Cerium-modified copper/hexagonal mesoporous silica catalyst for efficient dimethyl oxalate hydrogenation to ethylene glycol under moderate reaction conditions
WU Di,ZHANG Juan,HUANG Zhi-jun,CHEN Jian-gang,*
(1.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry,Chinese Academy of Sciences, Taiyuan 030001, China;2.University of Chinese Academy of Sciences, Beijing 100049, China;3.State Key Laboratory of Biomass Thermal Chemistry Technology, Wuhan 430223, China)
Abstract: A highly efficient cerium-modified Cu/hexagonal mesoporous silica (xCe-Cu/HMS) catalyst for the vapor-phase hydrogenation of dimethyl oxalate (DMO) into ethylene glycol (EG) was prepared using an ammonia evaporation method.The Ce promoter can significantly improve the performance of the catalyst,and the best catalytic performance was obtained after the introduction of 1.2% Ce promoter on Cu/HMS.The DMO conversion and EG selectivity got to 99.6% and 96.3%,respectively,under moderate conditions (200 °C,2.0 MPa,H2/DMO=100 and LHSVDMO=1.2 h-1).Characterization results revealed that Ce modification can enhance the interaction between Cu and the support,improve the dispersion of Cu on HMS,and maintain the appropriate ratio of Cu+/(Cu++Cu0).In this study,a simple and low-cost method was used to synthesize Ce-modified Cu-HMS catalysts,which showed excellent catalytic performance in conversion of DMO to EG under moderate conditions.
Key words: Cu/HMS;cerium promoter;dimethyl oxalate;hydrogenation;ethylene glycol
Ethylene glycol (EG) is an important raw material that has been widely used in modern industries such as the synthesis of polyester resins and fibres.It is also regarded as an effective additive,solvent,surfactant or antifreeze,in several chemical processes[1].
EG has attracted much attention in terms of academic and industrial applications that makes the annual consumption of EG have exceeded 20 million tons[2].Commercial EG is mainly produced from fossil fuels (i.e.coal,oil and natural gas) and biomass resources through the technology of ethylene oxidative hydration[3].Due to the depletion of fossil fuels and the increasing demand for EG in China,it is imperative to find alternative synthetic routes for the preparation of EG using cheap raw materials[4].A promising method is conversion of syngas obtained from coal gasification to EG.
Copper-chromium catalyst was firstly used for DMO hydrogenation to EG in the early stage of industrial application because of its good catalytic activity and high durability[5].However,chromium is highly toxic and even small amounts of chromium (VI)pose a significant risk to the workers and environment.Thus,the research has been tuned to develop chromium-free industrial application catalysts.The Cu/SiO2catalyst is shown to have similar catalytic activity to Cu-Cr in DMO hydrogenation,but it is safer and cheaper[6,7].Nevertheless,DMO hydrogenation on Cu/SiO2always requires rigorous reaction conditions(i.e.,reaction temperature: 220 °C;pressure: 3.0 MPa etc.) to obtain high conversion,selectivity and long lifespan that leads to huge energy consumption[8].Therefore,design and development of highly-efficient and low-cost catalysts to achieve DMO vapor-phase hydrogenation to EG under moderate reaction conditions is still a challenge.
Several studies have been conducted to clarify the role of copper in DMO hydrogenation.The catalytic activity and stability of Cu-based catalysts in DMO hydrogenation are closely related to the synergistic effect between the Cu0and Cu+species that depends on the ratio of Cu0and Cu+[9,10].Cu-based catalysts show high activity in hydrogenation of esters to alcohols,because they can promote the hydrogenation of C-O bonds,but hinder the activation of C-C bonds[11].
The Cu nanoparticles easily migrate,agglomerate and grow during the high-temperature reactions,due to the low Hüttig temperature of metallic Cu (451 K)[12].Therefore,silica-supported Cu-based catalysts usually rapidly deactivated and have poor catalytic stability.Various methods have been attempted to improve the stability of Cu active sites.The introduction of a second metal promoter is regarded as an convenient and feasible method to control the electronic and geometric structures of the Cu/SiO2catalyst.Metals and metal oxides,such as Ni,Ag,Zn and La2O3,have been used to enhance the physical and chemical properties as well as the catalytic performance of Cu/SiO2catalysts[4,13-15].
Preparation methods of Cu/SiO2catalysts for DMO hydrogenation were also intensively explored.The ammonia evaporation (AE),deposition-precipitation and ion exchange are commonly used[16-18].We recently successfully prepared Cu/SiO2catalysts by a homogeneous deposition-precipitation ammonia evaporation (AE) method.Using AE method could enhance the metal-support interactions due to the formation of a copper phyllosilicate [Cu2Si2O5(OH)2]phase[1,19],being in line with the observation of Chen et al.[16],Ce was usually served as a catalyst promoter due to its excellent promoting effect on hydrogenation reactions.Chen et al.[20]investigated the effects of Ce promoter introduced at different stages of AE process on the catalytic performance of Cu/SiO2catalysts.They demonstrated that simultaneously adding Cu2+and Ce3+at the mixing stage results in forming robust Cu-Ce composite oxides,thereby enhancing the synergy between Cu0and Cu+and improving the catalytic performance of catalyst.Ye et al.[21]studied the Cu-CeO2-SiO2catalyst for hydrogenation of methyl acetate to ethanol and they found that adding appropriate amounts of Ce to Cu-SiO2catalysts strengthen the interaction between the Cu species and support,diminish Cu crystallite size,improv the Cu dispersion and increase the surface Cu+species.Ai et al.[9]reported that the addition of Ce promoter to Cu/SiO2catalysts significantly increases the Cu dispersion (DCu) and retards the sintering of small-sized Cu species.Although the DMO hydrogenation reaction has been extensively investigated,the EG selectivity and catalytic stability of Cu-based catalysts still need to be improved,especially at moderate reaction conditions to enhance their viability in commercial application.
Herein,a series ofxCe-Cu/HMS catalysts were prepared using AE method and their catalytic performance in DMO hydrogenation to EG was investigated.The catalyst electronic structure and property were evaluated using H2temperature programmed reduction,N2adsorption-desorption,N2O chemisorption,powder X-ray diffraction,transmission electron microscopy,X-ray photoelectron spectroscopy,temperature-programmed reduction and Fouriertransform infrared spectroscopy to unravel the structureperformance relationship of the bimetallic system.
1 Experimental
1.1 Catalyst preparation
HMS was prepared according to a wellestablished procedure delineated by Tanev and Pinnavaia[22].In this method,tetraethylorthosilicate(TEOS) and dodecylamine (DDA) were used as silica source and template agent,respectively.Typically,5.04 g of DDA was dissolved in 53.33 g of deionized H2O and 39.42 g of ethanol under vigorous stirring.Then,21.39 g of TEOS was dropwise added into the above solution.The mixture was then stirred at 40 °C for 30 min.The obtained gel was aged for 18 h at ambient temperature. After that,the resulting solid was recovered by filtration,washed with deionized water,and dried at 100 °C,followed by calcination at 650 °C for 3 h.
xCe-Cu/HMS catalysts with 20% copper loading were prepared by the ammonia evaporation method.In a typical procedure,5.07 g of Cu(NO3)2·3H2O and a certain amount of Ce(NO3)2·6H2O were dissolved in deionized water.Then,12.6 mL of 28% ammonia solution was added and stirred for 5 min at room temperature. A certain amount of HMS was subsequently added to the above solution and stirred for another 4 h.The initial pH of the suspension was 11.0-12.0.The suspension was then preheated at 90 °C to evaporate ammonia and decrease the pH and consequently deposit copper and cerium species on silica.When the pH value of the suspension decreased to 6.0-7.0,the evaporation process was terminated.The solid was washed with deionized water and dried at 120 °C for 12 h,followed by calcination at 450 °C for 4 h.
1.2 Catalyst characterization
The elemental content of the catalyst was measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES) on the USA Agilent ICPOES720 instrument.0.1 g as-calcinedxCe-Cu/HMS samples were dissolved in concentrated hydrochloric acid and hydrogen fluoride,and the mixture was diluted with water to meet the detection range of the instrument.
The BET surface area and pore size distribution were measured using N2adsorption-desorption at-196 °C.Prior to the experiments,the samples were degassed at 300 °C for 3 h.The isotherms were measured using a Micromeritics ASAP 2010 system.The total pore volume was calculated from the amount of vapor adsorbed at a relative pressure close to unity assuming that the pores are filled with the condensate in the liquid state.The pore size distribution curves were calculated from the desorption branches of the isotherms using the Barrett-Joyner-Halenda (BJH)formula.
The powder X-ray diffraction (XRD) patterns were collected on a DX-2700 with monochromatic CuKα radiation operating at 40 kV and 30 mA,a scanning angle (2θ) from 5° to 85° and a scanning speed of 2(°)/min.
X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy measurements were carried out on the Thermo Scientific ESCALAB 250Xi equipment,which was employed to determine the surface composition and valence state of catalysts.The as-calcined samples were pressed into a thin disk and reduced with pure H2at 350 °C for 3 h,the samples were transferred into the XPS analysis chamber.
Transmission electron microscopy (TEM) images of the catalysts were obtained using a JEM-2100F microscope operated at 200 kV.
Fourier transform infrared (FT-IR) spectroscopy was performed in transmission mode from 4000 to 500 cm-1using a Bruker Vector 22 spectrometer equipped with a DTGS detector and a KBr beam splitter.Then,the TPR experiments were performed as follows: the fresh catalysts were firstly placed in a quartz reactor and then reduced by a 5% H2/N2gas mixture with a flow rate of 50 mL/min,ramping at 5 °C/min to the final temperature.
The number of surface metallic copper sites was determined by dissociative N2O adsorption at 50 °C.Catalysts were firstly reduced by a 5% H2/N2gas mixture with a flow rate of 50 mL/min,ramping at 5 °C/min to 350 °C for 10 min.Then,the sample was purged with Ar and cooled to 50 °C.Surface copper atoms were oxidized in a N2O (50 mL/min) at 50 °C for 0.5 h.Finally,the samples were flushed with 5% H2/N2to remove the oxidant for 1 h and then to start another TPR run.
1.3 Activity measurements
The catalytic activities for the vapor-phase hydrogenation of DMO was tested in a fix-bed stainless steel tubular reactor.The H2flow was controlled by a Brooks 5850E mass flow controller (MFC).The pressure was maintained at 2.0 MPa via a backpressure regulator,during the whole evaluation period.Afterin situreduction at 350 °C for 5 h with a H2flow rate of 100 mL/min,the catalyst bed was cooled to room temperature.Then,the fixed bed reactor was heated to 200 °C in steps of 2 °C/min.The liquid reactant is injected by the advection pump to adjust the liquid hourly space velocity (LHSV).After evaporation by preheater,the steam is mixed with H2.The products were collected in a cooled cold trap at 5 °C and analyzed by gas chromatography (Shimadzu GC-14C)using a capillary column (19,091n-213,HPINNOWAX,0.25 mm × 30 m) with a flame ionization detector (FID) and a packed column (TDX-101) with a thermal conductivity detector (TCD).
2 Results and discussion
2.1 Structural and textural properties of the xCe-Cu/HMS catalysts
The chemical compositions and structural properties ofxCe-Cu/HMS catalysts (xis the content of Ce promoter) are summarised in Table 1.The N2adsorption/desorption isotherm and corresponding pore size distribution are shown in Figure 1.AllxCe-Cu/HMS samples exhibited Langmuir type IV isotherms with an H1-type hysteresis loop,which corresponds to a typical large-pore mesoporous material with one-dimensional cylindrical channels.A capillary condensation of nitrogen with uniform mesopores existed in all samples,causing a sudden steep increase in the nitrogen uptake within a characteristic relative pressure (p/p0) range of 0.3-0.5.This suggests that all samples had a typical mesoporous structure with uniform pore diameters.Since HMS exhibits a uniform hexagonal mesopore array connected by smaller micropores,the wide hysteresis loop in the isotherms of all Cu-doped samples indicates long mesopores,which limits the emptying and filling of the accessible volumes[23].A slightly decrease in BET surface area was observed for all Cu-containing samples after ammonia evaporation.An evident effect on both pore volume and pore diameter is detected upon incorporating the copper and cerium species onto the silica substrate.It was found a redissolution and reprecipitation of the silica structure during the preparation process,along with the decrease of BET surface area and mesopore volume from 1117 to 299 m2/g and from 0.98 to 0.41 cm3/g,respectively,with the elevation of average pore diameter from 2.68 to 5.58 nm[24].In the process of catalyst preparation by AE method,the copper precursor (copper tetraammine complex,[Cu(NH3)4]2+) accessed the pores of the support and reacted with mesoporous surface silica to form copper phyllosilicate,which leads to the destruction of some mesopores.Chen et al.[16]revealed that SiO2was immersed in the [Cu(NH3)4]2+solution at the pH value of 11.0-12.0 at room temperature until adsorption and ion-exchange reached a dynamic equilibrium. It is generally accepted that there are two prerequisites for the formation of metal phyllosilicate: first,the presence of OH bridging ligands in the neutral metal complex and second,the generation of silicic acid by dissolving silica particles in a basic solution[25].Zhao et al.[24]reported that the mesoporous structure of the support can be maintained in the aging stage,but it was destroyed in the evaporation stage. The presence of mesoporous structure ensured the uniform distribution of the copper precursor in the channels.Thus,the mesoporous structure is necessary for improving the dispersion and surface area of copper species in the aging stage.In addition,the average pore diameter increases with the increase of Ce loading.This indicated the expansion of some mesopores or the formation of accumulated pores by the collapse and reorganization of some mesopores.These changes resulted in the generation of more copper phyllosilicate in the catalysts with the increase of Ce loading[24].
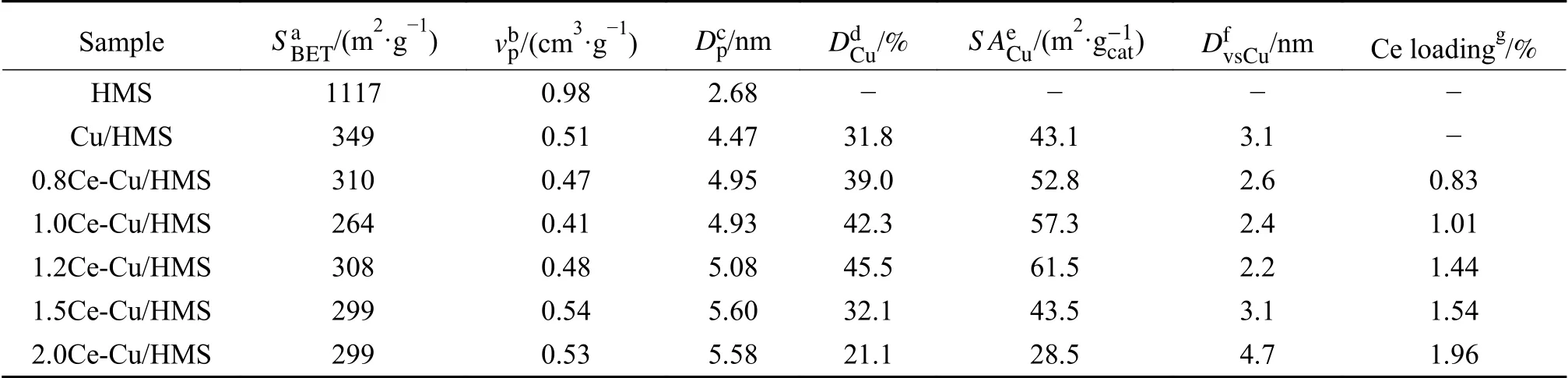
Table 1 Structural properties and chemical compositions of synthesized catalysts
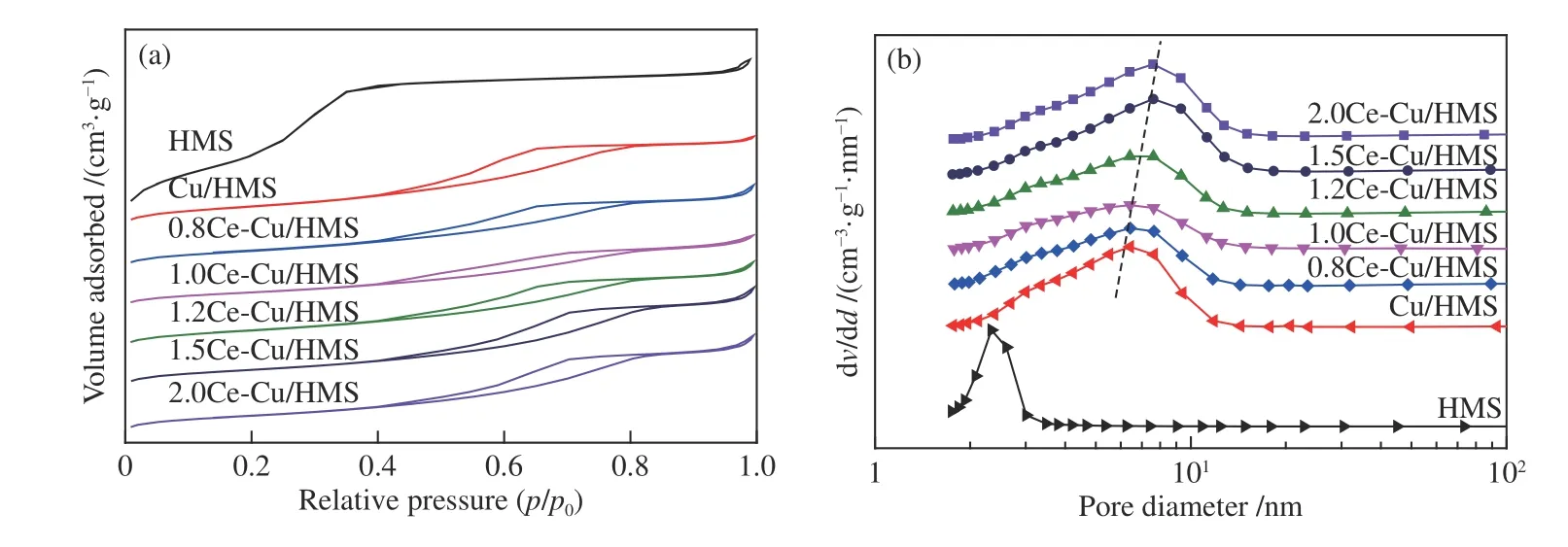
Figure 1 Nitrogen adsorption and desorption isotherms and pore size distribution of the catalysts
DCuand Cu surface area (SACu) are the main factors determining the catalytic activity of Cu/SiO2catalysts in ester hydrogenation reactions.The results of Table 1 indicates thatDCuandSACuare relatively improved with the introduction of Ce promoter in Cu/HMS catalysts.The 1.2Ce-Cu/HMS exhibits the highestDCuandSACu.However,further increase of the Ce contents leads to a gradual decrease inDCuandSACu.Notably,the copper average volume-surface area diameter (dvsCu) gradually decreased from 3.1 nm for Cu/HMS to 2.2 nm for 1.2Ce-Cu/HMS,and then inversely increase to 4.7 nm for 2.0Ce-Cu/HMS.These results suggested that doping Ce promoter could decrease the copper particle sizes and improve theDCu.The 1.2Ce-Cu/HMS catalyst exhibited the smallest particle size and maximum dispersion,which could be attributed to the most suitable interaction between Cu and Ce species.On the contrary,addition of too much or too few Ce species leads to the growth of copper particles and the decrease of dispersion.In summary,the textural structure of the catalysts was simultaneously determined by the copper and cerium species.
XRD characterisations were performed on the catalysts to understand the nature of the copper and cerium species after calcination at 450 °C for 4 h under an air atmosphere.Figure 2(a) shows the XRD patterns of the calcinedxCe-Cu/HMS catalysts.No significant diffraction peaks corresponding to copper and cerium species could be observed,which indicates the high dispersion of copper and cerium species prepared by AE method.The broad diffraction peak at 2θof around 21.7° can be attributed to amorphous silica[26].Figure 2(b)shows the XRD patterns of the Cu/HMS and 1.2Ce-Cu/HMS catalysts after the hydrogenation reaction.Although the sharp diffraction peaks ascribable to Cu0(JCPDS 65-9743) and Cu2O (JCPDS 05-0667) are detected on both of Cu/HMS and 1.2Ce-Cu/HMS catalysts after the reaction,the peak intensity of these Cu species are quite different in the two samples.The agglomeration and growth of copper particles are more evident on Cu/HMS than on 1.2Ce-Cu/HMS.
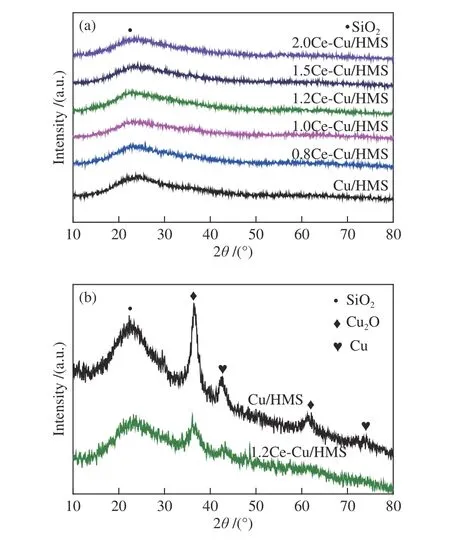
Figure 2 X-ray diffraction patterns of the (a) fresh catalysts and (b) used Cu/HMS and 1.2Ce-Cu/HMS catalysts
To further observe the surface morphology of the catalysts,the fresh and used Cu/HMS and 1.2Ce-Cu/HMS catalysts were characterized by TEM,and the relevant results were shown in Figure 3.The mean size of Cu particles over Cu/HMS catalyst was about 3.6 nm based on the statistical results of TEM images.However,after adding an appropriate amount of Ce promoter,the metallic Cu particles of 1.2Ce-Cu/HMS catalyst were more uniformly dispersed without obvious agglomeration,and the average diameter was about 2.4 nm.After reaction,the Cu particles evidently agglomerated and grew up to 5.9 nm on the surface of Cu/HMS catalyst,whereas for 1.2Ce-Cu/HMS catalyst,the size of copper particles was only slightly increased to 3.25 nm under the identical condition.
The H2temperature-programmed reduction is a useful technique to investigate the chemical properties,reducibility and metal-support interactions of metal oxide catalysts.As shown in Figure 4,the unmodified Cu/HMS catalyst exhibited an H2consumption peak at 290 °C.After doping with the cerium promoter,the H2consumption peak of thexCe-Cu/HMS catalysts gradually shifted into higher temperature.These results could be attributed to the intense interaction between the support,cerium oxide and copper oxide.Additionally,a small shoulder peak appeared near the main reduction peak onxCe-Cu/HMS catalysts and it may be due to the formation of a new copper species(copper phyllosilicate)[26].
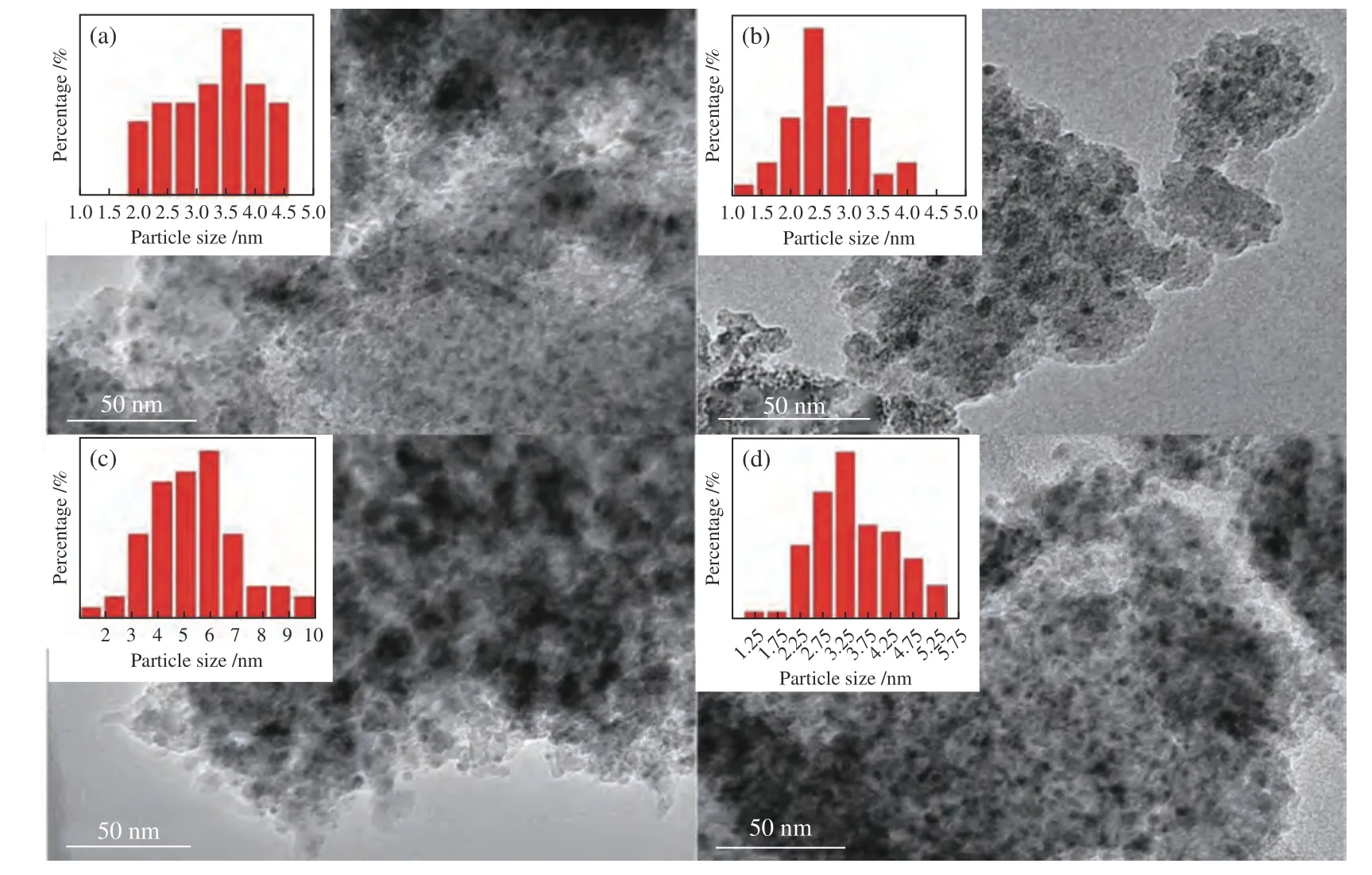
Figure 3 TEM images with the corresponding Cu particles size distribution diagrams of Cu/HMS(a): fresh;(b): used and 1.2Ce-Cu/HMS;(c): fresh;(d): used
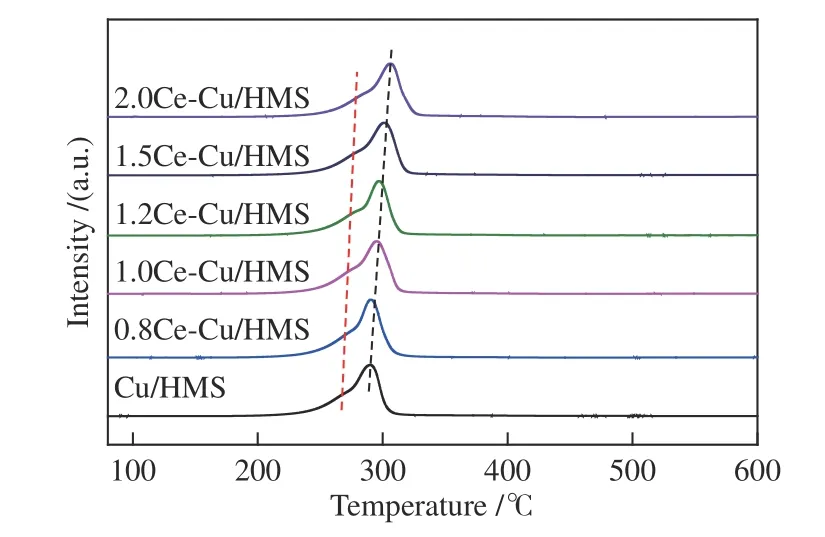
Figure 4 H2-TPR profiles of the calcined catalysts
Cu/SiO2catalysts prepared using different methods show different reduction behaviours.The reduction temperatures of catalysts prepared using precipitation-deposition method,salicylic acid complexation method and urea-assisted gelation method are 208,249 and 266 °C,respectively[2,15,27].Among them,using AE method can increase the interaction between the copper species and support by promoting the formation of more Cu-O-Si units and copper-phyllosilicate phase.Therefore,the main phase structure of copper species inxCe-Cu/HMS catalysts contains small CuO particles and some copper phyllosilicates.Cerium oxide has weakly basic,and hence,it can interact with SiO2support[28].Additionally,cerium also interacts with copper.Therefore,cerium additives enhance the interaction between the copper species and support.Combined with the XRD,it can be concluded that the highly dispersed CuO and copper phyllosilicate existed inxCe-Cu/HMS catalysts prepared using AE method.
2.2 Surface species and reducibility properties
The surface properties of the prepared coppercontaining HMS catalysts with different cerium loadings and copper supported on HMS catalysts were investigated using FT-IR adsorption analysis measured in a spectral range of 4000-400 cm-1(Figure 5).FT-IR is the most accurate and widely used characterisation technology to distinguish the copper phyllosilicate phase and evaluate the amount of copper phyllosilicate in the catalysts[29].A broad absorption peak was observed at 3430 cm-1,corresponding to the stretching vibration of the silanol and -OH groups in the support,and its width reflects the strength of the hydrogen bonding between the-OH groups.The adsorption bands of asymmetric and symmetric stretching vibrations of Si-O-Si appear at 1110 and 800 cm-1,respectively,in amorphous SiO2.The presence of theδOHband at 670 cm-1andυSiOband at 1040 cm-1on the low-frequency side of the νSiOasymmetric stretching band of SiO2at 1110 cm-1suggest the presence of the copper-phyllosilicate phase in the catalysts (Figure 5)[30].The overlap of the υSiOband of the copper phyllosilicate at 1040 cm-1and the υSiOband of the silica at 1110 cm-1enables a rough estimation of the relative content of the copper phyllosilicate using the intensity ratio of theδOHband at 670 cm-1to the υSiOband at 800 cm-1(I670/I800)[15,31].Note that the ratio ofI670/I800could only give a qualitative estimation of the amount of copper phyllosilicate because the extinction coefficients of the corresponding infrared bands are not known.Chen et al.[16]reported that the relative content of copper phyllosilicate in the calcined Cu/SiO2catalysts gets to the maximum value,when the AE temperature is 363 K.Compared to other catalysts,1.2Ce-Cu/HMS catalyst exhibits the largest value ofI670/I800(0.1084),which indicates the presence of more hydroxyl groups because of the formation of copper phyllosilicate.The calculation results are presented in the inset of Figure 5.The FT-IR results indicate that introducing Ce species into Cu/HMS catalysts significantly promotes the formation of the copper phyllosilicate phase.The mesoporous structure of HMS should play an important role in their formation,which will be further confirmed in the discussion section.
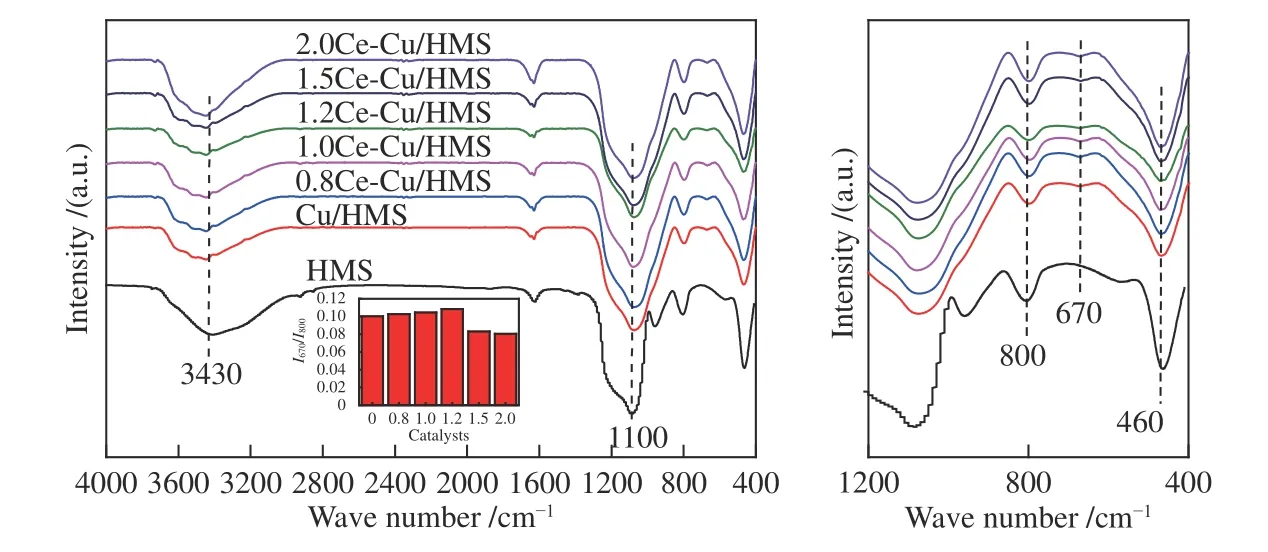
Figure 5 FT-IR spectra of the calcined catalysts
2.3 Surface composition and chemical state
The surface composition and chemical state of various catalysts were investigated using XPS.Figure 6 shows the Cu 2pXPS spectra of the fresh catalysts.Typically,the Cu 2p3/2binding energy (BE) of CuO is observed at 933.5 eV[32].The BE of the supported copper phyllosilicate is observed at 934.9 eV[6].The results of Cu XPS indicates the presence of copper as Cu2+in all samples.This was identified by the Cu 2p3/2peak at 933-936 eV and the 2pto 3dsatellite at 942-945 eV[33].Cu LMM X-ray Auger electron spectroscopy (XAES) is an efficient method to determine the minimal binding energies between Cu0and Cu+.Before the XPS measurements,all catalysts were activated using H2at 350 °C for 4 h.As shown in Figure 7,a broad and asymmetric peak is observed on the reduced Cu/HMS andxCe-Cu/HMS catalysts,which indicates the simultaneous existence of Cu0and Cu+species on these catalysts. The broad and asymmetric peak shape of Cu LMM XAES spectra indicated that chemical state of Cu species is complex in reduced Cu/HMS andxCe-Cu/HMS catalysts.The surface distribution of Cu+and Cu0was determined through the deconvolution of the Auger kinetic energy peak into two symmetric peaks at 913.3 and 916.2 eV,respectively[9].The synergistic effect between Cu+and Cu0on the surface of copper-based catalysts in the hydrogenation of DMO has been reported.In detail,the metallic copper species act as active sites for dissociating H2,while the Cu+species are electrophilic or Lewis acidic sites to polarise DMO molecules.Based on the deconvolution results,the Cu+/(Cu0+Cu+)intensity ratios are 32.83%,34.86%,45.68%,48.03%,41.05% and 31.82% for the samples containing Ce content of 0 (no Ce),0.8%,1.0%,1.2%,1.5% and 2.0%,respectively.The Cu+/(Cu0+Cu+) ratio firstly increased until the cerium content reached 1.2%,and then decreased with the increase in the cerium content.The Cu+/(Cu0+Cu+) ratio exhibited the same order as that of SCu,suggesting that the copper phyllosilicate content affects the ratio of Cu+to Cu0.
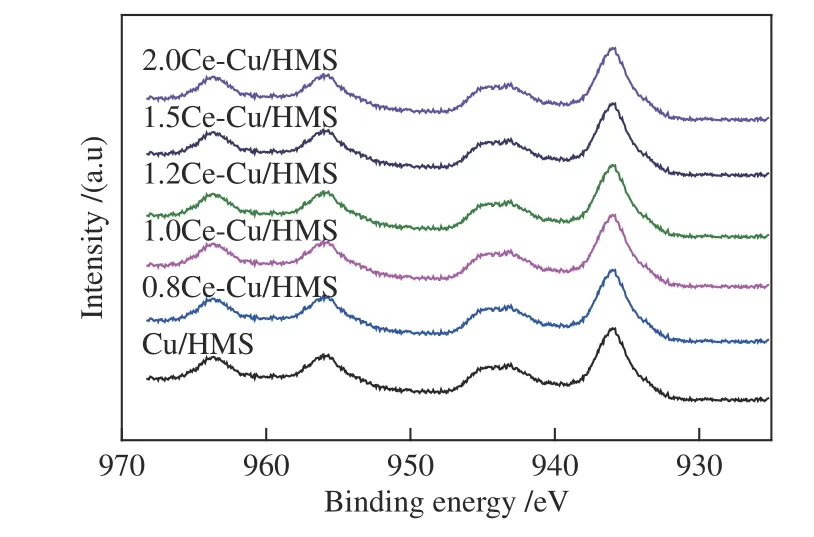
Figure 6 XPS spectra of the fresh catalysts

Figure 7 Cu LMM XAES spectra of the reduced catalysts(a) Cu/HMS,(b) 0.8Ce-Cu/HMS,(c) 1.0Ce-Cu/HMS,(d) 1.2Ce-Cu/HMS,(e) 1.5Ce-Cu/HMS and (f) 2.0Ce-Cu/HMS
2.4 Catalytic evaluation
Hydrogenation of DMO to EG [with methyl glycolate (MG) as a by-product] is a two-step continuous hydrogenation process. From a thermodynamic viewpoint,this reaction tends to generate EG because the equilibrium constant of the second step is much higher than that of the first step.Therefore,high EG selectivity can be obtained at high hydrogenation conversion rates.However,DMO hydrogenation is a rather complicated reaction.Since highly-efficient hydrogenation of DMO to EG at low energy consumption and moderate operation conditions is extremely desirable,a vapourphase hydrogenation of DMO to EG was performed over the developedxCe-Cu/HMS catalysts.Figure 8 shows the DMO conversion and product distribution over the catalysts under identical conditions of 200 °C;2.0 MPa;H2/DMO=100.Under optimum reaction conditions,the conversion of DMO firstly increased with the increase of Ce loading from 0.5% to 1.2%,and then dropped with a further increase of the Ce loading from 1.2% to 2.0%.The 1.2Ce-Cu/HMS catalyst exhibits a DMO conversion of higher than 99.6%,which is the highest value reported on Cu catalysts.1.2Ce-Cu/HMS sample also exhibits the highest EG selectivity of 96.3% with an LHSVDMOof 1.2 h-1.The main by-product in this process is MG,along with formation of tiny 1,2-butanediol and 1,2-propylene glycol.The selectivity of ethanol and 1,2-butanediol is very low,due to the lack of acidic and basic sites on SiO2.A similar trend is also observed in the activities of other Ce-containing catalysts.Adding more or less of Ce promoter results in a decrease in the EG selectivity,indicating that Cu/HMS containing 1.2% Ce is superior to other samples in DMO hydrogenation to EG.
The reaction performance of 1.2Ce-Cu/HMS catalyst in DMO hydrogenation reaction was further carried out under moderate conditions (200 °C,2.0 MPa,H2/DMO=100 and LHSVDMO=1.2 h-1) within 120 h(Figure 8).The DMO conversion and EG selectivity of 1.2Ce-Cu/HMS were nearly unchanged during entire period.Thus,Cu/HMS catalyst modified by appropriate amount of Ce shows good catalytic stability with high DMO conversion and EG selectivity within 120 h.The excellent catalytic performance of the optimised Ce-Cu/HMS catalyst confirms its potential for industrial applications.
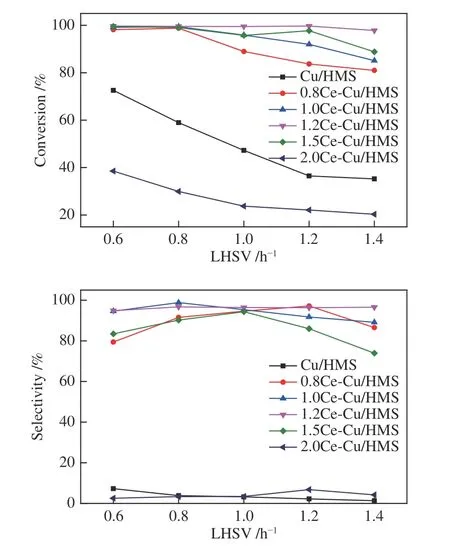
Figure 8 Catalytic performance of the xCe-Cu/HMS catalysts in DMO hydrogenation reaction 200 °C,2.0 MPa and H2/DMO=100
In order to understand more about the advantages of our preparedxCe-Cu/HMS catalysts,Table 2 shows a comparison of the performance of several reported catalysts for DMO hydrogenation reaction.It can be seen that most of the catalysts require harsh reaction conditions.In contrast,1.2Ce-Cu/HMS catalyst shows high DMO conversion and EG selectivity under more mild reaction conditions.
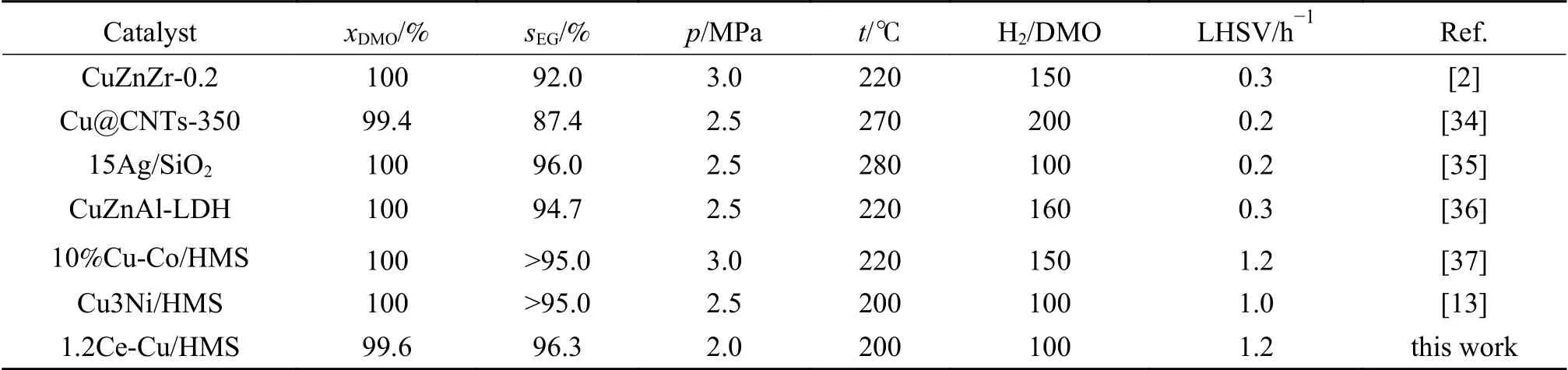
Table 2 Catalytic performance of several catalysts under different reaction conditions
2.5 Discussion
In this work,the structure of all catalysts was destroyed during the preparation process.As shown in Table 1,the specific surface area of the catalysts considerably decreased,whereas the pore volume and average pore size are inversely increased.Therefore,the copper precursors interacted with the support during the ammonia evaporation process,resulting in the destruction of catalyst structure and the formation of copper phyllosilicate.It is possible that there is a competitive adsorption between Cu2+and Ce3+that makes the copper precursors uniformly disperse in the channels of support[9,20].Simultaneously,theDCuandSACuobtained by N2O titration are crucial factors affecting the catalytic activity and stability.The highestDCuandSACuwere acquired on the 1.2Ce-Cu/HMS,thereby exposing more active sites.The addition of a small amount of cerium improves the dispersion of copper and facilitates the formation of Cu nanoparticles with smaller size.When cerium loading exceeded 1.2%,the surface of the copper species is covered by cerium species,resulting in the decrease of dispersion.The smaller Cu nanoparticles have stronger interact with the support,which,hence,forming more copper phyllosilicate during the ammonia evaporation process.Furthermore,the catalyst with a cerium content of 1.2% owns the highest content of copper phyllosilicate.The strong metal-support interaction could inhibit the migration of Cu species and depress the agglomeration of Cu nanoparticles.The presence of Ce is benefit to stable the Cu nanoparticles through suppressing the further reduction of Cu+.The 1.2Ce-Cu/HMS catalyst with mesoporous structure could maintain the high dispersion of small sized Cu nanoparticles,which is essential for improving the catalytic performance under moderate conditions.Considering the interaction between Cu species and Ce promoter,we speculate that the stabilizing effect of Ce promoter on the valence state of Cu species may contribute to enhance the catalytic stability of 1.2Ce-Cu/HMS catalyst.After the hydrogenation reaction,the copper particles of 1.2Ce-Cu/HMS are smaller than that of Cu/HMS.In conclusion,the main reason of Cu/HMS catalysts deactivation could be attributed to the aggregation and growth of copper particles.Doping a proper amount of Ce species into the Cu/HMS catalysts could not only promote the dispersion of Cu particles,but also inhibit the aggregation of these Cu species.
The synergistic mechanism of Cu0and Cu+has been demonstrated by the kinetic research of DMO hydrogenation[38].Since Cu+could not be detected on the sample prepared by impregnation of Cu on silica,the Cu+should be mainly derived from copper phyllosilicate,rather than from CuO[25].The Cu LMM XAES characterisation indicated that the proportion of Cu+species gradually increased with the increase of cerium loading from 0 to 1.2%,and then decreased with further increasing the cerium loading.Moreover,the DMO conversion and EG selectivity exhibited a similar trend to that of the cerium loading.A higher DMO conversion and glycol selectivity might originate from the synergistic effect between Cu0and Cu+.
3 Conclusions
This work proposed a simple and low-cost AE method to prepare Cu-based catalyst with excellent catalytic activity and stability for hydrogenation of DMO to EG under moderate reaction conditions.The FT-IR spectra results proved that introducing proper amount of Ce can make the copper precursor well distribute in the mesoporous silica,which,hence,leading to the formation of more copper phyllosilicate through interacting with the silica during the evaporation stage.In addition,the content of copper phyllosilicate has a positive effect on the ratio of Cu+/(Cu0+Cu+),and the Cu LMM XAES indicates that Cu/HMS with Ce loading of 1.2% has the highest copper phyllosilicate content and the highest ratio of Cu+/(Cu0+Cu+).Thus,the highest DMO conversion and high EG selectivity were achieved on 1.2Ce-Cu/HMS catalyst. Meanwhile,addition of cerium promoter can improve the copper dispersion and copper surface areas,and thus,leading to a high activity and stability of the catalyst.In a word,the catalyst with 1.2% Ce loading showed an outstanding EG selectivity of 96.3% under moderate reaction conditions,as it has higher copper dispersion,larger copper surface areas and proper ratio of Cu+/(Cu0+Cu+).This study also clarifies the positive effect of Ce promoter on the structure and performance of Cu/HMS catalyst in DMO hydrogenation to EG.Therefore,the insights shown in this work provide support and direction for the industrial production of EG by DMO hydrogenation.

