Doublet luminescence due to coexistence of excitons and electron-hole plasmas in optically excited CH3NH3PbBr3 single crystal
Jie Wang(王杰) Guang-Zhe Ma(马广哲) Lu Cao(曹露) Min Gao(高敏) and Dong Shi(石东)
1School of Optoelectronic Science and Engineering,University of Electronic Science and Technology of China,Chengdu 610054,China
2School of Materials and Energy,University of Electronic Science and Technology of China,Chengdu 610054,China
Keywords: semiconductor,crystal,surface,luminescence
1. Introduction
Research interests in metal halide perovskite semiconductors has been rekindled following the breakthroughs in solar-to-electric power conversion efficiencies achieved via the use of the prototypical hybrid methylammonium lead trihalide perovskites, CH3NH3PbX3(X= Br, or I), in photovoltaic cells.[1-3]A couple of solution-processed material processing protocols,including the well matured one-step and sequential spin coating,[4,5]strongly backgrounded the fast development of perovskite photovoltaics in the past decade.[6-9]More recently,while perovskite photovoltaic technologies are approaching the edge of commercialization,[10]other applications of the halide perovskites for lasing,[11,12]light-emitting diodes,[13,14]photodetectors,[15,16]x-ray detectors[17,18]were lightened simultaneously.
Driven by the pursuit for ever-increasing device performances, intensive research efforts were devoted to improved solution-processed materials processing by scientist with diverse academic backgrounds.[19-22]Most remarkable among the advances in material processing was the growth of high-quality single crystals that enabled improved understanding on the optoelectronic superiorities of halide perovskites.[23,24]To the delight of fundamental researchers interested in catching the intrinsic material physics, room-temperature solution-grown CH3NH3PbBr3and CH3NH3PbI3single crystals demonstrated extraordinarily low trap-sate densities that are superior to a wide array of established inorganic semiconductors.[23]Therefore, the extrinsic effects associated with defects, surface roughness and midgap states get largely suppressed when running spectroscopic characterizations.[25-27]Indeed, new optical phenomena shown by high-quality halide perovskite single crystals other than their polycrystalline thin-film counterparts are being observed.[28]One of the most intriguing phenomena is the occurrence of doublet luminescence shown the front-face PL from high-quality single crystals of the most studied prototypical CH3NH3PbBr3and CH3NH3PbI3perovskites.[23,28,29]Although repeatedly observed,the underlying physical mechanism that leads to the occurrence of such doublet PL remains poorly understood. A crystal-clear elucidation on the physical origin of the doublet PL is an outstanding quest,in view of its direct consequence on diverse optoelectronic functionalities.
We were thus motivated to revisited the spectroscopic properties shown by one of the most studied prototypical hybrid metal halide perovskites, CH3NH3PbBr3, in its single crystal form. Practically,single-crystal samples with variable surface roughness were prepared and used for spectroscopic characterizations. By interpreting the spectroscopic data according the established physical theorems in semiconductor optics, we figured out the physical origin of the doublet luminescence, i.e., the coexistence of surface-specific excitons and electron-hole plasma inside the crystal bulk of optically excited CH3NH3PbBr3single crystal.
2. Materials and methods
2.1. Materials
The two CH3NH3PbBr3perovskite precursors,CH3NH3Br and PbBr2were purchased from Xi’an Polymer Light Technology Corp.The solvent, N, Ndimethylformamide (DMF) was purchased from Shanghai Titan Scientific Co.,Ltd.
2.2. Methods
2.2.1. Crystallization
Room-temperature slow solvent evaporation from the precursors’ solution in DMF yielded high-quality CH3NH3PbBr3single crystals. In particular, PbBr2and CH3NH3Br (1:1 by molar~1.0 M) were dissolved in DMF in screw capped vials, stirred for 3 hours, filtered through a syringe filter(Ø=0.45µm),and eventually yields a colorless and transparent solution. Then, the solvent was allowed to evaporated out of the solution phase at room temperature and 1.5 atm in a nitrogen-filled gloved box. The crystallization procedure is schematically shown in Fig.1(a).
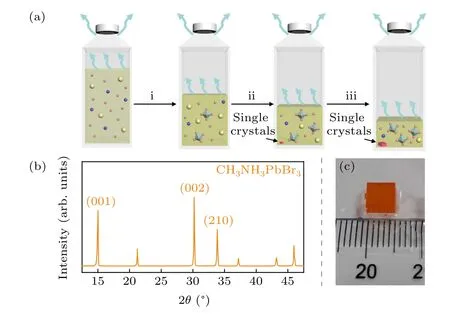
Fig.1. (a)Schematic diagram of the crystallization processes. Yellow sphere represents CH3NH+3, blue sphere represents Pb2+, pink sphere represents Br-. (b)Experimental powder XRD profile of CH3NH3PbBr3 single crystal ground into powders. (c)Photograph of one piece of CH3NH3PbBr3 single crystal with ~5 mm dimensions.
2.2.2. Structural and spectroscopic characterizations
The phase purity of the as-grown CH3NH3PbBr3single crystals was confirmed by x-ray diffraction(XRD)which can give detailed lattice information. The diffractometer,Maxshima XRD-7000 that equips with the standard Cu-Kα1excitation radiation source and a diffracted beam monochromator, was used. The surface morphologies of the as-grown pristine single crystals were characterized by Asylum MFP-3D atomic force microscopy(AFM).The absorbance of highly transparent CH3NH3PbBr3single crystals was recorded in a transmission mode using Shimadzu UV-2600 spectrometer.The front-face steady-state PL and time-resolved photoluminescence (TRPL) were recorded by FluoTime 300 fluorometer(PicoQuant)equipped with a time-correlated single photon counting(TCSPC)detector. The crystal sample was photoexcited by a picosecond pulsed diode laser(PicoHarp, LDH-DC-405M,exciting at 400 nm,repetition rate: 0.2 MHz).
3. Results and discussion
Figure 1(a) schemes the crystallization procedure via slow solvent evaporation out of the precursor (PbBr2and CH3NH3Br, 1:1 by molar) solution in DMF. Slow solvent evaporation at room temperature allows for balanced ionic incubation in the solution phase, whereby freely solvated Pb2+and Br-ions self-assemble into the energetically more stable octahedral corrodinates,[PbX6]4-. Once crystallization starts when both precursors saturates along with solvent evaporation,the solution phase remains invariable in concentration afterwards. This leads to invariable stoichiometric conditions surrounding the enlarging crystal surfaces all around. In this scenario,by gingerly screwing down the cap without causing too much disturbance,freshly incubated perovskite single crystals would be obtained. In the presence of passvation by the constituent ions in the solution, the crystal surface is expected to be characterized by naturally orientated surface dangling bonds.
The crystal phase purity of the room-temperature grown single crystals was confirmed by powder XRD. A random batch of the as-grown single crystals were collected, grinded into powders, and then mounted in the sample holder of the diffractometer. The collected XRD data was shown in Fig.1(b). Characteristic diffractions at 2θ=15.1°,30.2°,and 33.8°, which are assigned to the characteristic (001), (002),and (210) planes of the cubic CH3NH3PbBr3single crystal,are observed.[3]All diffractions were precisely assigned to the its well-known room-temperature cubic lattice symmetry,indicating crystal phase purity. Figure 1(c) shows a representative photograph of one piece of the as-grown pristine CH3NH3PbBr3single crystal. Notably,the sharp edges of the regular square shapes nicely reflect its cubic crystal symmetry.
The surface morphologies of freshly grown pristine CH3NH3PbBr3single crystals were characterized by AFM.One best-case top-view morphology showing rather smooth surface and least density of surface nanoparticles is shown in Fig. 2(a). One moderate-case and one worst-case morphologies showing increased surface roughness are selectively provided in Figs.2(b)and 2(c),respectively.--
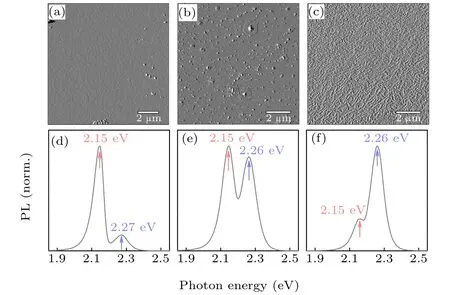
Fig.2. (a)-(c)Pristine surface morphologies of three CH3NH3PbBr3 single crystal samples viewed under AFM. (d)-(f) Front-face PL spectra corre sponding to the crystal surface morphologies shown in(a)-(c),respectively.
When recording the luminescence spectra generated by front-face photoexcitation, the illuminated surface was oriented about 30°from the incident excitation beam. In this way, the incident light got distributed over large surface area and moreover,strong reflection of light entering the emission monochromator was avoided.[31]The collected PL spectra as a function of photon energy with two components were shown in Figs. 2(d)-2(f) for each case. Notably, the high-energy PL component peaks at 2.26 eV and the low-energy one at 2.15 eV.The peak positions of both components remain invariable regardless of their relative intensities. Similar doublet PL was also observed for high-quality CH3NH3PbI3single crystal in previous studies.[28]Interestingly,the high-energy PL component augments dramatically along with increased surface roughness, indicating that the high-energy PL is attributable to surface-specific emission,while the low-energy PL component is attributable to bulk-specific luminescence.
We further found that by having a piece of high-quality single crystal kept in the saturated mother solution that contains abundant constituent ions,the low-energy PL component became significantly augmented over the high-energy one. In this case, a basically mono-peak PL with asymmetric spectral line-shape, as shown in Fig. 3(a), was observed. The mono-component bulk-dominated PL,peaking at 2.15 eV,carries a long PL tail that extends toward lower photon energy.In addition, a full width at half-maximum (FWHM) value,Γ~75 meV,is determined. In general,every solid has a surface connecting its bulk to the outer world. For the case with a high-quality CH3NH3PbBr3single crystal, an ideal crystal surface is expected to be an atomically flat surface that carries abundant dangling bonds orienting naturally along the lattice axis without causing lattice deviations and subsequent midgap states formation. To maintain the natural orientation of the dangling bonds, passivation - most preferred by the constituent ions from the outer surroundings over the crystal surface-is required.[32,33]Otherwise,structural deviation at the lattice peripheries are inevitable. In our case, the precursor ions in the colorless saturated mother solution help complete the octahedral coordination at the perovskite lattice periphery and thereby suppress structural distortions. The diagram shown in Fig.3(b)schematically illustrates the passivation effect of the ion-abundant mother solution.
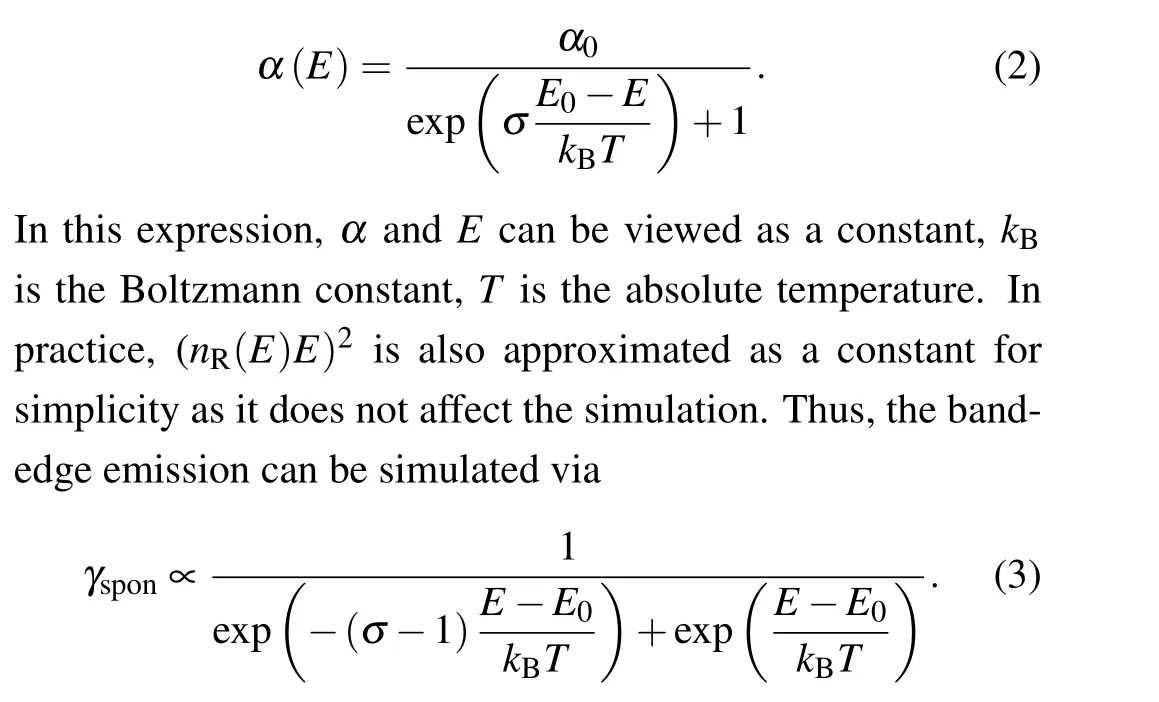
The occurrence of the asymmetric bulk-PL from highquality CH3NH3PbBr3single crystal is a universal phenomenon as it has been repeatedly observed previously.[23,34]In this regard, the physical mechanism that leads to the occurrence of the asymmetric PL should be,in principle,rooted in the framework of the intrinsic material physics. The direct bandgap nature defines that the radiative recombination within the lattice bulk of CH3NH3PbBr3single crystal is an inverse process of ligth absorption, which can be expressed via the established van Roosbroeck-Shockley relation[35,36]

In this expression,γspon(E) represents the spontaneous emission from the bandedge, and its spectral line-shape is, therefore, correlated with the distribution of the electronic density of states(DOS)at the bandedge. One accessible spectroscopic parameter related to the bandedge DOS is the dimensionless steepness factor,σ, the value of which can be directly determined from the absorption edge according to its correlation with the absorption coefficient,α(E),via
Figures 3(c)-3(e)show the simulatedγspon(E)corresponding toσ=1.5,2.0,and 3.0 respectively. In this scenario,the lineshape of spontaneous emission spectrum is subjected to the value ofσ.The slope ofγspon(E)at the high-energy side is determined bykBT,while at the low-energy side it is determined bykBT/(σ-1).In Fig.3(c),the slope at low-energy region is smaller than that at high-energy region,therefore,the observed PL spectrum is asymmetric and is characterized by a long tail that extends to lower energies. Whenσ=2.0, the simulated spectrum becomes symmetric as displayed in Fig.3(d). When increasingσto 3.0, the simulated spectrum becomes asymmetric again,but it is characterized by a long tail that extends toward higher energies as shown in Fig.3(e).the lowerEBthan the room-temperature thermal energy,kBT~26 meV,it is within conceivable expectation that the concentration of excitons in optically excited CH3NH3PbBr3singlecrystal bulk should be sufficiently low because of thermal dissociation. Thus, many-body electron-hole (e-h) plasma occurs in the excited phase. On the other hand, it has been repeatedly confirmed that the surface-abundant polycrystalline thin film shows much stronger excitonic feature, and this indicates the co-existence of excitons and e-h plasmas.[39]In a general scenario, the crystal surface behaves as giant lattice defects whereby excitons are apt to adhere.
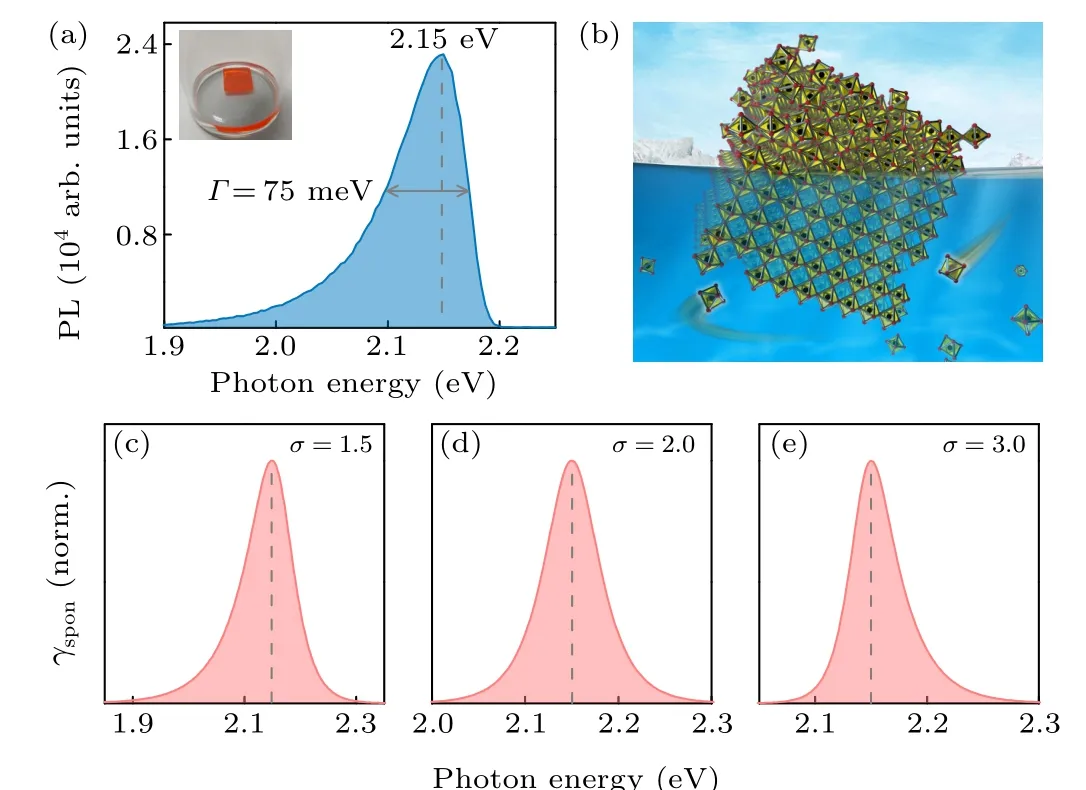
Fig. 3. (a) Asymmetric bulk-specific PL spectrum. The peak position and the FWHM are also reported. Inset: photograph of solution-passivated CH3NH3PbBr3 single crystal. (b) Schematic crystal lattice diagram showing largely suppressed deviation when passivated in mother solution. (c)-(e)Simulated bandedge emission spectral line-shapes corresponding to σ =1.5,2.0,and 3.0,respectively.

Fig.4. (a)Normalized logarithm absorbance(blue markers)as a function of photon energy. The black solid line shows the linear fti to the linear region of absorption tail,which yields EU=18 meV.(b)Absorbance data(grey makers) along with Elliott fti (black solid line) overlaid, the blue and the pink areas reflect the contributions to the absorbance from a sum of discrete excitonic states and band-to-band continuum excitations,respectively.
Based on the above-made discussions, it is clear that the asymmetric bulk luminescence with long tail that extends to lower energies corresponds toσvalues between 1.0 and 2.0.This is equivalent to set the values of the Urbach energy,EU, between 26 meV and 13 meV, according to their relationship,σ=kBT/EU. To confirm this point, we recorded and plotted the logarithmic absorbance against photon energies as shown in Fig. 4(a). Linear fit of the absorption edge yieldsEU= 18 meV, which corroborates our attribution of the low-energy PL to the intrinsic bulk emission from bandedge states. Although the absorbance of the CH3NH3PbBr3single crystal does not develop into a sharp excitonic peak as that observed in its polycrystalline thin film counterpart,the absorbance data was fitted well with the established Elliott formula.[37,38]The absorbance data along with the Elliott fit were shown in Fig.4(b). By performing the Elliott fit,contributions to the overall absorption intensity from discrete excitonic states below the gap and band-to-band continuum transitions are decoupled. As shown in Fig. 4(b), absorption by a sum of discrete excitons peaking atEX=2.194 eV and band gap energy ofEG=2.206 eV were determined. This in turns leads to the determination of the exciton binding energy,EB=12 meV, according toEB=EG-EX. In view of
The co-existence of excitons and e-h plasmas in optically excited CH3NH3PbBr3single crystal was further verified by TRPL measurement. Figure 5 shows the TRPL traces probed under picosecond pulsed photoexcitation at 400 nm.Apparently, the TRPL traces can be fitted well with a biexponential function. The bi-exponent fit (blue solid line)was plotted together with the TRPL traces in Fig. 5. The close to unity value of the reduced chi square,χ2R, and the symmetric distribution of the standard deviation,δk, jointly indicate the accpetalbe goodness-of-fit (GOF). Two lifetime components,τ1~2791±166 ns representing the lifetime of diffusive e-h plasma andτ2~179±81 ns representing the liftime of surface-specific excitons, were obtained. In addition, the remarkably low intensity ratio ofτ2further reflects sufficiently low density of excitons in optically excited highquality CH3NH3PbBr3single crystal.
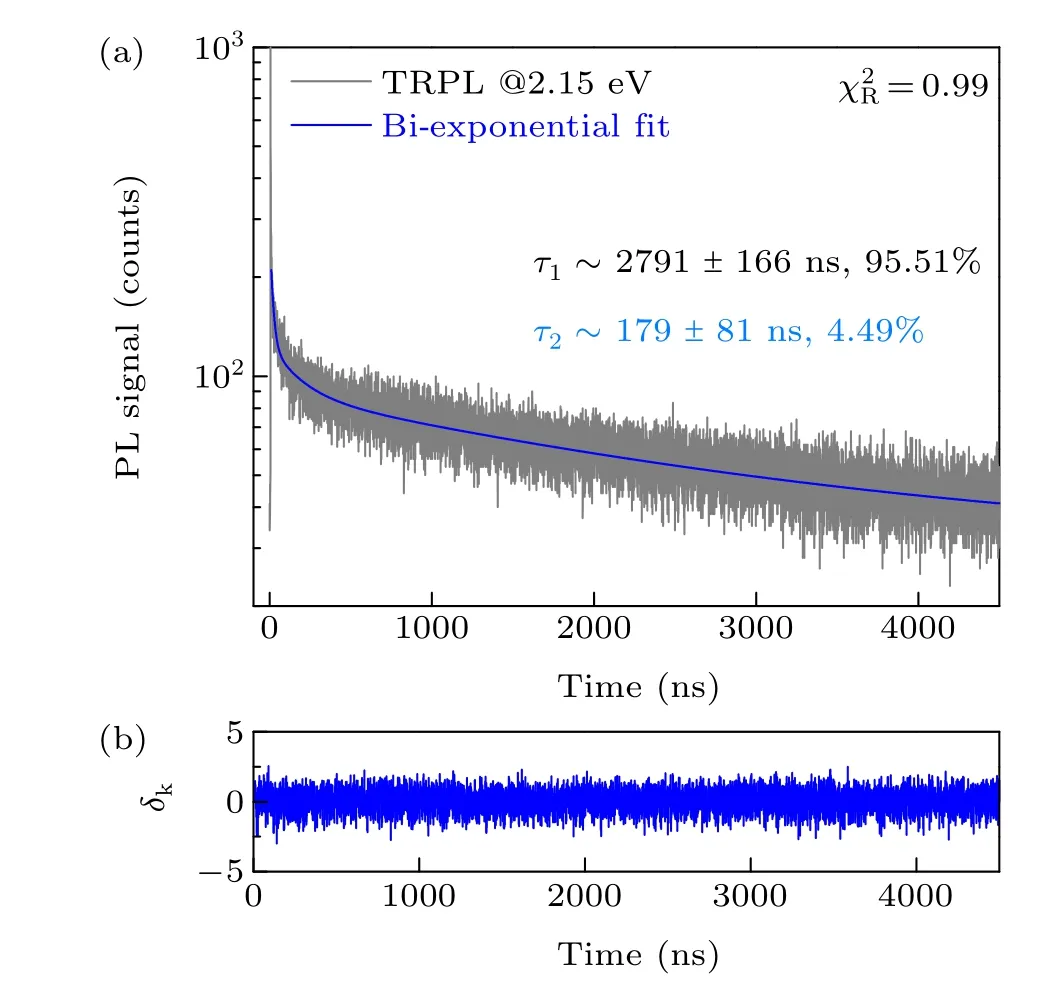
Fig.5. (a)TRPL traces(grey)recorded at 2.15 eV for CH3NH3PbBr3 single crystal along with bi-exponential fit (blue solid line). The yielded two lifetime components and the reduced chi-square are also reported. (b)Standard deviations traced along with bi-exponential fit,showing high-level GOF.
4. Conclusion and outlook
In summary,we developed a room-temperature solutionbased crystallization that affords high-quality CH3NH3PbBr3single crystal with atomically flat pristine surfaces. The largely suppressed pristine crystal surface roughness enabled us to observe the unmasked intrinsic luminescence with asymmetric spectral line-shape from the crystal bulk. The agreement between the experimental spectroscopic data and theoretical simulations validates our attribution for the doublet luminescence. Our study confirms that the low-energy PL component with asymmetric spectral line-shape is only attributable to the intrinsic bulk emission from the band-edge states, while the high-energy PL component is attributable to surface-specific emission by excitons.Coexistence of excitons and e-h plasmas were indicated by plausible spectroscopic features. Further efforts aimed at revealing more in-depth intrinsic material physics based on high-quality single-crystal material platform are strongly encouraged.
Acknowledgment
Project supported by the National Natural Science Foundation of China(Grant No.51872038).
- Chinese Physics B的其它文章
- Quantum walk search algorithm for multi-objective searching with iteration auto-controlling on hypercube
- Protecting geometric quantum discord via partially collapsing measurements of two qubits in multiple bosonic reservoirs
- Manipulating vortices in F =2 Bose-Einstein condensates through magnetic field and spin-orbit coupling
- Beating standard quantum limit via two-axis magnetic susceptibility measurement
- Neural-mechanism-driven image block encryption algorithm incorporating a hyperchaotic system and cloud model
- Anti-function solution of uniaxial anisotropic Stoner-Wohlfarth model

