Targeting neuroinflammation after therapeutic hypothermia for perinatal hypoxic-ischemic brain injury
Kelly Q.Zhou,Joanne O.Davidson
Hypoxic-ischemic encephalopathy (HIE) has an incidence of 1–3 in 1000 term births in highincome countries (Zhou et al.,2020).The standard treatment for these infants is therapeutic hypothermia.Although therapeutic hypothermia significantly reduces the risk of death and disability for infants with HIE,it is still only partially protective as up to 45% of infants still develop disability despite treatment (Zhou et al.,2020).The current therapeutic hypothermia protocol is optimal for widespread use in infants with moderate to severe HIE.However,it is possible that a more tailored approach for individual babies,including stratification of the cooling regimen for the severity of HIE and the identification of reliable biomarkers to guide treatment,may improve efficacy in the future.Current research efforts are now focused on finding add-on treatments to hypothermia that can provide additive neuroprotection (Zhou et al.,2020).This endeavor has proven to be very difficult,as many therapeutic agents have been tested in preclinical and clinical studies,but have shown a lack of additive neuroprotection to therapeutic hypothermia (Zhou et al.,2020).Therefore,a better understanding of the mechanisms of injury that are not targeted by therapeutic hypothermia is needed,in order to find the appropriate add-on treatment that has complementary mechanisms of action.
Therapeutic hypothermia works through many mechanisms of action [as reviewed in Wassink et al.(2019)].Based on studies undertaken in fetal sheep,the window of opportunity for effective intervention with therapeutic hypothermia has been shown to be 6 hours after hypoxiaischemia,consistent with an acute sentinel event(Zhou et al.,2020).Based on these studies,the clinical guidelines state that treatment must be started within 6 hours of birth.However,for some babies with HIE,the actual therapeutic window of opportunity may be substantially shorter,as the hypoxic-ischemic insult could have evolved progressively over the course of labor,rather than having been an acute sentinel event,making these babies less likely to benefit from therapeutic hypothermia (Davidson et al.,2022).Therefore,to improve therapeutic hypothermia,the challenge is to find mechanisms of injury that are not completely attenuated by hypothermia.In particular,we must find the mechanisms of injury that may have a substantially longer therapeutic window of opportunity for treatment than hypothermia.Therefore,babies born with ischemic brain injury that has already evolved beyond the actual therapeutic window for hypothermia may still benefit from this potential additional/alternative treatment.
One such mechanism of injury that is a promising therapeutic target is chronic inflammation (Figure 1).The inflammatory response following hypoxicischemic brain injury is crucial for the clearance of cellular debris and the facilitation of tissue repair.However,increasing evidence has shown that chronic inflammation that persists even after treatment with therapeutic hypothermia is a likely contributor to neurodevelopmental deficits after HIE.Many clinical trials have shown that in babies and children who had HIE,higher levels of plasma cytokines are consistently associated with adverse outcomes (Foster-Barber et al.,2001;Zareen et al.,2020).A prospective study of 73 term infants who experienced perinatal asphyxia showed that higher levels of interleukin-1,interleukin-6,and tumor necrosis factor in heel-stick blood samples collected on the 1stor 2ndday from birth were associated with a higher risk of death or diagnosis with cerebral palsy at 1 year old (Foster-Barber et al.,2001).Interestingly,inflammation persists in children who had HIE,even at 4–7 years of age (including those treated with hypothermia),including elevated levels of plasma cytokines,such as granulocyte-macrophage colony-stimulating factor,tumor necrosis factor-β,interleukin-2,interleukin-6,and interleukin-8,compared with control children (Zareen et al.,2020).
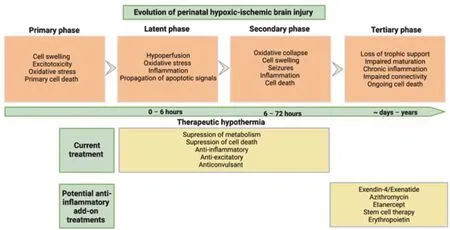
Figure 1|Schematic diagram showing the evolution of perinatal hypoxic ischemic brain injury.
Recently,we have shown that not only is there an increase in the number of microglia– resident immune cells in the brain but also an increase in the ratio of CD86 (classical activation “M1”marker) to CD206 (alternative activation “M2”marker) expression by microglia,in near-term fetal sheep at 7 days after global cerebral ischemia(Zhou et al.,2022).To our best knowledge,this was the first study to report microglial phenotypic markers in a large animal model of perinatal hypoxic-ischemic brain injury.These data indicate that there is a predominance of “pro-inflammatory microglia” during this tertiary phase of perinatal hypoxic-ischemic brain injury.Strikingly,treatment with therapeutic hypothermia in this paradigm only partially reduced this ratio of CD86 to CD206 expression in the parasagittal cortex,but not in the intragyral white matter of the parasagittal gyrus,highlighting that there are likely spatiotemporal differences in the inflammatory response (Zhou et al.,2022).Furthermore,higher numbers of microglia were correlated with worse electroencephalogram power recovery at 7 days after global cerebral ischemia,suggestive of a poorer recovery of brain activity (Zhou et al.,2022).Although electroencephalogram power was significantly higher in hypothermia treated fetuses compared to normothermic fetuses,it did not recover to sham control level (Zhou et al.,2022).This deficit could be due to a combination of mild residual neuronal loss and persistent microgliosis.
A study of postnatal day 9 mice showed a significant increase in the number of CD86 positive cells,and no difference in the number of CD206 positive cells in the hippocampus at 7 days after exposure to unilateral common carotid artery ligation and hypoxia,compared with sham operated animals (Seitz et al.,2021).In contrast to our study in fetal sheep (Zhou et al.,2022),hypothermia was associated with no significant difference in the CD86 positive cells and an increase in CD206 positive cells,compared with sham control animals (Seitz et al.,2021).However,there was no direct comparison for the expression of these markers in the hippocampus between the normothermia and hypothermia groups in this study.A key point of difference between these two studies is that hypothermia was started immediately after hypoxia-ischemia in the mouse study,whereas in the fetal sheep study,hypothermia was only started at 3 hours after the end of ischemia,to reflect a more clinically relevant delay.Furthermore,the duration of hypothermia was only 4 hours in the study of Seitz et al.(2021),compared to the clinical protocol of 72 hours used in the fetal sheep study.Additionally,the expression of CD86 and CD206 was assessed in the hippocampus in the neonatal mouse,whereas the areas assessed in fetal sheep were the cortex and the intragyral white matter of the parasagittal gyrus,suggesting there could be area-specific effects of hypothermia.
Interestingly,other studies have reported that there is a population of cells that express both CD86 and CD206 markers,highlighting the microglial M1/M2 classification likely oversimplifies the microglial responsesin vivo(Hellstrom et al.,2016;Seitz et al.,2021). Assessing a wider array of markers or the transcriptome of myeloid cells after hypoxia-ischemia with and without therapeutic hypothermia in future studies may better capture these complex changes.This approach will provide a more complete picture of the role of inflammatory response in ischemic brain injury and how it is modulated by therapeutic hypothermia.
Overall,these clinical and preclinical data show that there is chronic inflammation after perinatal hypoxic-ischemic brain injury,and highlights that there is potentially a long window of opportunity for targeting this neuroinflammation.These data support the hypothesis that modulating inflammation in addition to therapeutic hypothermia may augment neuroprotection following perinatal hypoxic-ischemic brain injury.However,it is important that the approach is not to achieve blanket suppression of inflammation,but specifically to target the deleterious effects of inflammation without suppressing the beneficial effects.For example,when microglia were depleted in a postnatal day 10 mouse model of hypoxia-ischemia,there was an exacerbation of brain injury,indicating that the net effect of the microglial response is beneficial (Tsuji et al.,2020).A potential approach may be to use drugs that can modulate the polarization of microglia from a more “M1” phenotype to an “M2” phenotype.
The timing of when to target inflammation is also a critical factor to consider.Wassink et al.(2021)recently investigated the effect of recombinant erythropoietin,which has anti-apoptotic and anti-excitotoxic effects,as an additive treatment to hypothermia.Treatment with both hypothermia and recombinant erythropoietin was started at 3 hours after the end of ischemia,and despite a trend toward a reduced number of microglia in the combined treatment group,combined treatment did not provide additive neuroprotection compared with hypothermia alone.Although microglial phenotypic markers were not assessed in this study,these data suggest that the widespread suppression of inflammation during the latent and secondary phase of injury,while treating with therapeutic hypothermia,may not enhance neuroprotection.
We propose that targeting chronic inflammation after the end of treatment with therapeutic hypothermia and the resolution of the secondary phase of injury may be associated with greater beneficial effects.Future studies should aim to better characterize the evolution of the inflammatory state in the brain and identify when the peak pro-inflammatory state occurs after hypoxia-ischemia,with and without treatment with therapeutic hypothermia.This would allow for more targeted treatment of the deleterious effects of inflammation,which may help to further reduce brain injury after hypoxia-ischemia in the perinatal brain.
In conclusion,increasing evidence from both clinical and preclinical studies has shown that chronic inflammation may be associated with ongoing deficits,despite treatment with hypothermia,suggesting that targeting inflammation may provide additive neuroprotection to hypothermia.The aim should be to attenuate the deleterious effects of inflammation,without compromising the beneficial effects.We suggest that it is plausible that this balance may most likely be achieved by targeting inflammation after treatment with therapeutic hypothermia.However,further studies are needed to better characterize the evolution of the inflammatory response after hypoxia-ischemia and to identify the peak of the pro-inflammatory response,which may be the optimal time for administering immunomodulatory therapies.
This work was supported by the Health Research Council of New Zealand (No.17/601 to JD) and the Marsden Fund (No.17-UOA232 to JD).
Kelly Q.Zhou,Joanne O.Davidson*
The Department of Physiology,The University of Auckland,Auckland,New Zealand
*Correspondence to:Joanne O.Davidson,PhD,joanne.davidson@auckland.ac.nz.
https://orcid.org/0000-0002-6781-4258(Joanne O.Davidson)
Date of submission:August 23,2022
Date of decision:September 23,2022
Date of acceptance:October 3,2022
Date of web publication:November 18,2022
https://doi.org/10.4103/1673-5374.360174
How to cite this article:Zhou KQ,Davidson JO(2023) Targeting neuroinflammation after therapeutic hypothermia for perinatal hypoxicischemic brain injury.Neural Regen Res 18(6):1261-1262.
Open access statement:This is an openaccess journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

