Whole Brain Imaging of Larval Zebrafish during Rheotaxis
WU Yu-Bin,ZHANG Ren-Chang,LI Da-Guang,QI Ke-Xin,CHAI Yu-Ming,SHEN Chen,SI Guang-Wei*,WEN Quan
(1)Chinese Academy of Sciences Key Laboratory of Brain Function and Diseases,Division of Life Science,University of Science and Technology of China,Hefei 230026,China;2)State Key Laboratory of Brain and Cognitive Science,Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China;3)Hefei National Laboratory for Physical Sciences at the Microscale,Center for Integrative Imaging,University of Science and Technology of China,Hefei 230026,China;4)School of Data Science,University of Science and Technology of China,Hefei 230026,China)
Abstract Objective Rheotaxis,namely to orient and swim against the water flow,is a conserved behavior across most fish and amphibians.While the study of rheotaxis behavior has a relatively long history,and in recent years the behavioral algorithm of rheotaxis has been described,how distributed neural circuits integrate multisensory information,make decisions,and generate counterflow motor sequences remain largely unknown.Whole brain calcium imaging of larval zebrafish during rheotaxis would provide a unique opportunity to tackle this difficult problem.Methods To this end,we developed a microfluidic device that can precisely control the water flow and elicit rheotaxis behavior.By integrating the chip with a customized light field tracking microscope,we built a system to record whole brain neural activity in freely behaving larval zebrafish during rheotaxis.Results Larval zebrafish showed reliable rheotaxis behavior in the setup,represented by prominent positional holding and counterflow swimming bouts in water flow.In the meanwhile,we successfully recorded zebrafish whole brain neural activity,from which a few brain regions were identified whose calcium signals strongly correlated with rheotaxis behavior.Conclusion Our study,for the first time,demonstrates a method for imaging whole brain neural activity in larval zebrafish while the animal is performing rheotaxis.Future analysis and modelling of the neural activity and behavioral data will deepen our understanding of sensorimotor transformation in this important naturalistic behavior.
Key words rheotaxis,larval zebrafish,microfluidics,light field microscope
Rheotaxis,an innate and conserved behaviour in many aquatic animals[1],is of great importance to animal survival.For example,salmon would swim against water stream to return to their birthplace to lay eggs[2];small fish living on plankton often hold their position by facing upstream to intercept food carried by the current[3].The commonality of rheotaxis and the fact that it involves the integration of multiple sensory(visual,hydromechanical,etc.)cues[4]have drawn the attention of many systems neuroscientists.
The sensory basis of rheotaxis has been investigated extensively.It has been shown that fish can detect flow-induced displacement of themselves by using visual cues[5].Researchers have also discovered that when deprived of visual cues,larval zebrafish can still consistently perform body orientation and position holding in water flow by using the mechanosensory information detected by the lateral line[6].Despite these insights,it remains unclear how larval zebrafish exploit visual and hydromechanical cues in their brains to reach the adaptive decision to swim against the direction of the water flow.Most studies on rheotaxis of larval zebrafish were carried out either by behaviour tests[4,6-7]or by brain imaging of immobilized fish[8-9],neither of which alone is sufficient to reveal the neural mechanisms underlying the generation of rheotaxis.
Whole brain imaging in a freely behaving animal is an emerging frontier in systems neuroscience,holding the potential for revealing brain dynamics and naturalistic animal behaviors and their relationships at multiple spatiotemporal scales[10].Continuous efforts have been made to image larval zebrafish’s whole brain activity while the animal is performing behaviors,ranging from navigation to foraging,but all in zero-flow environment[11-15].Microfluidics,with the advantages in precise stimuli control,has proven to be an ideal platform to study sensorimotor transformation in brains of small organisms[16],including larval zebrafish[17-19].Previous microfluidic applications have demonstrated reliable and precise control of hydrodynamics surrounding larval zebrafish[8,20].However,the existing microfluidic designs either immobilize the fish in the channel[8]or are not compatible with the tracking microscope[20].
In this study,we developed a novel microfluidic structure,and integrated it with the extended field of view light field microscope(XLFM)[12]to study rheotaxis of freely-swimming larval zebrafish.We showed that larvae zebrafish could display rheotactic behavior in the presence of 488 nm blue laser illumination and movements of the tracking stage.We successfully captured whole brain activity of larval zebrafish while it was performing rheotaxis and identified a few brain regions that are possibly involved in this behavior.
1 Materials and methods
1.1 Zebrafish preparation and behavioral experiments
Zebrafish(Danio rerio)larvae of either sex,carrying the transgene elavl3:H2B-GCaMP8s(a gift from Dr.Ahrens Misha at Janelia Research Campus,HHMI)were generated and maintained at 28.5°C on a 14 h on/10 h off light cycle.All Experiments were conducted in zebrafish of nacre background[21].Larval zebrafish were maintained at 28.5℃in 0.5×E2 embryo media and fed with paramecium.Adult zebrafish were maintained,fed,mated as previously described[12].
Larvae zebrafish at five to nine days postfertilization(d.p.f)were transferred into our custombuilt microfluidic chamber hooked up to a glass container filled with embryo media.The container was fixed at a certain height and water flow was driven by compressed air.The flow rate was tuned using a pressure regulator(MFCS,Fluigent Inc.)and monitored in real time by a flow sensor(Flow unit,Fluigent Inc.).We used a perfusion controller(Valvelink8.2,Autom8 Inc.)to switch on and off the flow,synchronized with image acquisition.The microfluidic chip was placed upside down on the stage and illuminated by a homemade IR LED ring(Figure 1a).During experiments,we delivered water flow from one side of the arena to elicit rheotactic behavior in larval zebrafish.We used a customized tracking system to track the movement of larval zebrafish and a low magnification camera underneath the microfluidic chamber to perform behavior recording[12].
1.2 Microfluidic device
The microfluidic device has a behavior arena with dimensions of 2 cm(W)×5 mm(D)×0.8 mm(H)(Figure 1b),allowing larval zebrafish to freely swim and turn inside the chip.Pillars are constructed at each end to prevent fish from escaping.A side channel is designed to load fish into the arena.The layout is designed using SolidWorks(Dassault Systèmes,Inc.),and the design file is publicly available(File S1).
To fabricate this device,the design file was 3D printed(MicroArch s140,Boston Micro Fabrication)with HTL resin to make a high-resolution master mold.Then,the reagent polydimethylsiloxane(PDMS)elastomer base and curing agent were mixed at a 10∶1(w/w)ratio and de-bubbled,and the mixture was cast onto the 3D-printed master mold with a negative replica design of the device followed by degassing for around 30 minutes.The PDMS was cured at 65°C overnight,after which it was cut out and peeled off the master mold.Inlet and outlet with a diameter of~1 mm were punched respectively at either side of the chamber.A hole of~4 mm in diameter was made at the inlet of the loading channel.Finally,the PDMS was bonded to a flat glass slide with the aid of air plasma and baked at 65℃overnight.
1.3 Whole-brain calcium imaging and image reconstruction
Neural activity was recorded with our custombuilt light field microscope as previously described[12].Calcium indicator across the whole zebrafish brain was excited simultaneously by a 488 blue laser with an illumination area of~1.44 mm in diameter.The fluorescence emission was collected by a high NA objective and transmitted through a dichroic mirror and a pair of achromatic lenses before reaching the customized lenslet array.The dichroic mirror and achromatic lenses were placed in a way that the objective’s back pupil is conjugated onto the lenslet array.The customized lenslet array consists of an aluminum plate with 27 holes and 27 customized micro-lenses housed within these holes.An sCMOS camera was positioned behind the lenslet array to collect transmitted fluorescence.We developed an iterative reconstruction algorithm to reconstruct 3D fluorescence stacks(600×600×250)from the acquired 2D images(2 048×2 048).This algorithm was derived from the classical Richardson-Lucy deconvolution method,which requires an experimentally measured point spread function(PSF)of the XLFM system.
1.4 Volumetric image registration and neural activity analysis
The whole brain neural activity was extracted from the reconstructed 3D image stacks acquired by the XLFM system.First,we cropped and resized the original image stack(from 600×600×250 to 308×315×210)and aligned them to the standard atlas“zbb”[22].Next,to obtain relatively stable and reliable regions of interests(ROI)across volumes,we performed 3D intensity-based rigid registration on each volume by using the computational morphometry toolkit(CMTK)[23]with the criteria of“correlation ratio”[24].After voxel registration,we computed pairwise correlation between nearby voxel intensities.The correlation map was used to cluster and segment voxels(watershed algorithm)into consistent ROIs across all volumes.The fluorescence signals,averaged over voxels within each ROI,were calculated.Finally,we adopted the“OASIS”[25]deconvolution method to denoise and to infer neural activity from the fluorescence time sequence.
1.5 Tracking
To track the position and heading of larval zebrafish onx-yplane under our high NA imaging objective,a high-speed camera(2 ms exposure time,300 fps or higher,Basler aca2000-340kmNIR,Germany)and horizontally-motorized stages were used as previously described[12].First,with the aid of OpenCV 3.4,a C/C++based real time system was used to detect and identify head positions,after which the error signal between actual head position and the set point was transmitted into the PID to generate output signals that control the movements of a motorized stage.
To track the axial movements of larval zebrafish,the principle of light field microscope was applied.We used an autofocus camera behind a twodimensional microlens array to capture quintuple images of larval zebrafish from different perspectives.Movements of zebrafish alongzaxis will cause the inter-fish distance,namely the distance between the centroids of the fish head in the left and right subimages,to change.We used pairwise correlation between sub-images to compute the distance shift.As the distance varies linearly with axial movements,thezposition of larval zebrafish can be calculated by multiplying the inter-fish distance with a pre-factor.The error signal between the actual axial position and the set point was used to generate an output signal that drives a piezo-coupled fish container.The autofocus control system was developed using LabVIEW.
1.6 Microparticle imaging velocimetry
Particle tracking velocimetry(PTV)was used to map the flow velocity profile in the behavior arena.The microfluidic chip was set up in a configuration similar to the experimental conditions.0.45-μmdiameter fluorescent polystyrene latex microbeads(NFPPS,Spherotech Inc.)were suspended at a concentration of 105/ml.The trajectory of the fluorescent microbeads was imaged with a spinning disk confocal microscope(Dragonfly,Andor Inc.)and analyzed with custom MATLAB(Natick,MA,US)code.
1.7 Statistics and cross-correlation
We used bootstrap to test whether the difference between bouts number under flow-on and flow-off conditions are statistically significant for individual fish.We calculated the difference between the means of two bout number distributions;then the two distributions were shuffled and a new difference in means was calculated.This procedure was repeated 10 000 times and theP-value,namely the probability of finding the difference of the means in the original dataset,was calculated.The results were presented in the form of the average bouts per second±SEM(standard error of mean).A similar approach is used to test the statistical difference between the fraction of bout time under flow-on and flow-off conditions.
In order to find out which brain regions are related to rheotactic behavior,we labeled each calcium image volume with‘0’or‘1’according to whether a rheotactic behavior was present or not at that volume,resulting in a binarized time series.We then calculated the Pearson’s linear correlation coefficients between the calcium signal and the binarized time series using student’s t distribution[26].The correlation coefficients were calculated for calcium signal in each segmented brain region(or ROI),and we ranked the correlation coefficients of each ROI from high to low,with the highest reaching 0.49.Regions with correlation coefficients above 0.29 were presented in Figure 4.
2 Results
2.1 Combining microfluidics device with the extended field of view light field microscope(XLFM)to study rheotaxis
The investigation of brain-wide activity during rheotaxis requires the ability to effectively elicit rheotactic behaviour in larval zebrafish while performing calcium imaging in freely moving animal.To this end,we designed a 3D-printed microfluidic device that could be integrated with our custom-built light field microscope(XLFM)(Figure 1a).
Experimental procedures are shown in Figure 1c.We loaded a 5-9 d.p.f.zebrafish larva,through a side channel,into a microfluidic chamber,the size of which was designed to allow fish swimming and turning freely within the chip(Figure 1c).The microfluidic chip was connected to an embryo media source for water delivery,controlled by a pinch valve.When the valve turned on,a flow velocity gradient perpendicular to the flow direction was immediately established inside the chamber(Figure 1c).The flow velocity profile,measured by Particle Tracking Velocimetry,exhibited a symmetric parabolic shape(Figure 1d),consistent with fluid mechanics calculation.By adjusting the pressure applied to the liquid source,we found that a net flow speed around 1.8 mm/s in the chamber was enough to evoke rheotactic behavior in zebrafish.

Fig.1 Experimental setup for whole brain imaging of larval zebrafish during rheotaxis
2.2 Prominent counterflow swimming behavior could be evoked in our whole brain imaging system
During rheotaxis,larval zebrafish first reorients itself along the water current,and then displays position holding by performing counterflow swim bouts[4].Here,we identified and labelled video frames based on behavior types(Figure 2a).Larval zebrafish exhibited bouts toward or away from the water flow,and also made turns within the microfluidic channel(Figure 2b,Video S1 and Video S2).After the flow turned on,the fish reoriented itself from downstream to upstream direction,followed by frequent upstream bouts(Figure 2a).The behavior sequence demonstrated typical rheotactic behavior.
To further examine whether zebrafish could reliably exhibit counterflow swimming behavior characteristic of rheotaxis under our imaging set up,we compared swim bouts in the presence and absence of water flow,as well as toward and away from the applied current direction.To rule out potential bias,we compared the frequencies of swim bouts(left panels in Figure 3a-d)under flow-on and flow-off conditions when larval zebrafish was facing the same direction.We present results when the flow and the fish movement were in opposite directions(Figure 3a,c)or in the same direction(Figure 3b,d)respectively,finding that counterflow swim bout frequency was significantly higher than that in the absence of water flow,consistent with positive rheotaxis behavior.We also calculated the fraction of time larval zebrafish spent on swimming against the water flow,and found clear position holding during the application of water flow(right panels in Figure 3a-d)
2.3 Whole brain imaging of larval zebrafish during rheotaxis
Next,we conducted whole brain calcium imaging while water flow was applied to elicit rheotaxis.The zebrafish brain exhibited rich spatiotemporal neural dynamics while the flow switched on and off(Video S3).Using crosscorrelation analysis between behavior and neural activity,we identified several brain regions that showed significantly elevated activity during periods of counterflow swim bouts.Regions activated were found in telencephalon,optical tectum and hindbrain(Figure 4,color coded areas).Figure 4 below shows calcium signal from one example fish.

Fig.2 Behavior sequence of larval zebrafish rheotaxis
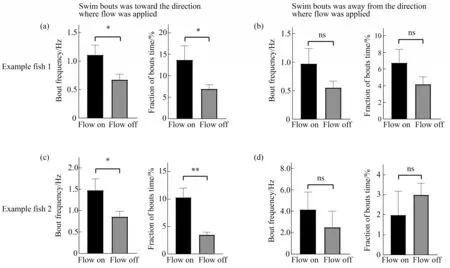
Fig.3 Larval zebrafish display prominent rheotactic behavior
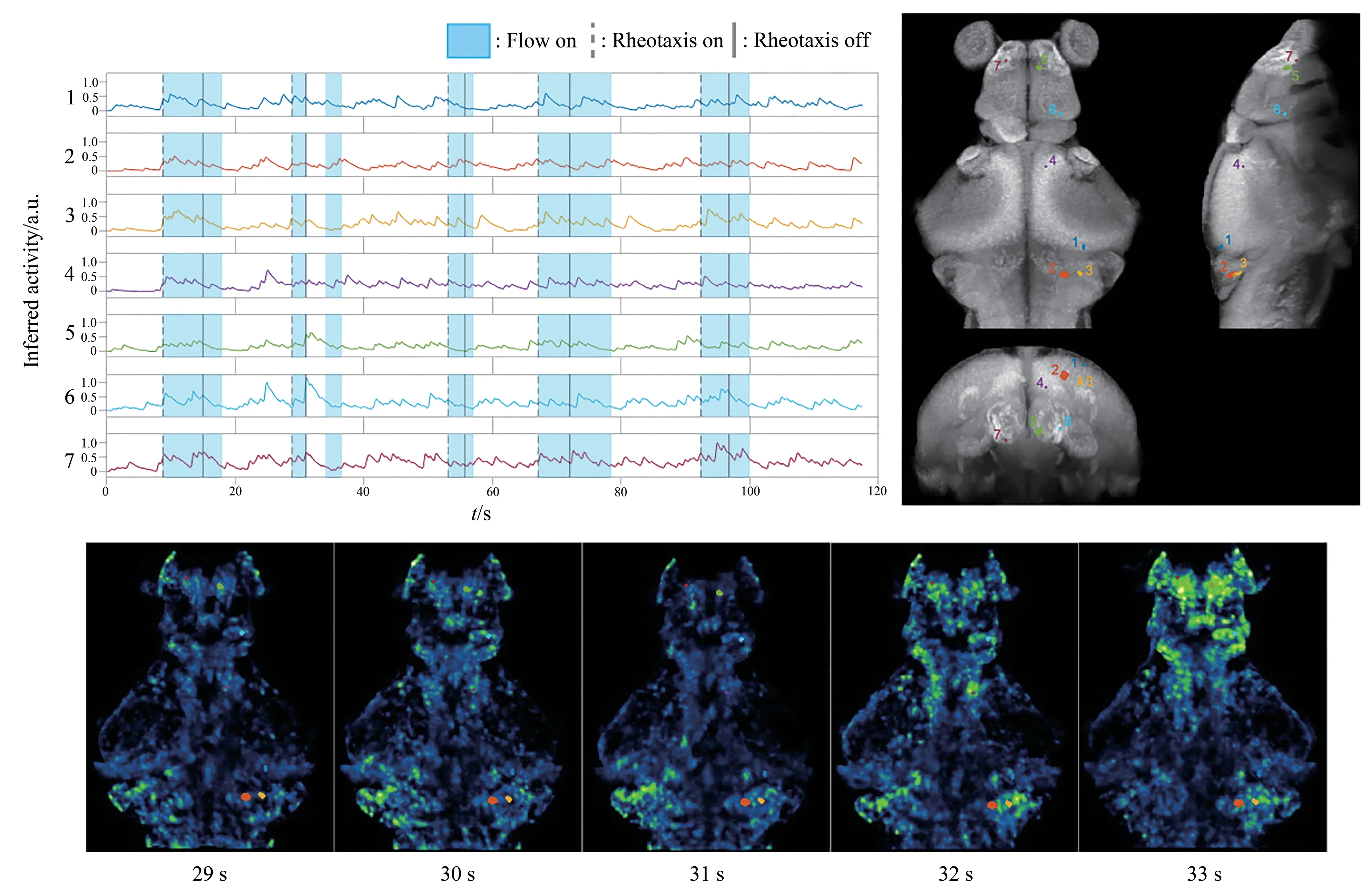
Fig.4 Whole brain imaging of larval zebrafish during rheotaxis
3 Discussion
We have developed an integrated system in which both the rheotactic behavior and brain-wide calcium activity of larval zebrafish could be recorded simultaneously.The design of microfluidic device enabled us to expose larval zebrafish to precisely controlled water flow,and it turned out that both the blue laser,which could interfere the visual system,and the movement of our tracking stage had little effect on the rheotaxis behavior.We successfully recorded brain-wide neural activity during rheotaxis and located brain regions with rising activity correlated with rheotactic behavior.
An animal’s brain usually has dynamic internal states that switch between each other[15].Larval zebrafish clearly showed two distinct phases when engaging in and disengaging from rheotaxis,characterized by counterflow swim bouts,suggesting that state-encoding neural population may be responsible for the maintenance of rheotactic behavior.The brain regions identified in our study showed elevated calcium signals not only during rheotaxis but also at times when water flow was not applied(Figure 4),indicating that these regions may not be involved in state encoding,and their activity may just reflect a correlation with swim bouts.Thus,more experiments and more sophisticated analysis are needed to reveal brain regions that are essential for triggering and maintaining rheotaxis.
We observed prominent counterflow swimming sequences and position holding behavior during the application of water flow in our microfluidic device.We did capture several reorientation events in larval zebrafish,but overall the event was not commonly seen.As larval zebrafish could perform reorientation in much narrower microfluidic chip[20],the size of our chip is big enough for zebrafish larvae to freely turn and swim.We reasoned that the less frequent occurrence of turning events might be attributed to the relatively slow speed of water flow.Although our applied water flow could trigger prominent counterflow swim bouts,the elicitation of turning behavior may require higher flow speed,as previously reported[20].Besides,we found that the frequency of rheotactic behavior dropped as time went by,and near the end of an experiment,it became relatively hard to elicit typical rheotactic behavior with the applied water flow.We suspect that zebrafish larvae would become more and more familiar with the microfluidic chamber and fatigue may build up during an experiment.To more effectively evoke rheotactic behavior in larval zebrafish,we will redesign the microfluidic chip,making it compatible with higher speed water flow in the future study.
To our knowledge,the current study was the first to report brain-wide neural activity during rheotaxis in larval zebrafish.Before our study,possible behavior mechanisms as well as circuit models have been proposed[6,9].Our set up could therefore provide a useful platform to test existing models and uncover unknown brain regions and neural mechanisms for sensorimotor transformation during zebrafish rheotaxis.
4 Conclusion
By integrating microfluidics with light field tracking microscopy,we successfully recorded whole brain neural activity in larval zebrafish while the animal was performing rheotaxis behavior in a precisely controlled flow environment.Larval zebrafish exhibited current-evoked position holding behavior and persistent counterflow swim bouts.Neural activity in three brain regions,namely telencephalon,optical tectum,and hindbrain,were found to be highly correlated with the rheotaxis behavior.Further imaging data analysis and modeling will help elucidate neural mechanisms for sensorimotor transformation in this important naturalistic behavior.
SupplementaryAvailable online(http://www.pibb.ac.cn or http://www.cnki.net):
PIBB_20220354_File S1.stl
PIBB_20220354_Video S1.avi
PIBB_20220354_Video S2.avi
PIBB_20220354_Video S3.mp4

