Interaction of an unwetted liquid Li-based capillary porous system with high-density plasma
Yingwei GAO(高英玮),Zongbiao YE(叶宗标),*,Jianxing LIU(刘建星),Hengxin GUO(郭恒鑫),Shuwei CHEN(陈曙嵬),Bo CHEN(陈波),Jianjun CHEN(陈建军),Hongbin WANG(王宏彬) and Fujun GOU(芶富均),*
1 Key Laboratory of Radiation Physics and Technology,Ministry of Education,Institute of Nuclear Science and Technology,Sichuan University,Chengdu 610064,People’s Republic of China
2 College of Physics,Sichuan University,Chengdu 610064,People’s Republic of China
Abstract This study examined the effects of plasma irradiation on an unwetted liquid lithium-based capillary porous system(Li-CPS).The Li-CPS was irradiated with high-density Ar plasma using a linear plasma device at Sichuan University for Plasma Surface Interaction.The high-speed camera,Langmuir probe,and multi-channel spectrometer were used to characterize the effects of plasma irradiation.Upon Ar plasma irradiation,liquid Li drops were formed on the surface of the unwetted Li-CPS.Immediately after this irradiation,the drops fractured and were ejected into the plasma within~20 ms scale,which is not observed before to the best of our knowledge.Related results showed that the ejection behavior of Li could effectively cool electron temperature and reduce incident heat flux by~30%and correspondingly matrix temperature~150°C,revealing an enhanced vapor shielding effect.The involved internal mechanism and physical processes deserve further investigations.
Keywords:unwetted Li-CPS,plasma,liquid Li,vapor shielding effect,drop
1.Introduction
Divertor is crucial to meet the regular operation of tokamak devices for future applications of fusion energy.Suffering from the considerable impact of complicated particles irradiation environment on divertor,liquid lithium(Li)has come as an alternative plasma-facing material and has been widely investigated in recent years[1,2]because of its self-healing ability to irradiation and good compatibility with D/T plasma[3,4].At present,experimental studies of liquid Li have been carried out in CDX-U/LTX[4],FTU[5],T-11M[6],NSTX[7],EAST[8],and TJ-Ⅱstellarator[9],and the results verified its feasibility as the first wall of future fusion reactors.
Owing to the magnetohydrodynamic effect in complex electromagnetic fields[10,11],a capillary porous system(CPS)was introduced onto the surface of liquid Li and restrained its splashing behavior[12,13]through capillarity.Evtikhinet al[14]tested the interaction of magnetized plasma streams with Li-CPS targets in the plasma accelerator QSPA and observed the formation of a dense ionized Li radiation layer(~10-15 mm thick)in front of the target.They found that the Li ionosphere could absorb and radiate most of the incident plasma energy(~97%-99%),thus creating a radiation buffer zone to protect the substrate.Our group[15]also showed that Li vapor can form a shield against the loaded heat carried by incident plasma.However,most reported studies on Li-CPS were investigated under wetted condition(CPS is wetted by liquid Li)before plasma irradiation,little attention was paid to an unwetted Li-CPS,which is common after an extreme plasma event such as edge localized modes(ELMs)[14,16].It is currently unclear whether unwetted Li-CPS-based component could survive under plasma circumstances.
In this work,unwetted Li-CPS is prepared and irradiated under high-density plasma by the linear plasma device of Sichuan University Plasma Surface Interaction(SCU-PSI)to disclose its corresponding interaction with plasma.Meanwhile,the evolution process of the Li-CPS under plasma irradiation is studied and the underlying mechanism is also discussed.
2.Experiment
As shown in figure 1,the Li-CPS consisted of a Mo crucible,a Mo cap(external diameter:55 mm;inner diameter:25 mm),Li,and a tungsten mesh(wire diameter:~60 μm;pore size:~100 μm).The CPS consists of~100 μm capillary holes and can produce sufficient capillary force>10 kPa to support the stabilization of liquid Li.Commercial pure Li(99.99%)was obtained from Ganfeng Lithium Co.,Ltd,China.The tungsten mesh was acquired from Anping Hongyun Metal Products Co.,Ltd,China.The unwetted Li-CPS was prepared through the following steps:pure solid Li tablets were first added to a pre-cleaned hollow Mo crucible and incubated at~200 °C in an Ar-filled glovebox.Then,CPS was installed on the top of the Li-filled Mo crucible to cover the surface and subsequently was anchored with a Mo cap to seal firmly.After the entire system was naturally cooled down to room temperature,Li-CPS was removed from the glovebox for subsequent plasma irradiation experiment.For the preparation of wetted Li-CPS,it is obtained through heating treatment of unwetted Li-CPS at 500 °C under 10-4Pa for 1 h.
The fabricated Li-CPS was placed vertically,facing against the incident plasma in SCU-PSI.Figure 2 shows the schematic illustration of SCU-PSI as we reported before[17].The Ar plasma irradiation parameters were adjusted as follows:averageTe~0.5 eV,ne~2×1019m-3,power flux of 8.23 kW m-2with a diameter of~26 mm(measured by the Langmuir probe)under a magnetic fieldBof 0.2 T.During plasma exposure,the substrate temperature of Li-CPS was tested using thermocouples(thermocouples was set attached below CPS).The real-time optical emission spectrum was monitored in front of Li-CPS by the Avaspec-2048(Avantes OES).In addition,Memrecam ACS-1 high-speed camera was utilized to observe the interactions between plasma and liquid Li.
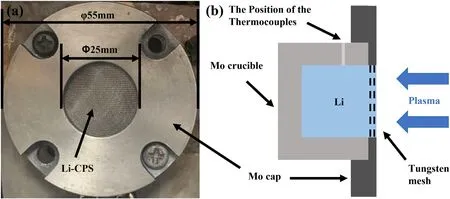
Figure 1.(a)Photograph and(b)schematic diagram of Li-CPS.
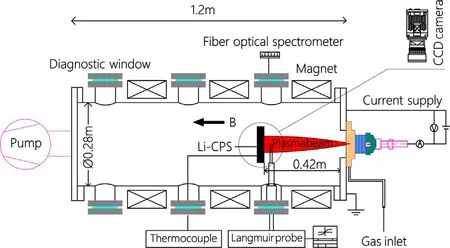
Figure 2.Schematic illustration of SCU-PSI.
3.Results and discussion
Figure 3 demonstrates the interaction between Ar plasma and Li-CPS with a fluence of 4.5×1021m-2.In addition to the source of particles,the incident Ar plasma also acted as a source of heat load from the right side.Liquid Li could evaporate from the surface of the Li-CPS by the heating function of plasma.At the initial phase,a superficial and shallow bright vapor layer of Li could be observed on both unwetted and wetted surfaces of the Li-CPS.As irradiation continued,it shows gradually enhanced brightness and increased vapor thickness on the surface of wetted Li-CPS.

Figure 3.Snapshots of the interaction between Li-CPS and plasma.The frame rate is 50 fps with a time resolution of 20 ms.#1,#2 and#3 represent independent eruption process of Li for unwetted Li-CPS,#4 displays typical irradiation process for wetted Li-CPS.
Meanwhile,it is interesting to find that there are Li drops formed on the surface of CPS during the irradiation process for all unwetted samples as shown in figure 3.The drop gradually swells under unremitting plasma irradiation,gradually the external Li drop enlarges to a hemispherical globule with an obviously enhanced shining surface of Li-CPS.Within only 20 ms,the Li drops fractured suddenly for all unwetted samples and were ejected into the plasma from the surface of the target with a violent burst of light along the jet direction.The entire process of drop formation and ejection from the Li-CPS target occurred within the timeframe of a few seconds.Subsequently,the injection area showed alternating strong and weak vapor layer,which has previously been reported as a typical mode of oscillation[18].Although independent experiments #1,#2,and #3 show varied mildly drop formation behavior with different drop forming sizes and speeds,which might come from the difference in weight of filled Li,the position of Mo crucible,plasma parameters,or even vacuum,the unstable Li drop ejection behaviors are similar for each experiment.For the wetted Li-CPS,there is no Li drop observed on the surface of capillary meshes.The brightness monotonously and persistently increases as time goes by,revealing a stably strengthened Li vapor and its interaction with incident Ar plasma.
The formation and ejection of liquid Li drops under plasma irradiation for unwetted Li-CPS are not discussed or understood up to now.Based on our experimental results,it appears that the observed phenomenon can be attributed to the unstable capillary force between the CPS and liquid Li.Due to no strong capillary force formed between the liquid Li and porous meshes for unwetted Li-CPS,liquid Li might be constrained by its surface tension and could not withstand instability of thermal equilibrium[19]and volume change,resulting in violent ejection as shown in figure 3.
Spectrum is an effective method to study the evolution of evaporated Li particles.Figure 4 shows the typically visible spectra of the Li-CPS under irradiation with Ar plasma.Before the ejection of the Li drop,only the Ar signals could be detected because of the limited evaporation of Li.However,after the ejection of the Li drop,the quantity and intensity of the Ar spectrum reduced remarkably,while those of the Li spectrum increased considerably.This change can be ascribed to the strong interaction and energy exchange between the Ar ions and evaporated Li atoms.The Li atoms could move down from an excited state and undergo deexcitation,thereby emitting the characteristic spectrum of Li.As shown in figure 4(b),two noticeable emission lines of Li atoms could be observed at 670.78 nm(3.38 eV)and 812.62 nm(1.9 eV).Moreover,a few Ar emission lines could also be identified within the range from 300 to 900 nm.Therefore,it can be speculated that the bright region observed in figure 3 resulted from the effect of Li evaporation.In addition,it is known that the intensity of the visible emission spectrum of Li atoms is proportional to the density of evaporated Li atoms[15].Therefore,the spectral intensity of the Li_670.78 nm spectrum emitted owing to de-excitation from the first excited state could reveal the time-dependent spatial evolution behavior of Li.

Figure 4.Spectra of unwetted Li-CPS under Ar plasma(a)before and(b)after Li drop ejection.
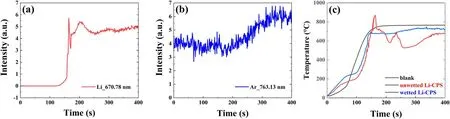
Figure 5.Spectral intensity of(a)Li_670.78 nm and(b)Ar_763 nm for unwetted Li-CPS#1,(c)sample temperature of blank CPS,unwetted Li-CPS #1,and wetted Li-CPS under the irradiation of Ar plasma.
Figure 5 shows the spectral intensity of Ar and Li,and the sample surface temperature of blank CPS,unwetted Li-CPS,and wetted Li-CPS under plasma irradiation along time.For blank sample,the temperature continuously increases to a plateau of~750 °C.After filled with Li,both unwetted Li-CPS and wetted Li-CPS show radiation power from the plasma due to vapor shielding effect and decrease of target temperature.The wetted Li-CPS shows similar temperature variation tendency to that of blank sample with attenuated plateau of~700 °C.At the range of 50-100 s,the phase change of Li(with melting point of 180 °C)slows down the temperature curve.As the surface temperature reached the melting point,the temperature curve started increasing steeply with time.This could be because the heat transfer mode changed from conduction(between porous mesh and solid Li)to convection(within liquid Li).The heat transfer coefficient and thermal conductivity were greatly enhanced because Li is a relatively good heat transfer medium that improves heat transfer effects within the entire target system.
Correspondingly,the spectral intensity of Li for unwetted Li-CPS increased sharply between 140 and 160 s as shown in figure 5(a),and a rapid increase in temperature(up to 850°C)was also observed.Meanwhile,Li drop fractured and was ejected from the surface of the Li-CPS.This event corresponded to a significant increase in the spectral intensity(spatial diffusion of Li atoms)and a remarkable decrease in temperature(heat loss from phase change).While it is found that,as shown in figure 5(b),the spectral intensity of incident Ar revealed independent variation trend and kept a relatively stable value during the entire irradiation process.Subsequently,the eruptible Li atoms displayed a typical oscillation mode due to the vapor shielding effect,as reported previously[18].The incident plasma brought in particles and heat,and the temperature of Li-CPS would rise then and induce evaporation of Li.The evaporation could take away the energy of substrate and result in a decrease of base temperature.In the meantime,the decreased temperature would weaken the evaporation of liquid Li and further lead to its elevation of temperature of Li-CPS under constant plasma irradiation.During the feedback process,the evaporated Li atoms can be excited and ionized and then de-excited and recombined with radiation of huge energy as discussed before.The above process would be oscillating quickly(the oscillation details could be traced from the bouncing temperature and spectrum curve along time,the intensity of Li,and the temperature of the substrate changed alternately)and reach a thermodynamic equilibrium as shown in figure 5.
Figure 6 shows the axial variation in the Li_670.78 nm spectral intensity and the corresponding fitting curve.Limited to size of inspection window,the detecting distance is confined in the range of 0-14 mm.As the figure shows,the spectral intensity of Li_670.78 nm receded as the distance from the sample increased.The spectral intensity decreased exponentially with increase in the distance from the crucible along the axis,satisfying the following equation[20]:
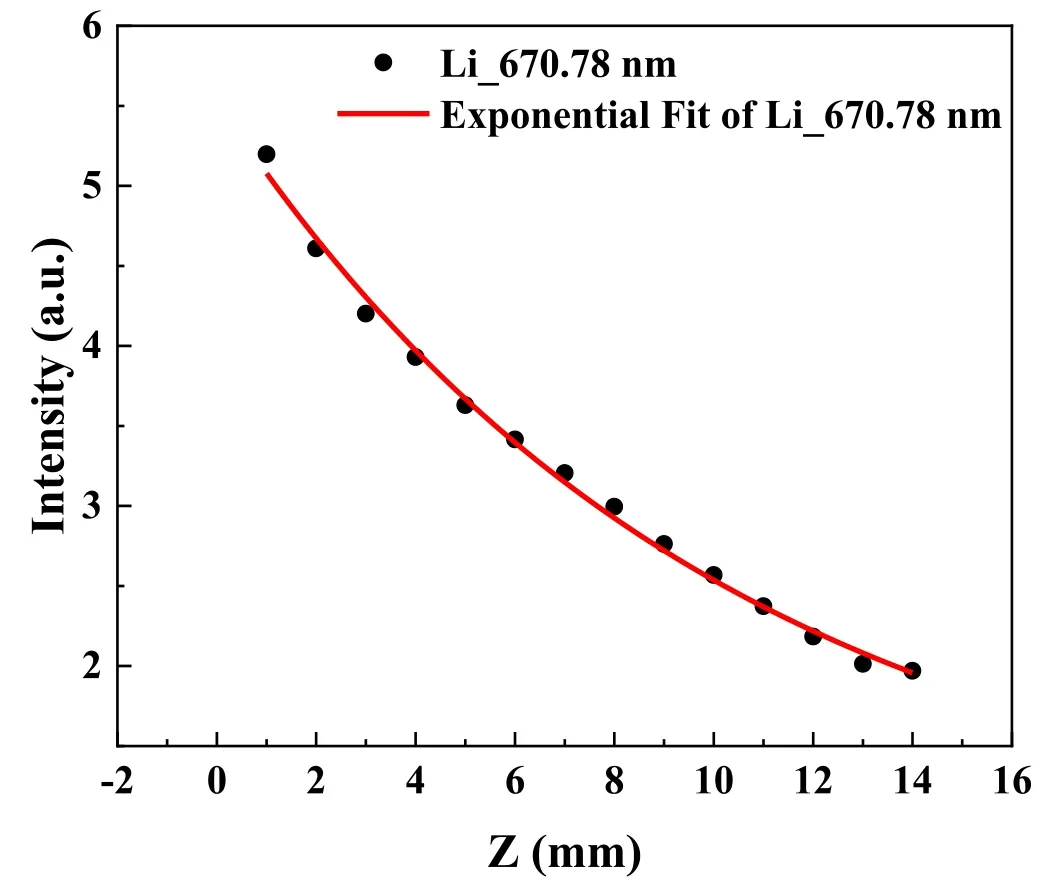
Figure 6.Axial distribution of Li_670.78 nm spectral intensity for unwetted Li-CPS #1.

Figure 7.Time-varying characteristics of(a)electron temperature,(b)electron density,(c)ion flux,and(d)heat flux on the front of unwetted Li-CPS #1 during Li evaporation.

wherezis the distance between the monitoring point and the sample target plate,C1andC2are the fitting constants of the curve,and λmfpis the free ionization path of the neutral particles.The best-fitting values of λmfp,C1,andC2were 10.13 mm,4.77,and 0.76,respectively.In contrast to estimation of mean free path of escaped Li atoms by high temperature,λmfp-2=<v>/<σv>izne,in which<v>is the average velocity of the neutral Li,<σv>iz=6.597×10-10cm3s-1is the collision rate coefficient which is available from the ADAS Database,the λmfp-2could be obtained as 1.68×102mm(higher than the observed value).The huge difference could also be attributed to the ejection of liquid Li.The possible reason we attribute to the estimated λmfp-2mainly depends on the surficial temperature,Teandne,and they reveal a limited change in value during the Li eruption process,as shown in figures 7(a)and(b).While for the spectrum intensity of Li,it is greatly affected by the eruption process and increased by several orders of magnitude after Li eruption,as shown in figure 5(a).The significantly enhanced Li vapor would exacerbate the degradation of Li spectral intensity along with axial distribution and induce the mismatched underestimated λmfp.
In addition,it is indicated that the concentration of the Li vapor shows a typical exponential damping tendency after formation as shown in figure 6.This provides a glimpse into the diffusion behavior of the Li vapor during plasma irradiation process.Combining with the consideration of lost fraction of particles flux,Γloss/Γ=exp(-L/λmfp),whereLrepresents the half height width of the plasma beam(~13 mm).Therefore,theΓloss/Γ≈0.28,which means that more than 20% of the evaporated Li are not redeposited on Li-CPS in consideration of experiment error.
It is known that the surface temperature of the sample decreases with an increase in the Li vapor concentration.According to the liquid Li evaporation rate formula[21]:

whereTis the sample temperature,MLiis the molar mass of liquid Li,andfandgare constants(equal to 8.0 and 8143.0 respectively).With an increase in the Li vapor concentration,the shielding effect is enhanced,leading to a reduction in the input heat load and a decrease in temperature through radiation of energetic Li particles[22].For the unwetted Li-CPS in our experiment,a significant amount of Li was injected into the plasma during plasma exposure,creating conditions conducive for the absorption of more incident energy and a lower temperature than that observed in a wetted Li-CPS.To clear up the power handling role of evaporation-condensation and radiation,the evaporation part of energy loss could be evaluated through temperature analysis.The evaporation flux[23]is known asand the evaporation power[24]can be calculated by multiplying the evaporation flux by the latent heat of vaporization and integrating over the target area aswhereNArepresents the Avogadro constant andYthe particle redeposition fraction.ΔHevapis the latent heat of vaporization.T(r)is the surface temperature.Under the redeposition of 72% and steady-state temperature of 700°C,which is significantly reduced in comparison to the temperature of a pre-wetted liquid metal-CPS[2]by about 100 °C,the〈Pevap〉could be obtained by 7.78 W.
For the part of ions,the variations in the electron temperature,electron density,ion flux,and heat flux near the sample surface at different time points were measured by a fast-reciprocating probe with data collection frequency of 0.2 Hz and detecting position of 25 mm away from the surface of target in the centre of Li-CPS,as shown in figure 7.
The electron temperature was relatively stable between 0 and 160 s,consistent with the weak spectral intensity of Li.As the drop fractured,the electron temperature dropped dramatically(between 160 and 350 s),and this corresponded to the ejection and oscillation behavior.Because of the low excitation energy of Li atoms,the evaporated neutral Li atoms likely collided with the incident plasma at multiple stages and absorbed the incident energy,thus becoming excited.This resulted in a decrease in the electron temperature of the incident plasma.Between 350 and 400 s,the electron reached a state of thermal equilibrium,corresponding to the stationary phase of the spectrum and substrate temperature.Finally,the electron temperature stabilized at~0.57 eV owing to the vapor shielding effect,with a delicate balance between the evaporation and re-deposition of Li.For electron density and ion flux,they are slightly increased along entire irradiation process,originating from the ionization of Ar to massively evaporated Li.For the heat flux,it can be found that for the unwetted Li-CPS,the steady heat flux decreases from initial~9 to~6 kW m-2,with heat flux shielding efficiency(as shown in figure 5)of>30%,which is rather high than our previous wetted result~12%[15],revealing an enhanced vapor shielding effect due to increased Li concentration in plasma for unwetted Li-CPS.Moreover,in consideration to that radiation of ions would be significant only forTe>10 eV[22],the excitation-de-excitation of Li should play the dominant role in the process of vapor shielding as energy dissipation of evaporation is also very limited.
4.Conclusions
The unwetted Li-CPS was irradiated under high-density Ar plasma using SCU-PSI.Drop formation and ejection were observed on the surface of unwetted Li-CPS during plasma irradiation,which significantly decrease incident electron temperature,heat flux,and matrix temperature.Mechanistically,the ejection behavior could be attributed to an unstable capillary force between liquid Li and CPS.Though the unwetted Li-CPS showed better vapor shielding effect than the wetted Li-CPS,it also caused an extra loss of liquid Li from matrix.Nevertheless,further investigations are required to reveal the intrinsic variable nature.
Acknowledgments
This work is supported by National Natural Science Foundation of China(Nos.11875198 and 11905151),China Postdoctoral Science Foundation(No.2019M663487),Sichuan Science and Technology Program(Nos.2021YJ0510 and 2021YFSY0015).
 Plasma Science and Technology2022年11期
Plasma Science and Technology2022年11期
- Plasma Science and Technology的其它文章
- Comparative study of pulsed breakdown processes and mechanisms in self-triggered four-electrode pre-ionized switches
- Wide-range characteristics of beam perveance and saddle point potential of LIPS-200 ion thruster
- Discharge and jet characteristics of gliding arc plasma igniter driven by pressure difference
- Investigation of stimulated growth effect using pulsed cold atmospheric plasma treatment on Ganoderma lucidum
- Understanding the changing mechanism of arc characteristics in ultrasound-magnetic field coaxial hybrid gas tungsten arc welding
- Nanosecond laser preheating effect on ablation morphology and plasma emission in collinear dual-pulse laser-induced breakdown spectroscopy
