Putting PLX5622 into perspective:microglia in central nervous system viral infection
Alanna G.Spiteri,Nicholas J.C.King
Elucidating the exact contribution of microglia to central nervous system (CNS) pathology has historically been extremely challenging.These resident parenchymal myeloid cells are considered to have critical roles as frontline responders during pathogen invasion and CNS perturbation.Thus,understanding the precise temporal kinetics of microglial function is central to the evolution of novel therapeutics for disease intervention and/or resolution (Spiteri et al.,2022a).The development of PLX5622,a colonystimulating factor 1 receptor (CSF-1R) inhibitor typically formulated into a rodent chow for simple oral administration has facilitated exploration of microglial functions in disease (Spangenberg et al.,2019).This molecule is widely used as a microglia-depletion agent,with a 20-fold greater selectivity for CSF-1R than other kinases,greater CNS penetrance and better depletion efficacy than previously developed CSF-1R inhibitors like PLX3397.Moreover,PLX5622 is more effective than other microglia depletion systems,including those using clodronate depletion,or CD11b-HSVTK mice that express the herpes-simplex virus thymidine kinase under the CD11b-promoter(Heppner et al.,2005),or CX3CR1CreER: iDTR mice that express Cre-recombinase under the CX3CR1 promoter and crossed with iDTR animals (Bruttger et al.,2015).These approaches require intracranial injections,bone marrow (BM)-reconstitutions,or administration of ganciclovir or tamoxifen,all of which produce non-physiological effects that may confound data interpretation.However,while PLX5622 has undoubtedly enhanced our understanding of microglia biology,studies investigating the potential off-target or indirect effects of this molecule are few and have focused on circulating blood leukocytes or splenocytes.Moreover,these studies have been confined to major cell subsets due to the limited array of cytometric parameters used,and thus the identification of smaller,equally important immune subsets have not been reported.More recently,ex vivoanalysis showed impairment in macrophages and lymphoid subsets 3 weeks post-PLX5622 treatment (Lei et al.,2020).Elucidating the precise impact of this molecule on the periphery is important,particularly if PLX5622 is used to study the role of microglia in diseases where peripheral immune cells demonstrably contribute to disease resolution and/or progression,as seen in viral encephalitis.Indeed,modulation of peripheral immune cell compartments by indirect effects of PLX5622 substantially impeded dissection of the role of microglia in a murine model of West Nile virus (WNV) encephalitis (WNE) (Spiteri et al.,2022b;Figure 1).
In WNE,viral infection of CNS neurons results in a massive 10-fold increase in the number of leukocytes isolated from the brain,with the majority of the infiltrate being Ly6Chiinflammatory monocyte-derived cells (MCs) from the BM (Getts et al.,2008;Spiteri et al.,2021).Ly6ChiMCs are recruited to the CNS in a pathogenic manner,producing nitric oxide and inflammatory mediators that induce tissue damage and mortality.Interventions that inhibit this inflammatory infiltrate reduce the disease phenotype and increase survival (Getts et al.,2008;Spiteri et al.,2022b).Intriguingly,and in stark contrast to previously published studies investigating viral encephalitis (Waltl et al.,2018;Wheeler et al.,2018;Funk and Klein,2019– a full list of studies is reviewed in Spiteri et al.,2022a),PLX5622 treatment 21 days prior to WNV-infection and following this,for 7 days post infection (dpi),ameliorated the disease phenotype by significantly reducing neuroinflammation and CNS infiltration of recruited MCs,demonstrating the unexpected therapeutic potential of this agent in CNS viral infection (Spiteri et al.,2022b;Figure 1).
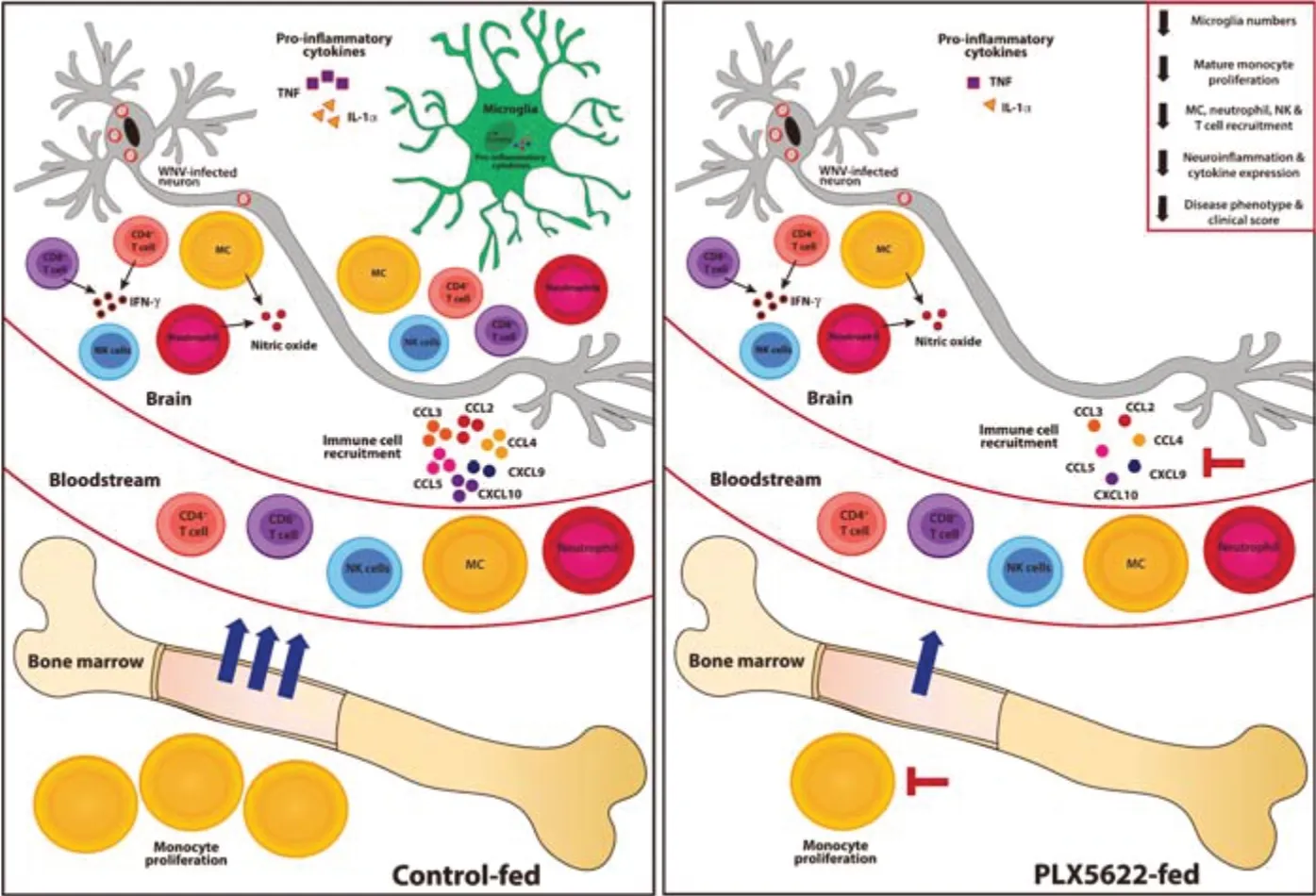
Figure 1|PLX5622 inhibits bone marrow monocyte production,reducing neuroinflammation and CNS infiltration following WNV infection.
Other studies using PLX5622 have suggested a neuroprotective role for microglia in CNS viral infection,with PLX5622-mediated microglial depletion enhancing mortality and/or morbidity(Waltl et al.,2018;Wheeler et al.,2018;Funk and Klein,2019– a full list of studies are reviewed in Spiteri et al.,2022a).Although they examined a broad range of viruses,these studies used nonlethal and/or less inflammatory murine models,which accordingly recruited a lesser CNS infiltrate from the periphery.The use of this limited set of experimental models of viral encephalitis has therefore resulted in a generalized view that this molecule is pathological in viral infection.In contrast,in severe virus-mediated neuroinflammation associated with substantial peripheral immune cell recruitment to the brain,PLX5622 clearly has a protective effect.This indicates a wider range of possible effects of this drug than previously appreciated and needs to be integrated into our thinking in the field.
This neuroprotective effect of PLX5622 during WNE was not explained by 1) repopulation of putatively protective microglia that can occur with the reduced chow consumption (and thus reduced PLX5622 intake) that accompanies increased disease severity,as 85% of microglia remained depleted,or 2) a reduction in viral load,as this was slightly increased in PLX5622-treated mice at 7 dpi.Remarkably,however,PLX5622 did not reduce the proportion of remaining microglia proliferating at 5 dpi,when microglial proliferation is maximal in undepleted,infected mice,uncovering a CSF-1Rindependent microglia proliferation mechanism during infection.
During WNV infection,mature BM monocyte numbers and proliferative status strongly correlate with CNS infiltration of inflammatory MCs and immunopathology.Thus,we examined changes in the peripheral myeloid compartment to elucidate the protective effect of PLX5622 in WNE.Detailed cytometric profiling demonstrated that,as well as depleting microglia,PLX5622 inhibits mature BM monocyte proliferation and production,which serendipitously reduced the massive infiltration of “emergency” MCs into the inflamed brain(Figure 1).This may not be surprising,as other cells besides microglia,including BM monocytes,express CSF-1R and rely on its signaling for survival and proliferation (Tushinski and Stanley,1983).The 70% reduction in recruited MCs significantly reduced neuroinflammation and ameliorated the disease phenotype following WNV infection(Figure 1).The reduction in disease score may also be due to the depletion of pro-inflammatory and/or chemokine-producing microglia,involved in immune cell recruitment.However,the impact of PLX5622 on the BM compartment also likely contributes to this.Thus,removal of PLX5622 from murine diets on the day of infection resulted in a significant rebound in BM monocyte proliferation by 7 dpi,enhancing CNS infiltration,neuroinflammation and disease scores.Moreover,reducing PLX5622 to a dose that does not deplete microglia still reduced BM monocyte proliferation and CNS infiltration.Finally,intraperitoneal injection of a monoclonal antibody targeting CSF-1R (i.e.,the same target as PLX5622) also reduced BM monopoiesis and MC recruitment without affecting microglial numbers,strongly supporting the peripheral action of PLX5622 on CSF-1R-expressing MCs.Antibody blockade however,did not alter disease scores,suggesting higher potency and therapeutic potential of PLX5622 over anti-CSF-1R antibody treatment.Importantly,intravenous dye administration used to track recently infiltrating cells into the CNS,showed that CSF-IR inhibition dose not affect MC trafficking from the blood,but rather reduces their production in the BM.Thus,administration of PLX5622,at doses that minimally deplete microglia,or modified to reduce blood brain barrier penetrance,highlights for the first time,the potential therapeutic value of PLX5622 in viral infection and monocyte-mediated diseases,particularly since it induces rapid and reversible microglia and monocyte modulation.However,the practical application of PLX5622 in the clinic is challenging and a better understanding of its effects on the CNS and periphery is still required.Nonetheless,this work also provides new insight into the importance of CSF-1-CSF-1R signaling in monopoiesis and the recruitment of inflammatory monocytes into inflamed tissues.
As well as reducing BM monopoiesis,PLX5622 also reduced the number and proliferative capacity of classical dendritic cells in mock-infected animals,as well as reducing plasmacytoid dendritic cell numbers in the infected BM.Other cell populations in the spleen and BM were unaffected in either mock-infected or WNV-infected mice at 7 dpi.This highlights the dependency of particular cell types on CSF-1R signaling for survival and proliferation,as well as advising judicious consideration of nonmicroglia-specific effects in interpreting data produced by PLX5622 treatment.
More sophisticated microglia depletion methods,such as the use of Csf1r∆FIRE/∆FIREmice,in which genomic depletion of the fms-intronic regulatory element in the Csf1r locus results in permanent depletion of microglia,could potentially address some of these issues.However,this approach also depletes a range of other tissue-resident macrophage populations besides microglia,as well as reducing the proliferative responses of extant BM macrophages (Rojo et al.,2019).This perturbation would,at the least,likely attenuate peripheral responses to severe inflammation in the CNS,like PLX5622,leaving accurate resolution of the roles of monocytes and microglia in WNE unresolved.
Our work has uncovered significantly broader effects of PLX5622 on the myeloid lineage than just microglia depletion,advocating caution in the interpretation of data as microglia-specific.Nevertheless,despite caveats,PLX5622 has undoubtably enhanced our understanding of microglia biology.As such,this simple,responsive,reversible,and temporally versatile drug will likely continue to be valuable to study the role of microglia in inflammatory diseasein vivo.Thus,in moving forward,we believe that data interpretation in future studies using PLX5622 should consider,1) non microglia-specific effects induced by PLX5622,2) non-physiological effects in compensating for microglia loss produced both by remaining microglia and other cells during PLX5622 treatment,3) the rapid repopulation of microglia with reduced chow consumption that accompanies increasing disease severity and 4)the use of additional corroborative experimental tools,includingin vivoimaging,fate-mapping,single-cell transcriptomic or cytometric analysis of non-treated mice to validate depletion data.
This work was supported by a grant from the Merridew Foundation and NH &MRC Project,No.1088242 (to NJCK).AGS was supported by the Australian Government Research Training Stipend Scholarship and The University of Sydney Postgraduate Merit Award.
Alanna G.Spiteri,Nicholas J.C.King*
Viral Immunopathology Laboratory,Infection,Immunity and Inflammation Research Theme,School of Medical Sciences,Faculty of Medicine and Health,The University of Sydney,Sydney,NSW,Australia (Spiteri AG,King NJC)
Sydney Cytometry,The University of Sydney and Centenary Institute,Sydney,NSW,Australia(King NJC)
Ramaciotti Facility for Human Systems Biology,The University of Sydney and Centenary Institute,Sydney,NSW,Australia (King NJC)
Charles Perkins Centre,The University of Sydney,Australia (Spiteri AG,King NJC)
The University of Sydney Institute for Infectious Diseases,The University of Sydney,Sydney,NSW,Australia (King NJC)The University of Sydney Nano Institute,the University of Sydney,Sydney,NSW,Australia(King NJC)
*Correspondence to:Nicholas J.C.King,MB,ChB,PhD,nicholas.king@sydney.edu.au.
https://orcid.org/0000-0002-3877-9772(Nicholas J.C.King)
Date of submission:July 25,2022
Date of decision:September 16,2022
Date of acceptance:October 9,2022
Date of web publication:November 18,2022
https://doi.org/10.4103/1673-5374.360170
How to cite this article:Spiteri AG,King NJC(2023) Putting PLX5622 into perspective: microglia in central nervous system viral infection.Neural Regen Res 18(6):1269-1270.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and buildupon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

