手性双硫脲催化不对称Pictet-Spengler环化反应
张 敏,甘 坤,刘 莉,陈治明
(贵州师范大学 化学与材料科学学院,贵州省功能材料化学重点实验室,贵州 贵阳 550001)
β-咔啉是构成许多天然产物和药物的结构单元[1],是一类广泛分布在自然界具有三环结构的生物碱[2-3],其具有抗菌、抗病毒、抗肿瘤、抗血栓以及中枢神经系统抑制等活性[4-7].天然β-咔啉类化合物主要存在于植物、微生物和海洋生物体中[8-9],但由于其结构复杂,不易获得,因此化学研究者越来越重视β-咔啉类化合物的合成和性能研究.早在1911 年,Pictet 和Spengler[10]首次报道了苯乙胺和甲醛在酸性条件下的缩合反应(P-S 反应),合成了四氢异喹啉.而后Tatsui[11]小组利用色胺为底物,经过相似的反应历程合成了1-甲基-1,2,3,4-四氢-β-咔啉,为以后合成β-咔啉类衍生物奠定了基础.1998 年,Nakagawa课题组[12]报道了首例对映选择性的Pictet-Spengler反应,该反应以硝酮为原料在二异松蒎基氯硼烷催化下发生P-S 环化反应,-78 ℃下反应6 h,合成了N-羟基四氢-β-咔啉化合物,但催化效果不理想.2004 年,Jacobsen[13]报道了单硫脲催化的不对称P-S 反应,首先通过色胺和醛缩合得到亚胺,然后再在乙酰氯和2,6-二甲基哌啶的作用下形成酰亚胺离子,以乙醚为溶剂,-30 ℃下反应24 h,得到N-β-乙酰化的四氢-β-咔啉化合物,可得较高的对映选择性,但底物普适性只适用于脂肪醛,对芳香醛则表现出较低的反应活性.List 等[14]、Hiemstra H 等[15]、Leighton L 等[16]先后报道了合成β-咔啉化合物的方法,虽然这些方法在合成应用中有着潜在的价值,但反应条件苛刻,污染环境.因此,需要进一步寻找简单而更有效的催化剂.
本文报道用联二萘酚酸为原料,通过多步反应成功合成了3 种新型轴手性双硫脲催化剂(1a~1c),合成线见图1.该催化剂具有绿色、高效[17-19]、多活性中心、多氢键且结构上下对称可调等优点,其中的联二萘酚酸部分、分子结构内的羟基部分和硫脲中的氢键部分可能共同与底物作用加快反应的进程,理论上可以高效地催化不对称反应.本文将新合成的双硫脲催化剂应用到色胺与芳香醛生成β-咔啉类化合物(4a~4r),取得较高的收率82%和最高达88%的对映选择性.
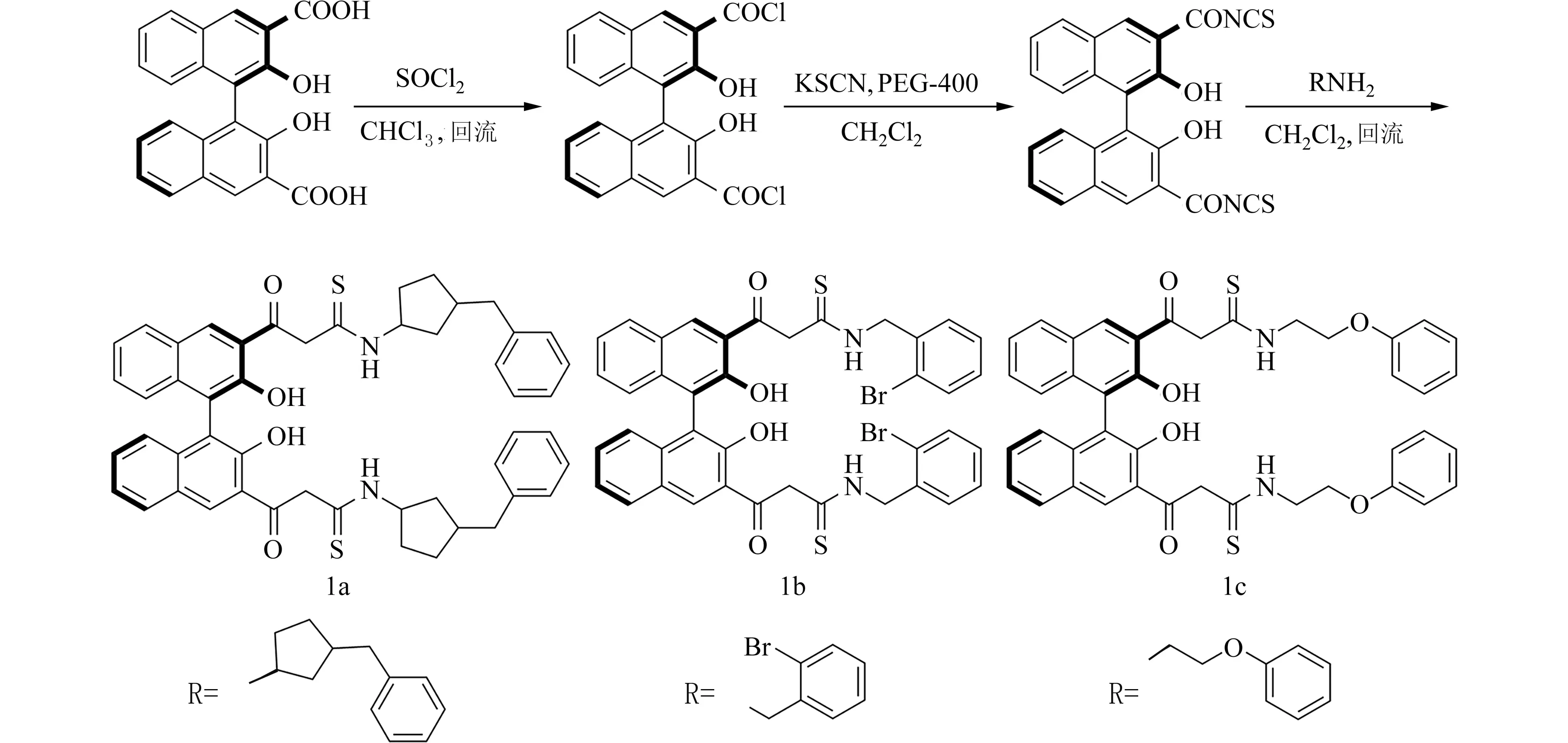
图1 催化剂的合成路线Fig.1 Synthesis route of catalysts
1 实验部分
1.1 仪器与试剂JEOL ECX 400MHz 核磁共振仪(TMS 为内标,美国Bruker 公司);EL104 型电子分析天平;RE-52 旋转蒸发仪(上海亚荣生化仪器厂);X-6 数字显微熔点测定仪(北京泰克有限公司);超高分辨飞行时间质谱(UHR TOF LC/MS Mass Spectrometer,Bruker 公司).
羟基-2-萘甲酸(阿拉丁化学试剂有限公司)、色胺 (国药集团化学试剂有限公司)、6-甲氧基色胺(上海阿拉丁生化科技股份有限公司)、苯甲醛(国药集团化学试剂有限公司)、o/p/m-氯苯甲醛(阿拉丁化学试剂有限公司)、苯甲酸(阿拉丁化学试剂有限公司)、甲苯(国药集团化学试剂有限公司)等试剂及 其他所需试剂和溶剂均为分析纯.
1.2 实验方法称取0.15 g (0.4 mmol)的(R)-2,2′-二羟基-1,1′-二萘-3,3′-二甲酸(根据文献[20]制备)于100 mL 圆底烧瓶中,并用20 mL CHCl3溶液溶解,随后加入1 mL 氯化亚砜,升温至80 ℃,回流搅拌6 h,直到气体全部逸出后减压蒸发出溶剂,得(R)-2,2′-二羟基-1,1′-二萘-3,3′-二甲酰氯待用.在装有磁力搅拌的50 mL 单口烧瓶中,依次加入0.1 g(1 mmol) 硫氰酸钾、PEG-400 和10 mL 纯化的二氯甲烷,搅拌直到PEG-400 均匀地分散在有机溶剂中后,将已制备好的(R)-2,2′-二羟基-1,1′-二萘-3,3′-二甲酰氯全部溶解在10 mL 二氯甲烷溶液中,用恒压滴液漏斗缓慢滴加到溶有硫氰酸钾的二氯甲烷溶液中,约5 min 加完,持续搅拌,TLC 检测酰氯消失,反应完成,有沉淀生成.旋蒸除掉溶剂,得黄色固体.最后在150 mL 三颈烧瓶中用15 mL THF 溶液溶解制备好的异硫氰酸酯,再在N2气保护下加0.1 mL (0.6 mmoL) 1-苄基吡咯烷-3-胺,磁力搅拌并在冰浴条件下反应12 h,TLC 检测反应完成,旋蒸除去溶剂,柱层析纯化(洗脱剂V石油醚∶V乙酸乙酯=8∶1),得黄色固体1a,产率68%,(图1).
以同样的方法制得催化剂1b~1c.
1a:黄色固体,产率68%,m.p.238.2~242.8 ℃;1H NMR (400 MHz,DMSO-d6)δ:8.68 (s),7.98 (s),7.31 (s),7.20 (s),4.81 (s),3.92 (t,J=184.8 Hz),2.77(d,J=213.9 Hz),1.34 (s),1.22 (s),1.00 (d,J=109.1 Hz),-0.01 (s).13C NMR (101 MHz,DMSO-d6)δ:166.74 (s),158.70 (s),128.57 (s),127.59 (s),54.30 (s),52.54 (s),46.61 (s),46.18 (s),41.25~40.78 (m),40.65 (s),40.34 (d,J=21.0 Hz),40.03 (s),39.82 (s),39.61 (s),39.40 (s),35.09~34.73 (m),32.07 (s),31.73 (s),30.76-30.24 (m),29.94 (s),29.58 (d,J=8.5 Hz),28.90 (s),23.74 (s),19.19 (s),14.09 (s),9.12 (s).HRMS(ESI) C48H46N4O4S2[M+H]+:计算值807.303 3,实测值 807.3041.
1b:黄色固体,产率73%,m.p.234.1~239.0 ℃;1H NMR (400 MHz,DMSO-d6)δ:11.37 (s),8.94~8.59 (m),8.42~8.05 (m),7.96 (d,J=6.6 Hz),8.05~7.27 (m),8.05~7.00 (m),6.83 (t,J=11.1 Hz),4.91(s),4.76 (s),4.46 (d,J=12.2Hz),4.19 (s),4.04 (d,J=47.9 Hz),3.02 (s),2.47 (s),1.92 (s),1.59 (s),1.35 (s),1.35 (s),1.30 (d,J=45.5 Hz),1.37~0.49 (m),-0.04(s).13C NMR (101 MHz,DMSO-d6)δ:48.98 (s),46.73(s),46.22 (s),43.01 (d,J=13.6 Hz),42.59 (s),42.32(s),41.60 (s),40.66 (s),40.45 (s),40.39~39.25 (m),31.73 (s),30.61 (d,J=12.8 Hz),30.34 (s),29.95 (s),29.57 (s),28.90 (s),14.45 (s),14.06 (d,J=8.2 Hz),9.14 (s).HRMS (ESI) C38H28Br2N4O4S2[M+H]+:计算值826.999 1,实测值826.9998.
1c:黄色固体,产率63%,m.p.217.8~222.1 ℃;1H NMR (400 MHz,DMSO-d6)δ:11.14 (s),8.74 (s),8.03 (s),7.00 (dd,J=117.2,66.1 Hz),6.88 (s),6.85(d,J=24.8 Hz),6.82 (s),4.76 (s),4.45 (s),4.13 (d,J=84.8 Hz),3.93 (s),3.91~3.72 (m),3.03 (s),2.47 (s),1.95 (s),1.55~0.27 (m),1.04 (t,J=77.6 Hz),1.04 (t,J=77.6 Hz),-0.04 (s).13C NMR (101 MHz,DMSOd6)δ:158.70 (s),45.37 (s),44.85 (s),40.03 (s),31.80(s).HRMS (ESI) C40H34N4O6S2[M+H]+:计算值731.199 3,实测值731.2002.
1.3 四氢-β-咔啉类衍生物的合成将1.00g (7.14 mmol)4-氯苯甲醛溶于10 mL DCM 中,用4 mol/L氢氧化钠水溶液洗涤3 次,每次用量10 mL.有机层用无水Na2SO4干燥,过滤浓缩高真空(133.3 Pa)得白色半晶固体备用.在装有磁力搅拌的三颈烧瓶中依次加入95 mg (0.1 mmol) 色胺,77 mg (0.55 mmol)4-氯苯甲醛,催化剂用量为x=20%(1c),在高真空下反应5 min,烧瓶用N2清洗,盖上橡皮隔膜,再加入10 mL 甲苯和12 mg (0.1 mmol) 苯甲酸,在室温下搅拌66 h,TLC 监测反应完成后用10 mL 饱和NaHCO3水溶液淬灭,然后用乙酸乙酯萃取,有机相用无水Na2SO4干燥,旋蒸除去溶剂,柱层析纯化(洗脱剂V石油醚∶V乙酸乙酯=2∶1),得产物4m(图2).用同样的方法制备4a~4r.
(R)-1-Phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4a):白色固 体,产率83%,ee 值84%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.59 (d,1H,J=8.0 Hz),7.56~7.53 (m,3H),7.48~7.46 (m,2H),7.33 (d,1H,J=8.0Hz),7.19 (t,1H,J=8.0 Hz),7.12 (t,1H,J=8.0Hz),5.96 (s,1H),3.70~3.65 (m,1H),3.62~3.57 (m,1H),3.35-3.26 (m,1H),3.22~3.18(m,1H).13C NMR (101 MHz,DMSO-d6)δ:18.6,56.0,108.0,112.1,118.7,119.5,122.4,126.3,129.1,129.3,130.2,130.6,135.3,137.1.HRMS (ESI) C17H16N2[M+H]+:计算值249.138 6,实测值 249.1391.
(R)-1-(2-Chlorophenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4b):白色固体,产率86%,ee 值89%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ(:7.63 (br s,1H),7.57 (d,1H,J=7.0 Hz),7.47 (dd,1H,J=8.0 Hz,1.2 Hz),7.27~7.23 (m,2H),7.18-7.10 (m,4H),5.70(s,1H),3.29~3.23 (m,1H),3.18~3.12 (m,1H),2.95~2.81 (m,2H).13C NMR (101 MHz,DMSO-d6)δ:139.1,135.8,133.8,133.0,130.1,129.8,129.1,127.7,127.0,121.8,119.4,118.2,110.9,110.8,53.6,41.7,22.4.HRMS (ESI) C17H15ClN2[M+H]+:计算值283.099 7,实测值283.1003.
(R)-1-(3-Chlorophenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4c):白色固体,产率84%,ee 值87%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.56~7.54(m,1H),7.53 (br s,1H),7.33~7.29 (m,3H),7.27~7.21 (m,2H),7.18 (dtd,1H,J=15.8 Hz,7.1 Hz,1.4 Hz),5.15 (s,1H),3.38~3.33 (m,1H),3.18~3.11 (m,1H),3.00~2.89 (m,1H),2.85 (dtd,1H,J=15.3 Hz,4.5 Hz,1.9 Hz).13C NMR (101 MHz,DMSO-d6)δ:143.8,135.9,134.7,133.4,130.0,128.5,128.4,127.2,126.6,121.9,119.5,118.3,110.8,110.5,57.5,42.6,22.4.HRMS (ESI) C17H15ClN2[M+H]+:计算值283.099 7,实测值283.1002.
(R)-1-(4-Chlorophenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4d):白色固体,产率82%,ee值85%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.58~7.55(m,1H),7.52 (br s,1H),7.36~7.33 (m,2H),7.30~7.27 (m,2H),7.26~7.24 (m,1H),7.20 (qd,2H,J=7.1 Hz,1.4 Hz),5.17 (s,1H),3.40~3.34 (m,1H),3.20 (ddd,1H,J=12.5 Hz,8.8 Hz,4.8 Hz),3.02~2.91 (m,1H),2.87~2.81 (m,1H).HRMS(ESI) C17H15ClN2[M+H]+:计算值283.099 7,实测值283.1006.
(R)-1-(2-Methoxyphenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4e):白色固体,产率76%,ee 值78%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.70 (br s,1H),7.54 (m,1H),7.31~7.27 (m,1H),7.25 (dd,1H,J=7.5 Hz,1.4 Hz),7.16~7.09 (m,2H),7.08 (dd,1H,J=7.5 Hz,1.4 Hz),6.98 (d,J=7.5 Hz,1H,),6.90 (td,1H,J=7.5 Hz,1.4 Hz),5.65 (s,1H),3.91 (s,3H),3.33 (dt,1H,J=12.5 Hz,5.3 Hz),3.18~3.12 (m,1H),3.00~2.80 (m,2H).HRMS (ESI) C18H18N2O[M+H]+:计算值279.149 2,实测值 279.1499.
(R)-1-(3-Methoxyphenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4f):白色固体,产率78%,ee 值80%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.55 (d,J=7.8 Hz,1H),7.52 (br s,1H),7.29~7.2 2 (m,2H),7.16~7.09 (m,2H),6.92~6.86 (m,3H),5.16 (s,1H),3.77 (s,3H),3.43~3.38 (m,1H),3.19~3.12 (m,1H),2.98~2.90 (m,1H),2.85~2.79 (m,1H).13C NMR(101 MHz,DMSO-d6)δ:160.0,129.8,121.7,120.7,119.4,118.2,113.9,113.8,110.8,110.0,58.1,55.3,22.3.HRMS (ESI) C18H18N2O[M+H]+:计算值279.149 2,实测值 279.1497.
(R)-1-(4-Methoxyphenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4g):白色固体,产率72%,ee 值79%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.57 (d,J=9.0 Hz,1H,),7.33 (d,J=8.0 Hz,1H,),7.19 (t,J=7.0 Hz,1H,),7.12 (t,J=7.0 Hz,1H,),7.07 (d,J=9.0 Hz,2H),5.89 (s,1H),3.86 (s,3H),3.67~3.63 (m,1H),3.59~3.54 (m,1H),3.11~3.07 (m,1H),3.30~3.24(m,1H),3.20~3.15 (m,1H).13C NMR (101 MHz,DMSO-d6)δ:18.3,39.8,51.4,107.6,111.1,115.2,117.8,119.2,119.6,120.2,122.3,130.3,131.4,137.1,155.9.HRMS (ESI) C18H18N2O[M+H]+:计算值279.149 2,实测值 279.1450.
(R)-1-(4-Nitrophenyl)-l-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4h):白色固体,产率86%,ee 值89%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:8.21 (d,J=8.8 Hz,2H),7.58 (d,J=8.5 Hz,1H,),7.53 (d,J=8.8 Hz,2H,),7.49 (br s,1H),7.27-7.24 (m,1H),7.20 (dtd,2H,J=8.5 Hz,7.0 Hz,1.5 Hz),5.28 (s,1H),3.34 (dt,1H,J=8.8 Hz,5.1 Hz),3.21 (ddd,1H,J=12.7 Hz,7.8 Hz,4.8 Hz),2.98-2.90 (m,1H),2.88 (dtd,1H,J=7.8 Hz,4.8 Hz,1.6 Hz).HRMS (ESI) C17H15N3O2[M+H]+:计算值294.123 7,实测值294.1243.
(R)-1-(2-(Trifluoromethyl)phenyl)-l-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4i):白色固体,产率88%,ee 值87%,(Chiral Pak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.77 (br s,1H),7.61 (d,2H,J=8.0 Hz),7.58~7.56(m,1H),7.42 (d,2H,J=8.0 Hz),7.21~7.13 (m,3H),5.18 (s,1H),3.33 (dt,1H,J=12.4 Hz,5.0 Hz),3.16~3.10 (m,1H),3.01~2.90 (m,1H),2.87~2.81(m,1H).13C NMR (101 MHz,DMSO-d6)δ:145.7,135.9,133.1,130.8 (q,J=32.3 Hz),128.8,128.2,127.1,125.6 (q,J=3.7 Hz),124.9 (q,J=234.8 Hz),121.9,119.5,118.2,110.8,110.5,57.3,42.2,22.3.HRMS (ESI) C18H15F3N2[M+H]+:计算值317.126 0,实测值317.1268.
(R)-2,6-Dimethoxy-4-(2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indol-1-yl)phenol(4j):白色固体,产率79%,ee 值78%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.58 (d,1H,J=9.0 Hz),7.34 (d,1H,J=8.0 Hz),7.19(t,1H,J=8.0 Hz),7.12 (t,1H,J=8.0 Hz),6.74 (s,2H),5.86 (s,1H),3.84 (s,6H),3.74~3.70 (m,1H),3.62~3.57 (m,1H),3.31~3.26 (m,1H),3.21~3.16(m,1H).13C NMR (101 MHz,DMSO-d6)δ:20.4,42.9,56.8,109.7,113.3,116.6,120.1,121.5,124.5,128.0,128.1,129.7,133.1,139.3,163.3.HRMS (ESI)C19H20N2O3[M+H]+:计算值 325.154 7,实测值325.1554.
(R)-1-(4-Methylphenyl)-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole(4k):白色固体,产率65%,ee 值71%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.57 (br s,1H),7.55~7.53 (m,1H),7.21~7.17 (m,4H),7.15~7.09 (m,3H),5.14 (s,1H),3.40~3.35 (m,1H),3.16~3.10 (m,1H),2.97~2.89 (m,1H),2.85~2.79(m,1H),2.36 (s,3H).HRMS (ESI) C18H18N2[M+H]+:计算值263.154 3,实测值 263.1545.
(R)-1-(4-Fluorophenyl)-7-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4l):白色固体,产率84%,ee 值82%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.48 (br.s.,1H),7.41 (d,J=7.41,1H),7.27 (m,2H),7.02 (t,J=8.5 Hz,2H),6.79 (dd,J=2.5 Hz,6.5 Hz,1H),6.73 (s,1H),5.11 (s,1H),3.79 (s,1H),3.32 (m,1H),3.11 (m,1H),2.88 (m,1H),2.77 (m,1H),1.94(br.s.,1H).13C NMR (101 MHz,DMSO-d6)δ:163.5,161.5,156.3,137.7 (d,J=2.5 Hz),136.6,132.8,130.5(d,J=8.25 Hz),121.5,118.8,115.5,(d,J=21 Hz),110.1,108.9,94.9,57.2,55.6,42.7,22.4.HRMS (ESI)r C18H18N2[M+H]+:计算值263.154 3,实测值263.1545.
(R)-1-(4-Chlorophenyl)-7-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4m):白色固体,产率81%,ee 值83%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.48 (br.s.,1H),7.41 (d,J=8.5 Hz,1H),7.32 (d,J=8.5 Hz,2H),7.25 (d,J=8.0 Hz,2H),6.79 (dd,J=8.5,2.5 Hz,1H),6.73 (d,J=2.0 Hz,1H),5.11 (s,1H),3.80 (s,3H),3.32 (m,1H),3.12 (m,1H),2.89 (m,1H),2.78 (m,1H),1.80 (br.s.,1H).13C NMR (101 MHz,DMSO-d6)δ:157.1,141.5,137.4,134.2,133.6,130.3,129.2,122.4,119.4,110.7,109.2,95.1,57.4,56.6,43.4,22.7.HRMS (ESI) C18H17ClN2O[M+H]+:计算值313.110 2,实测值 313.1108.
(R)-1-(2-Bromophenyl)-7-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4n):白色固体,产率80%,ee 值81%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.64 (d,J=7.9 Hz,1 H),7.58 (br.s.,1 H),7.43 (d,J=8.5 Hz,1 H),7.21 (d,J=7.3 Hz,1 H),7.17 (t,J=7.5 Hz,1 H),7.11 (dd,J=7.5,1.3 Hz,1 H),6.80 (dd,J=8.6,1.9 Hz,1 H),6.75 (d,J=1.6 Hz,1 H),5.63 (s,1 H),3.81 (s,3 H),3.18~3.26 (m,1 H),3.07~3.17 (m,1 H),2.84~2.92 (m,1 H),2.77~2.84 (m,12 H),2.01(br.s.,1 H).13C NMR (101 MHz,DMSO-d6)δ:156.4,140.9,136.7,133.1,131.8,130.4,129.4,127.6,124.3,121.6,118.8,110.8,108.9,94.9,56.1,55.7,41.6,22.5.HRMS (ESI) C18H17BrN2O[M+H]+:计算值357.059 7,实测值 357.0604.
(R)-1-(3-Bromophenyl)-7-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4o):白色固体,产率79%,ee 值84%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.44 -7.50 (m,3 H),7.42 (d,J=8.5 Hz,1 H),7.26 (s,1 H),6.80 (dd,J=8.6,2.3 Hz,1 H),6.76 (d,J=2.2 Hz,1 H),5.10 (s,1 H),3.81 (s,3 H),3.29~3.37 (m,1 H),3.09~3.16 (m,1 H),2.86~2.93 (m,1 H),2.75~2.82 (m,1 H),1.84 (br.s.,1 H).13C NMR (101 MHz,DMSO-d6)δ:156.4,144.4,136.7,132.3,131.4,131.3,130.3,127.1,123.0,121.7,118.8,110.4,109.0,95.0,57.5,55.7,42.6,22.4.HRMS (ESI) C18H17BrN2O[M+H]+:计算值357.059 7,实测值 357.0602.
(R)-1-(4-Bromophenyl)-7-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4p):白色固体,产率76%,ee 值83%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.47 (d,J=8.2 Hz,2 H),7.44 (br.s.,1 H),7.42 (d,J=8.6 Hz,1 H),7.20 (d,J=8.3 Hz,2 H),6.79 (dd,J=8.6,2.2 Hz,1 H),6.74 (s,1 H),5.10 (s,1 H),3.80 (s,3 H),3.30~3.37 (m,1 H),3.09~3.17 (m,1 H),2.84~2.93(m,1 H),2.74~2.82 (m,1 H),1.81 (br.s.,1 H).13C NMR (101 MHz,DMSO-d6)δ:156.4,141.0,136.7,131.9,130.2,122.0,121.7,109.0,94.9,57.4,55.7,42.6,22.4.HRMS (ESI) C18H17BrN2O[M+H]+:计算值357.059 7,实测值 357.0604.
(R)-1-(4-Methoxyphenyl)-7-methoxy-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(4q):白色固体,产率68%,ee 值78%,(ChiralPak AD-H,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:8.26 (br.s.,1 H),7.40 (d,J=8.6 Hz,1 H),7.16 (d,J=8.5 Hz,2 H),6.81 (d,J=8.5 Hz,2 H),6.77 (dd,J=8.5,1.8 Hz,1 H),6.60 (d,J=1.6 Hz,1 H),4.99 (s,1 H),3.77 (s,3 H),3.68 (s,3 H),3.22~3.35 (m,1 H),2.99~3.12 (m,1 H),2.82~2.93 (m,1 H),2.71~2.81(m,1 H),1.81 (br.s.,1 H).13C NMR (101 MHz,DMSO-d6)δ:159.3,156.1,136.6,134.0,133.5,129.6,121.7,118.5,113.9,108.7,94.8,57.3,55.5,55.2,42.6,22.4.HRMS (ESI) C19H20N2O2[M+H]+:计算值309.159 8,实测值309.1606.
(R)-1-Phenyl-7-methoxy-2,3,4,9-tetrahydro-1Hpyrido[3,4-b]indole (4r):白色固体,产率87%,ee 值82%,(ChiralPak ADH,25% MeOH,3 mL/min,230 nm,tR(major)=2.79 min).1H NMR (400 MHz,DMSO-d6)δ:7.31 (6H,m),6.77 (1H,s),6.56 (1H,d,J=9 Hz),5.12 (1H,s),3.76(3H,s),3.24 (1H,m),3.02 (1H,m),2.86 (1H,m),2.77(1H,m).13C NMR (101 MHz,DMSO-d6)δ:156.2,141.9,136.6,133.2,128.1,121.1,118.7,110.0,108.8,94.9,58.1,55.7,42.8,22.5.HRMS (ESI) C18H18N2O[M+H]+:计算值279.149 2,实测值279.1499.
2 结果与讨论
2.1 催化剂及用量对反应的影响催化剂及其用量不仅能加快反应速率,降低反应活化能,而且还能在一定程度上提高反应产率.在室温下,用甲苯为溶剂,苯甲酸为添加剂,以6-甲氧基色胺和4-氯苯甲醛的P-S 反应为模板,反应66 h,对催化剂1a~1c的催化性能及用量进行考察,结果见表1.
从表1 中数据可知,所合成的3 种催化剂均能有效催化6-甲氧基色胺和4-氯苯甲醛的不对称Pictet-Spengler 反应,但由于催化剂所连接的基团不同从而使催化结果有差异,当1a和1b作为催化剂时,产率和ee 值都较低.其可能原因是受苯环上取代基的影响,吡咯烷的原子太过于接近,导致两个相邻的原子之间形成重叠的电子云,产生斥力,从而影响了反应的活性.而1b由于苯环上的取代基Br 为强吸电子基,使得苯环上的电子密度降低而影响了催化活性.1c中的O 与苯环形成了p-π共轭体系,增加了苯环的电子云密度,诱导催化反应的进行.因此,1c在催化体系中催化活性相比于其他催化剂强,选用该催化剂为该反应的最佳催化剂.序号4~8 为催化剂用量对4m产率及立体选择性的影响,随着催化剂用量的增加,反应的产率和对映选择性都随之增加,但当x(催化剂)增加到25%时产率和对映选择性不再有大幅度的变化(序号7~8),因此考虑到经济环保的原则,确定催化剂的最佳用量为x=20%.
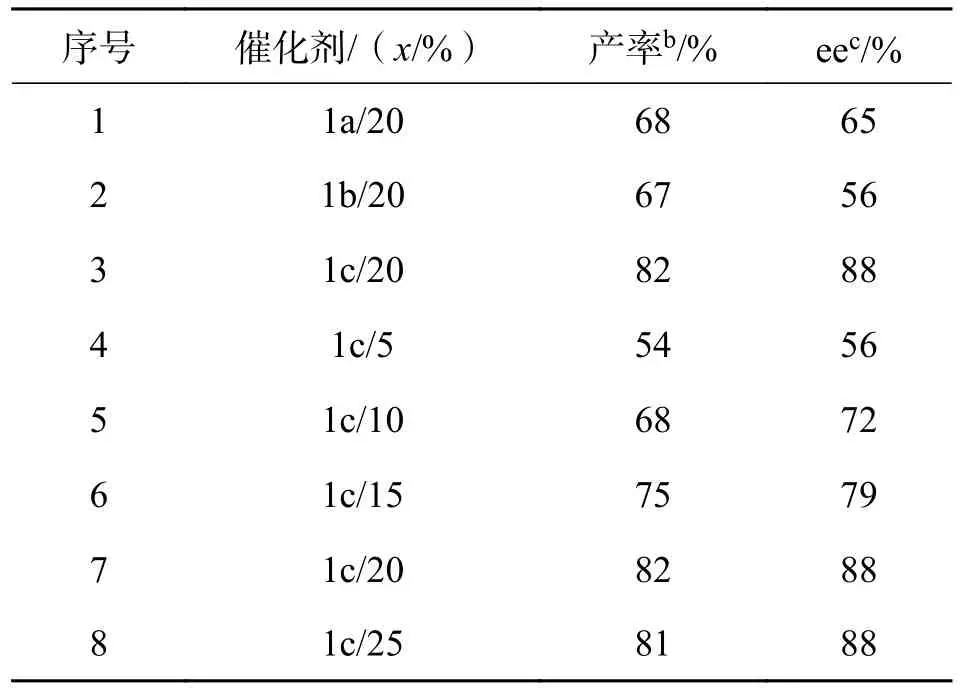
表1 催化剂及用量筛选aTab.1 Selection of catalyst and dosagea
2.2 不同溶剂对反应的影响以6-甲氧基色胺和4-氯苯甲醛的反应为例,在x=20%催化剂1c 催化反应下,考察不同温度对反应的影响,结果见表2.
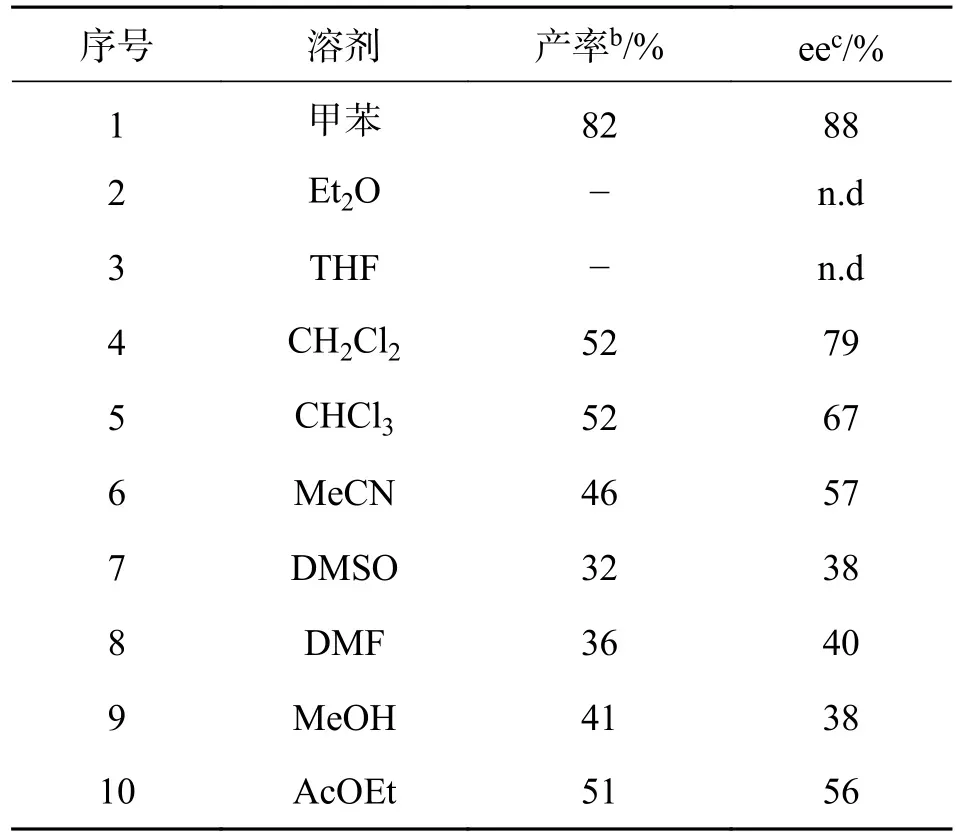
表2 溶剂的筛选aTab.2 Selection of solventsa
由表2 可知,不同的溶剂对反应的影响不同,其中使用甲苯(极性为2.4)作为反应溶剂时反应效果最佳,目标产物的最终产率为82%,ee 值为88%(序号1).当用DMSO(极性7.2)、DMF(极性6.4)、MeCN(极性6.2)等强极性溶剂时(序号6~8),产率和ee 值都较低,这可能是因为强非质子极性溶剂的分子具有极性,产生了溶剂化效应.而Et2O(极性2.9)、CH2Cl2(极性3.4)、CHCl3(极性4.4)、THF(极性4.2)以及AcOEt(极性4.3)等溶剂(序号2~5,9,10)的极性略高于甲苯,但从实验数据来看,效果并没有甲苯的好,因此,该反应的最佳溶剂为甲苯.
2.3 时间及温度对反应的影响以x=20%的催化剂1c 催化6-甲氧基色胺和4-氯苯甲醛的不对称Pictet-Spengler 反应为例,考察不同的反应时间及温度对产率和ee 值的影响,结果见表3.
由表3 可知,温度对该反应的影响较明显.在低温时(序号1~2),目标产物的ee 值达到中等(68%和71%),但其产率较低.随着温度的升高,产物的产率和ee 值也随之升高,当升至30 ℃时产率变化不再明显(序号2~7),因此,该反应的最佳温度为25 ℃.反应进行24 h 后,产率达到57%,ee 值达到中等(74%),延长反应时间,产率和ee 值也随之增加,延长至72 h 后,产率变化不再明显,ee 值也保持不变,因此,反应的最佳时间为66 h.
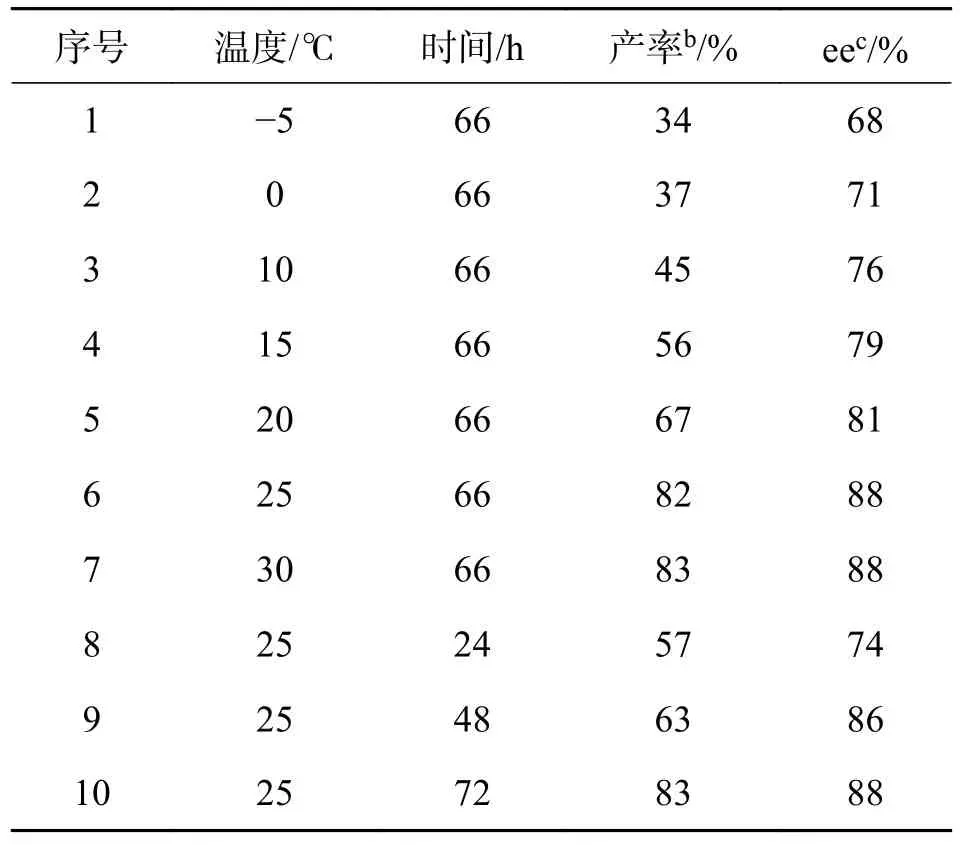
表3 时间及温度对反应的影响aTab.3 The influence of time and temperature on the reactiona
2.4 反应底物的扩展确定最佳反应条件后,本文对反应底物进行扩展,研究催化剂对该类反应的普适性.在室温下,以甲苯为溶剂,苯甲酸为添加剂,x=20%1c 为催化剂,对6-甲氧基色胺和芳香醛的Pictet-Spengler 反应底物进行扩展,反应通式见图3,结果见表4.
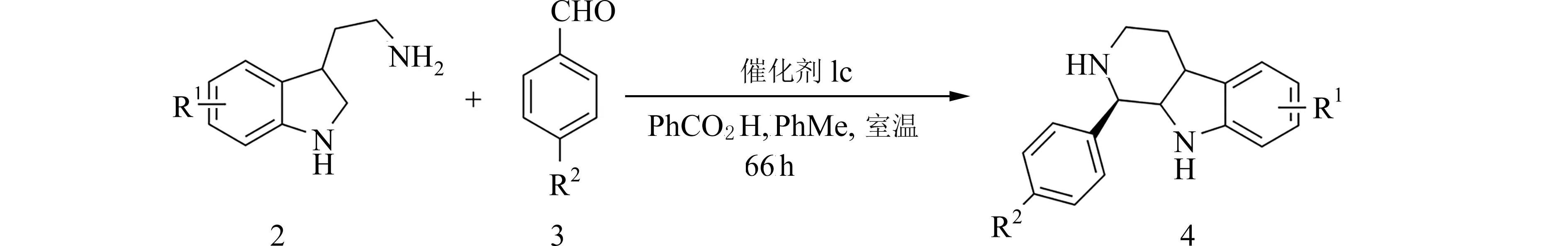
图3 底物扩展反应通式Fig.3 The general equation of expansion of reaction substrates
由表4 可知,色胺以及6-甲氧基色胺与不同取代基的芳香醛都能有效地反应,均能获得中等以上的收率(58%~87%)及中等以上的ee 值(54%~89%),存在差异的原因可能是芳香醛上取代基的不同.当取代基为给电子基时,目标产物的产率和ee 值较高(序号5,6,7,11,17),分别是79%~87%和80%~89%,这可能是因为给电子基(—CH3、—OCH3)使C= C 键的极化程度减小,增加了苯环上的电子云密度,降低了反应的活化能;当取代基为吸电子基(如氟、氯、溴、硝基)时,由于吸电子能力的不同结果也有所不同,吸电子能力强的苯环上的电子云密度减小得更多,引起的共轭和诱导效应使C= C 键的极化程度增强,从而影响了收率和ee 值(序号2~4、8~9、12~16).综上,不论吸、给电子取代基,都证明了催化剂1c对该反应有良好的催化活性,并且还具有很好的普适性.
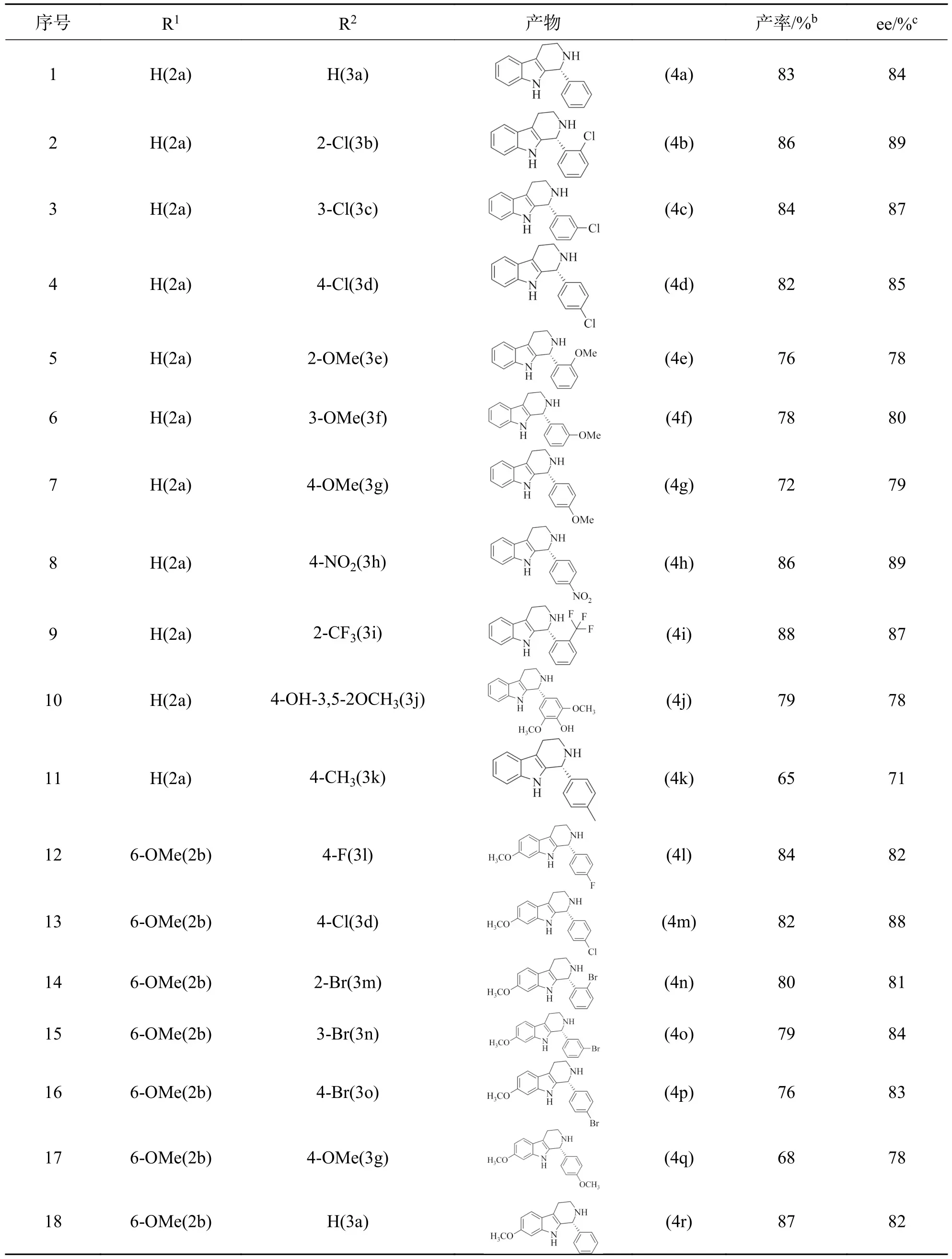
表4 反应底物扩展结果aTab.4 Results of expansion of reaction substratesa
3 结论
本文成功合成了C2轴手性双硫脲催化剂1a~1c,并将其应用于色胺和苯甲醛衍生物的Pictet-Spengler 反应合成四氢-β-咔啉衍生物4a~4r.结果表明,在室温(25 ℃)下,甲苯为溶剂,苯甲酸为添加剂,催化剂1c 的用量为x=20%,Pictet-Spengler环化反应取得较高的收率82%和较高的对映选择性88%.该方法具有操作简单,催化剂用量少,反应条件易控和对环境友好等优点,为合成β-咔啉化合物提供了一种有效的方法.

