectively.The principal product distribution of the MCH transformation contains C3‒C7alkanes, cycloalkane isomers (ethylcyclopentane and dimethylcyclopentanes),and C7aromatics.Small amounts of C2‒C7alkenes as well as methane, ethane and benzene are also produced.Transformation of MCH over acidic zeolite catalysts is generally recognized to follow the monomolecular protolysis mechanism and the classical bimolecular mechanism(carbenium ion chain mechanism) via carbenium ions[17‐20].The MCH transformation can be initiated via the protonation of molecules by the strong Brønsted acid sites to form carbocation, the carbonium transition state[21‐22].Furthermore,the strong Lewis acid sites can facilitate the formation of carbenium ions by hydride abstraction.Based on the product distribution obtained from MCH transformation, severaltypes of reactions taking place on the three DAY zeolite catalysts can be predicted. Table 3 Products distribution obtained at a reaction time of 5 min and 45 min over the three DAY samples As showed in Figure 3, MCH transformation can undergo C‐C bonds cleavage by protolytic cracking of ring or methyl side chain via tertiary (a) and secondary(b) carbocation intermediates.Because MCH has one primary, five secondary and one tertiary carbon atoms,the strong Brønsted sites can cleave the C‐H bond via the protonation of the tertiary hydrogen giving methylcyclohexyl species (c)[21].If the terminal methyl group is cleaved, dealkylation reaction can occur to produce methane and the products derived from the cyclohexyl carbenium ions, such as cyclohexene,cyclohexane, methylcyclopentane, methylpentanes,methylpentenes, as well as the hydrocarbons with less than six carbons, would be generated.The protolytic cracking of C‐C bond on the ring can be resulted from ring‐opening and the carbocation intermediate.The results have shown that heptene can emanate from proton transfer and desorption, heptane can originate from hydride transfer, and hydocarbons with less than seven carbon atoms can be produced from cracking and re‐cracking followed by the isomerization reaction.Compared with the cleavage of C‐C bond, the process of C‐H bond cleavage is less favorable.The methylcyclohexyl generated from the protonation of the tertiary hydrogen in MCH can isomerize and lose a proton to give methylcyclohexene and then would continuously throw a hydrogen giving methylcyclohexadiene and finally producing toluene via successive hydride transfer and deprotonation reactions.In these processes, the formation of methylcyclohexyl carbocation is a key factor and then the isomerization reaction would yield alkyl cyclopentyl carbocation.Alkylcyclopentenes and alkylcyclopentanes can be obtained by desorption of these carbocations, and/or are further cracked to give branched C7carbocations,followed by going through re‐cracking by the β‐scission to produce the hydrocarbons with less than seven carbons,including primarily C3andi‐C4hydrocarbons. The distribution of all reaction products can be classed into three categories, viz.: isomers (marked as “I”), C2‒C7cracking products (marked as“C”), aromatics (marked as“A”).The yields of products obtained during cracking,aromatization and isomerization reactions measured in 5 min and 45 min are presented in Figure 4 for all the DAY zeolite samples.Regardless of different properties of zeolite samples, cracking is the main reaction with an initial product yield of about 86%.The yield of isomerization products increases with the reaction at the expense of cracking products yield, while a decrease of the product yield obtained via cracking is accompanied by the zeolite deactivation.Due to high activation energy of protonation in the first step, the MCH transformation is dependent on both the concentration and strength of the acid sites[20].The isomerization, cracking and aromatization reactions are the relatively acid strength demanding reactions, in which the activated complexes producing isomerization and dehydrogenation products are different from those evolving to form cracking products.The MCH cracking needs stronger acid sites than isomerization and dehydrogenation in the transformation process[8]. Figure 3 Mechanism of methylcyclohexane transformation reaction Figure 4 Product yields of three categories products on the three DAY samples Figure 5(a) shows the relationship between the MCH cracking product yield and the amount of very strong,strong, and total Brønsted acid sites, which are determined by the hydroxyl group vibration bands at 3 600 cm‐1(very strong acid sites), and the adsorbed PyH+at 423 K (total acid sites) and 623 K (strong acid sites),respectively.It is obvious that the cracking yields increase with an increasing number of the total strong and very strong Brønsted acid sites, and more importantly, the strong dependence on very strong acid is confirmed.On the contrary, the reduction of strong Brønsted acid sites would result in the increase of isomerization products.On the other hand, the main effect of reaction time on the product yield is the decrease in the overall yield and the relative percentage of the C and I products.Despite the significant increase in isomers products, the yields of aromatics, which are related mainly to toluene, are lower than the yields of cracking and isomerization products formed over the three catalysts throughout the reaction time.Toluene is formed in the MCH transformation via three successive bimolecular hydride transfer and deprotonation reactions, hence, the low toluene selectivity on Y zeolite can originate from its adherent micropore structure, and the decreased yield on the three zeolites,including DAY‐1, DAY‐2, and DAY‐3, can be related to their decreasing acid density and increasing diffusion of reaction products ascribed to the increase of mesoporosity.With the reaction taking place over the catalysts for 45 min, the reduction of acid sites located in their micropores is essentially caused by the formation of a large amount of coke, resulting in the decrease of corresponding yields of aromatics on the three DAY zeolitic samples.On the other hand, the aromatics formation also can occur via hybrid transfer between the reactants and alkenes.Normally, a reactant molecule should produce two alkenes according to the MCH cracking stoichiometry.Therefore, the production of aromatics is less favored under a small cracking yield.The formation of aromatics is associated with the consumption of the produced alkenes. The intermediate carbocations formed by protolytical cracking of C‐C bonds on the ring can turn into heptene and heptane, or can be further cracked into lower aliphatic products.Low amounts of C7products indicate the high reactivity of bothn-heptane andn‐heptene in the zeolite samples, because of their further cracking in the reactions.With the reaction taking place for 5 min on the fresh zeolite samples, including DAY‐1, DAY‐2, and DAY‐3, the percentage of C7products increases, whereas that of C2‒C6products decreases, which implies the increase of secondary cracking.This case can be explained by the decreased amount of strong Brønsted acid sites and the diffusion length with an increasing mesoporosity of zeolite catalysts including DAY‐1, DAY‐2, and DAY‐3.The existence of less acid sites in zeolite and shorter diffusion length can be favorable to the desorption ofC7products and avoidance of further cracking.Another obvious characteristic is the formation of high percentage ofi‐C4.Endocyclic protolytic scission and ring‐opening of MCH on strong Brønsted acid sites can lead to formation ofn‐heptyl species, cracking of which can produce propylene and linear butane/butene.The branched C4species is resulted from the isomerization of the heptyl ions without cracking.Dimethylcyclopentanes as isomers of MCH would undergo endocyclic protolytic scission and ring‐opening and then β‐scission, resulting in isobutane/isobutene and propane/propylene.However, a great extent ofi‐C4products obtained on the DAY zeolite samples are resulted from the cracking of methylcyclohexane based on the large quantities of isobutane and propane in the products, because of low concentration of dimethylcyclopentanes.Because the C‐H bond cleavage needs stronger acid sites than C‐C bond, a decreasing yield ofi‐C4products obtained on catalysts including DAY‐1, DAY‐2, and DAY‐3 means their degressive strong acid sites, as shown in Figure 5(b).Therefore, high quantity ofi‐C4is an indicator of the great importance of protolytic cracking of C‐H bonds, because the protolytic cracking of C‐H bonds can result in the formation and cracking of methylcyclohexane isomers, and finally the formation ofi‐C4.This phenomenon reflects the existence of a high percentage of very strong acid sites in zeolites.Furthermore, the alkenes/alkanes ratio in the cracking products formed over the three zeolite samples is very low, indicating a high rate of hydrogen transfer (HT)occurring during the transformation reaction.The more facile the HT steps are, the lower the alkenes/alkanes ratio in the products is.Conventionally, the hydride transfer is defined as a bimolecular reaction, in which the hydride is transferred between the hydride donor and the acceptor.As expected, the alkenes/alkanes ratio increases over the catalysts (including DAY‐1, DAY‐2 and DAY‐3) in 5 min and 45 min of reaction, reflecting the dependence of hydride transfer on the concentration of the Brønsted acid sites. Figure 5 The changes of cracking yields (a) and i-C4 selectivity (b) with the amounts of very strong, strong and total Brønsted acid sites in DAY samples In this paper, three commercial dealuminated Y zeolites(DAY) with increased Si/Al ratio and mesoporosity were explored on the basis of the pyridine‐IR, OH‐IR spectroscopy, and model methylcyclohexane (MCH)transformation to evaluate the zeolite acid properties.MCH transformation was used as a probe reaction of zeolite acid catalyst behaviors.The activity, yields and selectivity(product distribution) of MCH transformation are correlated to the acidity and pore structure of the different Y zeolite samples, therefore the behaviors of MCH transformation can reflect the concentration, strength, and accessibility of the acid sites within zeolite.TOF activity of MCH transformation over the DAY zeolites with a high mesoporosity confirms the importance of the accessibility to acid sites.Upon analyzing the product distributions and yields of cracking products, aromatics, and isomers products over the three different fresh and used DAY zeolite samples, one can predict the concentration and strength of the acid sites of zeolite catalysts according to the selectivity (yield) of cracking products in MCH transformation.For the obtained cracking products, the amount of heptane and heptane (C7) as well as alkenes/alkanes (C2‒C7) ratio increase with a reduced acid sites and an increased diffusion, and on the contrary, C2‒C6yielddecreases, among which, the quantity ofi‐C4produced can be an unique measure for a high percentage of very strong acid sites, as evidenced by a wavenumber of 3600 cm‐1identified by the hydroxyl‐IR spectroscopy. Acknowledgments:This work was financially supported by the National Natural Science Foundation of China (Grant No.21978192), the SINOPEC Technology Project (No.117009‐1), and the Shanxi Province Key Innovative Research Team in Science and Technology (No.2014131006).
3.2 FT-IR characterization of the DAY samples


3.3 MCH transformation test on DAY samples




4 Conclusions
- 中国炼油与石油化工的其它文章
- Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
- Synthesis of Bimodal Mesoporous TiO2 -PTA/BMMS and Its Enhanced Performance in the Photocatalytic Oxidative Desulfurization
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Preparation of Ultra-High Molecular Weight Polypropylene Using Ziegler-Natta Catalyst via Combining Internal Electron Donor and Cocatalyst Loading
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts

