Nanosized amorphous nickel-boron alloy electrocatalysts for hydrogen evolution reaction under alkaline conditions
WU Mei-xia,CHEN Yan,LI Sen,YANG Xiao-meng,LI Jing-wei,SHANG Jian-peng,*,GUO Yong,LI Zuo-peng,*
(1.School of Chemistry and Chemical Engineering, Shanxi Datong University, Datong 037009, China;2.718 Research Institute of CSIC, Handan 056027, China)
Abstract: Hydrogen production from electrolyzed water driven by sustainable energy is an effective way to achieve the hydrogen economy with zero carbon emission.Alkaline electrocatalytic hydrogen evolution reaction (HER) is one of the main energy transforming processes in the field of electrolytic water technology.The development of active and cost-effective nonprecious catalytic electrodes is of great importance to alkaline hydrogen evolution reaction.Amorphous nanosized nickelboron alloy particles (NiB-COS) have been obtained by using chitosan oligosaccharides (COS) as a stabilizer via chemical reduction method.The as-prepared electrocatalysts have been used for the hydrogen evolution reaction (HER).The electrocatalysts have been characterized by using X-ray diffraction (XRD),transmission electron microscopy (TEM),inductively coupled plasma analysis (ICP) and X-ray photoelectron spectroscopy (XPS).NiB-COS displays a significant increase in hydrogen evolution reaction properties in alkaline media,affording low overpotentials of 49.4 mV at 10 mA/cm2 and 15.1 mV onset overpotential for the hydrogen evolution reaction.Tafel slope of NiB-COS is 86.1 mV/dec.Remarkably,the formation of a nickel-boron alloyed phase and the decrease of particle size are helpful to improve HER activity of NiBCOS.The experimental data indicated that the NiB-COS showed excellent long-term stability as a very active electrocatalyst.
Key words: hydrogen evolution reaction;NiB-COS;overpotential;Tafel slope;alkaline water electrolysis
Energy is a necessity for the economic and social development of the world.However,about 65 percent of the global energy demand is fulfilled by nonrenewable fossil fuels[1-3].Hydrogen,as a clean,efficient and zero carbon energy carrier,has been recognized as one of the new commanding heights of technology and industrial competition in the future.Hydrogen production by water electrolysis is a necessary way to realize zero carbon hydrogen emission economy.Alkaline water electrolysis is the most mature and economical technology among various water decomposition technologies,which has been commercialized for more than 100 years[4-6].Electrocatalytic hydrogen evolution reaction (HER) in alkaline environments is one of the effective means to accomplish the conversion,storage and transportation of clean electric energy.
In this paper,we report the synthesis and spontaneous assembly of NiB-COS nanoparticles into uniform aggregates using chitosan oligosaccharides as a protective agent. These nano aggregates are potentially interesting for the electrochemical reduction of water. The experimental results show that amorphous NiB-COS shows superior electrocatalytic performance for HER,and it is even better than that of Pt catalyst under some conditions.Simple preparation of amorphous NiB-COS alloy nanoparticles and their high activity in alkaline conditions provide advantages for their application in feasible and economical electrochemical hydrogen production systems.
1 Experimental
1.1 Preparation of catalysts
All the reagents were of analytical grade and used without further purification.NiB-COS catalyst was prepared by the chemical reduction method.In a typical synthesis of the catalyst,a certain amount of chitosan oligosaccharides (COS) was dissolved in deionized water,then NiCl2·6H2O aqueous solution was added to the above mixture with stirring.Afterward,an aqueous solution of KBH4was added dropwise to the above aqueous solution under vigorous stirring in argon atmosphere.The molar ratio of Ni∶B was 1∶2,and the reaction temperature was 20 °C.The reaction lasted until no bubbles were observed.The mixture was separated by suction filtration and the resulting NiBCOS catalyst was washed with distilled water and absolute alcohol,respectively,and stored in absolute alcohol before use.For comparison,NiB catalyst were prepared by chemical reduction without chitosan oligosaccharides. In addition,NiB-COS/NiB and NiB/NiB composite catalysts are obtained by loading NiB-COS and NiB on NiB using the impregnation reduction method,respectively.
1.2 Catalyst characterization
X-ray powder diffraction (XRD) patterns of samples were tested by a Rigaku D/max-2500 diffractometer using a CuKα radiation.The chemical composition of sample was determined by inductively coupled plasma (ICP) analysis using an IRIS Advantage spectrometer.The surface area was obtained by the BET method via N2adsorption at 373 K on an automatic surface area and pore size analyzer(Micromeritics ASAP 2010).X-ray photoelectron spectroscopy (XPS) was analyzed by a Kratos AXIS Ultra DLD spectrometer with AlKα radiation (Ar ion gun etching,10 min).The transmission electron microscope (TEM) was performed by a Philips FEI Tecnai 20 device system.Samples for TEM were supported on a copper grid with carbon film by dipping the grid in the sample suspension.
1.3 Electrochemical measurements
5 mg of amorphous alloy NiB-COS catalysts were dispersed in 625 μL deionized water,then 125 μL Nafion D-520 (5% in water and 1-propanol) was added into the above mixture in a 5 mL centrifuge tube.The centrifuge tube was sonicated for 60 min at room temperature.Next,10 μL suspension was dropped onto a 3 mm diameter glassy carbon electrode (GC 0.07 cm2).Finally,the electrode was dried under a 300 W infrared lamp for at least 0.5 h.The hydrogen evolution reaction performance measurement was carried out on an electrochemical workstation (CHI760E) using linear sweep voltammetry (LSV) in 1 mol/L NaOH solution saturated with N2.In order to avoid the influence of temperature,a circulating water bath was used to control the temperature of the electrolytic cell at room temperature.A standard three-electrode system using GC as working electrode was employed.A Pt wire and a Hg/HgO electrode filled with 1 mol/L NaOH electrolyte were served as counter electrode and reference electrode respectively.The potentials in our previous work[27]were calibrated by the Nernst equation:E(RHE)=E(Hg/HgO)+0.098+0.059pH.The durability of the catalysts was examined by scanning 500 cycles rate at 50 mV/s.
2 Results and discussion
The phase purity and crystallinity of the asprepared bulk NiB and NiB-COS catalysts were examined by XRD patterns,and the results are shown in Figure 1.As a reference,the XRD patterns of pure chitosan oligosaccharides was also indicated in Figure 1(a).It should be noted that diffraction peaks of chitosan oligosaccharides were observed at 2θ=10.4°and 19.9°.As shown in Figure 1b and 1c,the XRD patterns of the samples both exhibited a broad diffraction peaks at 45° without containing distinct peak corresponding to a crystalline phase,which are matched well with the typical diffraction peaks of the amorphous nickel-metalloid alloy[28]. This is a confirmation of single phase formation of NiB-COS.There are obvious differences between the peak intensities of the two samples,indicating that the disorder degree of NiB-COS powder is more serious than that of NiB powder.The dispersion peak of amorphous boron oxide appeared near 22° in the XRD patterns of each NiB amorphous alloys sample,which were considered in the literature to be inevitable during the reduction process in aqueous solution.These results suggest that the introduction of chitosan oligosaccharides couldn’t change amorphous structure of NiB particles.
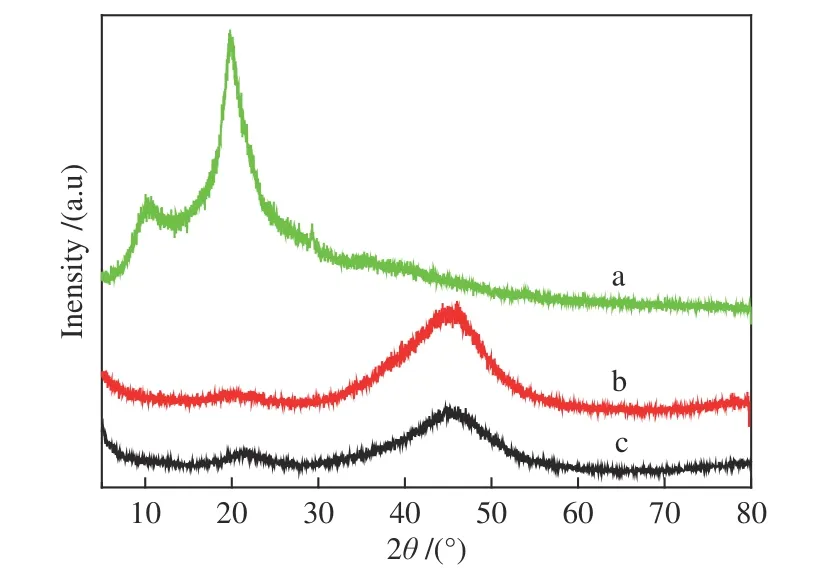
Figure 1 XRD patterns of chitosan oligosaccharides(a),NiB-COS (b) and NiB (c)
The morphology,dispersion,and particle size of NiB particles of the as-prepared catalysts were firstly characterized by TEM measurements and the representative images are shown in Figure 2.As can be seen from Figure 2,the NiB particles (Figure 2(a))have an approximately spherical morphology,and their average particle size is about 20 nm.The nanoparticles are not highly dispersed and tend to be interconnected to reduce the surface energy.The reason is that the surface energy of nanoparticles increases with the decrease of particle size,which is the characteristic of nanoparticles.The as-prepared NiB-COS sample has a net-like morphology (Figure 2(b)).It can be seen from TEM micrographs that the nano-aggregates are composed of many interconnected fine particles with narrow range of particle size between 3 to 5 nm,which indicate that the chitosan oligosaccharides inhibited the growth of NiB cluster.The amorphous structures of the two samples were further characterized by selected area electron diffraction (SAED),as illustrated inset in Figure 2.The SAED images of NiB and NiB-COS are composed of wide halos and dispersion rings rather than obvious points,which is consistent with the XRD results.

Figure 2 TEM images of NiB (a) and NiB-COS (b)
In addition,chemical compositions and surface areas of the catalysts were analyzed by ICP analysis and BET (Table 1).Table 1 summarizes the bulk compositions of NiB samples from water and COSwater bath respectively by chemical analysis.It is worth mentioning that the atomic ratios of the two NiB catalysts are slightly different,which may be the result of different preparation methods.Comparing the surface areas of NiB and NiB-COS by BET,it is obviously found that theSBETof NiB-COS is much higher since the particle size of NiB-COS particles is much smaller than that of NiB,which was inferred that the introduction of chitosan oligosaccharides can increase the exposure of surface active metallic nickel sites (Table 1). However,the surface of NiB nanoparticles may be mainly occupied by boron in the amorphous NiB alloy prepared from pure water bath.
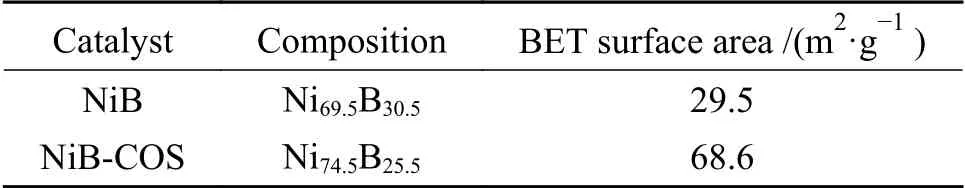
Table 1 Surface areas and bulk composition of the NiB and NiB-COS catalysts
Furthermore,the valence states of the elements on the surface of the materials were analyzed by XPS.The XPS spectra showed that the surface of the amorphous catalyst contained predominantly Ni and B.As can be seen from Figure 3(a),the Ni 2pregion of the XPS spectra shows that Ni exists mainly on the catalyst surface in the form of element Ni0,which is a slight deviation from the standard binding energy of Ni.Figure 3(b) shows the level of B 1s,the peaks at 188.3 and 192.6 eV are attributed to the elemental boron and trace amount of oxidized B (BOx)[29],respectively.The by-products of boron oxide are also produced in the process of chemical reduction because boron oxide is unavoidable in all kinds of preparation methods.From the XPS results obtained,it can be found that there are nickel and boron elements which could form amorphous nickel boron alloys in the catalyst samples.As compared to the binding energy of pure B (187.1 eV,B 1s)[30],that of the elemental boron in NiB-COS is positively shifted by about 1.0 eV,suggesting the electron donation from B to the vacantdorbitals of Ni.As a result,the former is electron deficient while the latter is electron rich,that is,so-called “B protection Ni”[31].Elemental nickel is prone to deactivation owing to corrosion and oxidation in the hydrogen evolution reaction.It has been proved that the electron-enriched Ni atom sites are highly active for hydrogen evolution reaction.The high covalence of Ni-B bond and the electron donating effect ensure the uniform dispersion of unsaturated active Ni sites,which may be the reason why NiB-COS have excellent HER activity.
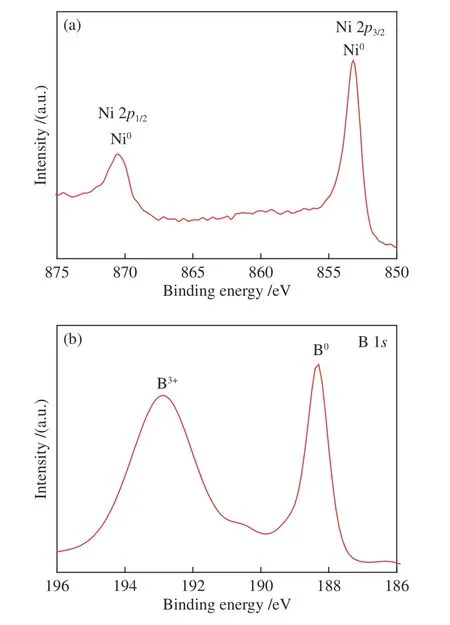
Figure 3 XPS spectra of NiB-COS(a): Ni 2p;(b): B 1s
In order to examine electrocatalytic HER activities of as-prepared electrocatalyts,NiB and NiB-COS catalysts were added dropwise on a glassy carbon electrode (GCE) to form semicircular microspheres maintaining the same loading amount of about 0.5 mg/cm2,and the electrocatalytic HER activities were investigated under alkaline medium (1 mol/L NaOH).A typical three electrode system was employed and the scanning rate of 10 mV/s was set during the HER activity measurement.Linear sweep voltammetry (LSV)curves of NiB-COS,NiB-COS loaded on NiB (NiBCOS/NiB),NiB,NiB loaded on NiB(NiB/NiB) and commercial platinum black were tested for comparison.HER performance of those electrocatalysts is listed in Table 2.Platinum black catalyst indeed shows excellent electrocatalytic HER activity in alkaline solution.The onset potential (ηonset) of platinum black is 56.5 mV.However,the pure NiB and NiB/NiB composite show relatively poor HER performance,and they demand 387.1 and 398.5 mV overpotential respectively to reach the specific current density (η10).It can also be seen from the polarization curves (Figure 4(a)) that the catalytic activity of NiB-COS/NiB electrode is close to but slightly inferior to platinum black.Encouragingly,the electrocatalytic activity of NiB-COS electrode is better than that of platinum black (η10=137.0 mV),and its overpotential is only 49.4 mV at current density of 10 mA/cm2.It is worth noting that the overpotential of NiB-COS is 149.6 and 222.3 mV at current density of 50 and 100 mA/cm2,while the overpotential of platinum black electrode is 264.1 and 353.2 mV,respectively.Obviously,the electrocatalytic activity of NiB-COS for HER at high current density is still better than that of platinum black. Meanwhile,the Tafel slope(Figure 4(b)) of NiB-COS (86.1 mV/dec) is a little smaller compared with Pt black (120.2 mV/dec),indicating that its reaction kinetics is faster,and its ratedetermining step may be Volmer reaction[32].In addition,we also noticed that the over potential of NiB-COS/NiB electrode was 170.9 mV when the current density was 10 mA/cm2,which was lower than that of NiB/NiB electrode under the same conditions,suggesting that the smaller the nanoparticles,the more active sites exposed,and the higher the electrocatalytic activity for HER.
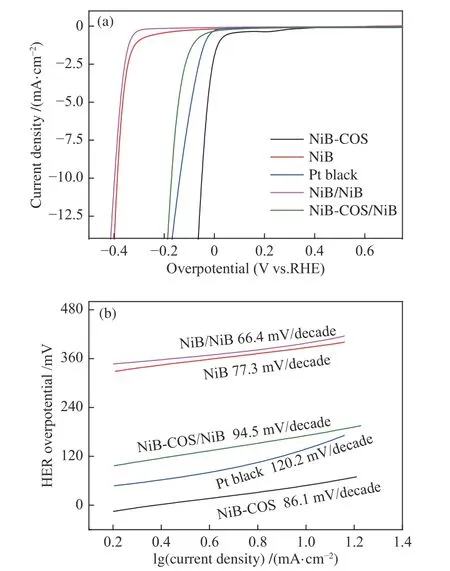
Figure 4 (a) Polarization curves and (b) corresponding Tafel plots of the different electrocatalysts in 1 mol/L NaOH alkaline solution
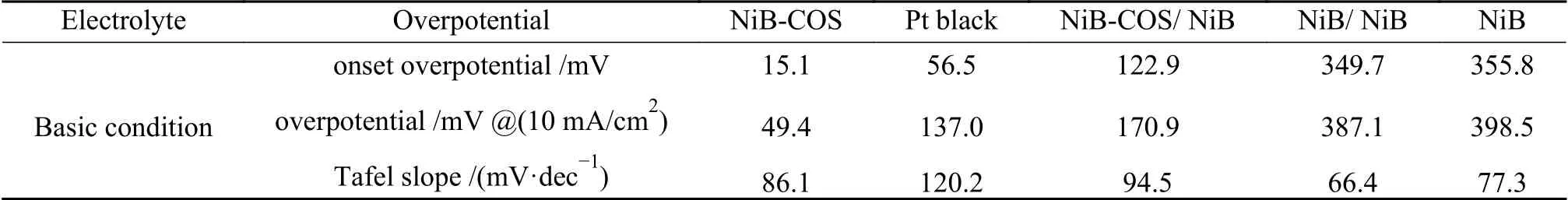
Table 2 HER performance of NiB-based electrocatalysts in basic solution
The CV curves (Figure 5(a) and 5(b)) of NiB and NiB-COS electrocatalysts were tested at different scan rates in non-faradic region.The ECSA of the catalyst was evaluated by calculating the CDI through the slope of the linear relationship of current density versus scan rate.ECSA is generally proportional to theCdIof the electrocatalyst.As shown in Figure 5(c),the NiB-COS possesses a significantly higherCdl(0.00836 mF/cm2)than NiB (0.00512 mF/cm2),indicating the larger ECSA of NiB-COS.The enlarged ECSA of NiB-COS illustrates that more active sites are exposed and its intrinsic activity is significantly improved,which is benefited from amorphous structure and small particle sizes of NiB-COS. Electrochemical impedance spectroscopy (EIS) is adopted to further scrutinize the HER kinetics behavior.As shown in Figure 5(d),the NiB-COS reveals a smaller charge transfer resistance than the NiB,which can increase the electron transfer rate,thus enhance the kinetic catalytic rate of HER electrocatalytic reaction.The above result may be attributed to the appropriate particles size of NiB-COS and the electron interaction between Ni and B.Anyway,these properties of NiB-COS catalyst are superior to those of other non-precious metal HER catalysts,such as Ni2P[33],supported NiP[34-36],N-doped graphene[37],Fe-doped NiP[38].
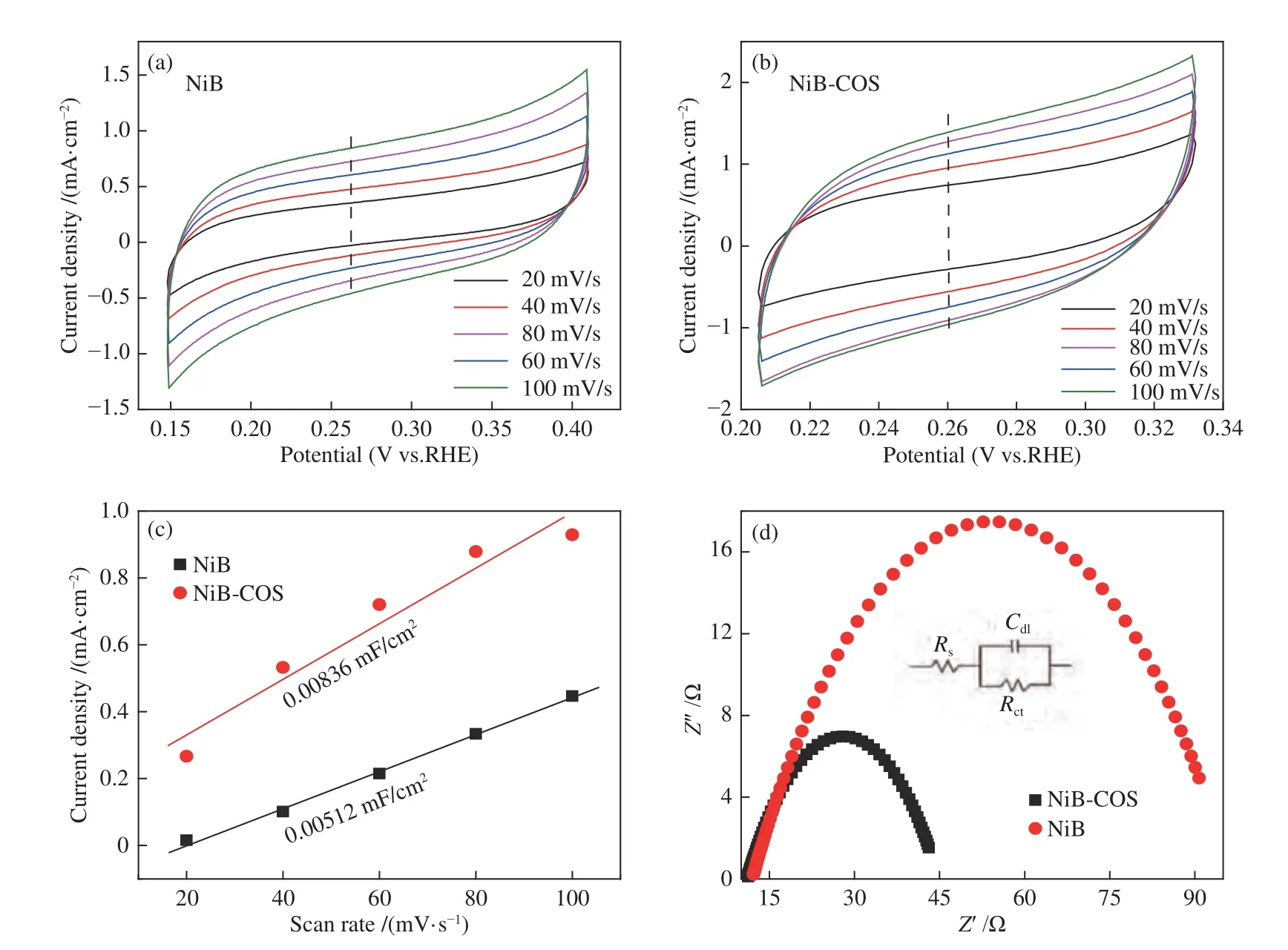
Figure 5 (a) CV curves of NiB electrocatalysts at different scan rate;(b) CV curves of NiB-COS electrocatalysts at different scan rate;(c) Linear fitting of the capacitance currents versus CVs scan rates;(d) EIS of NiB and NiB-COS electrocatalysts
The stability of electrocatalysis is another key factor to evaluate the performance of electrocatalysis for practical applications. In view of this,the durabilities of the Pt black,NiB and NiB-COS in alkaline media by long-term cycling tests were further probed.As displayed in Figure 6,Pt black and NiBCOS both afforded similar LSV curves after 500 cycles as initial,with negligible loss of the cathodic current.However,NiB showed poor durability with 90 mV over potential increasing at 10 mA/cm2,which might be caused by the partial aggregation of NiB nanoparticles.The high activity and favorable durability of the NiBCOS electrode makes it promising as a highly efficient HER electrocatalyst for alkaline water electrolysis.
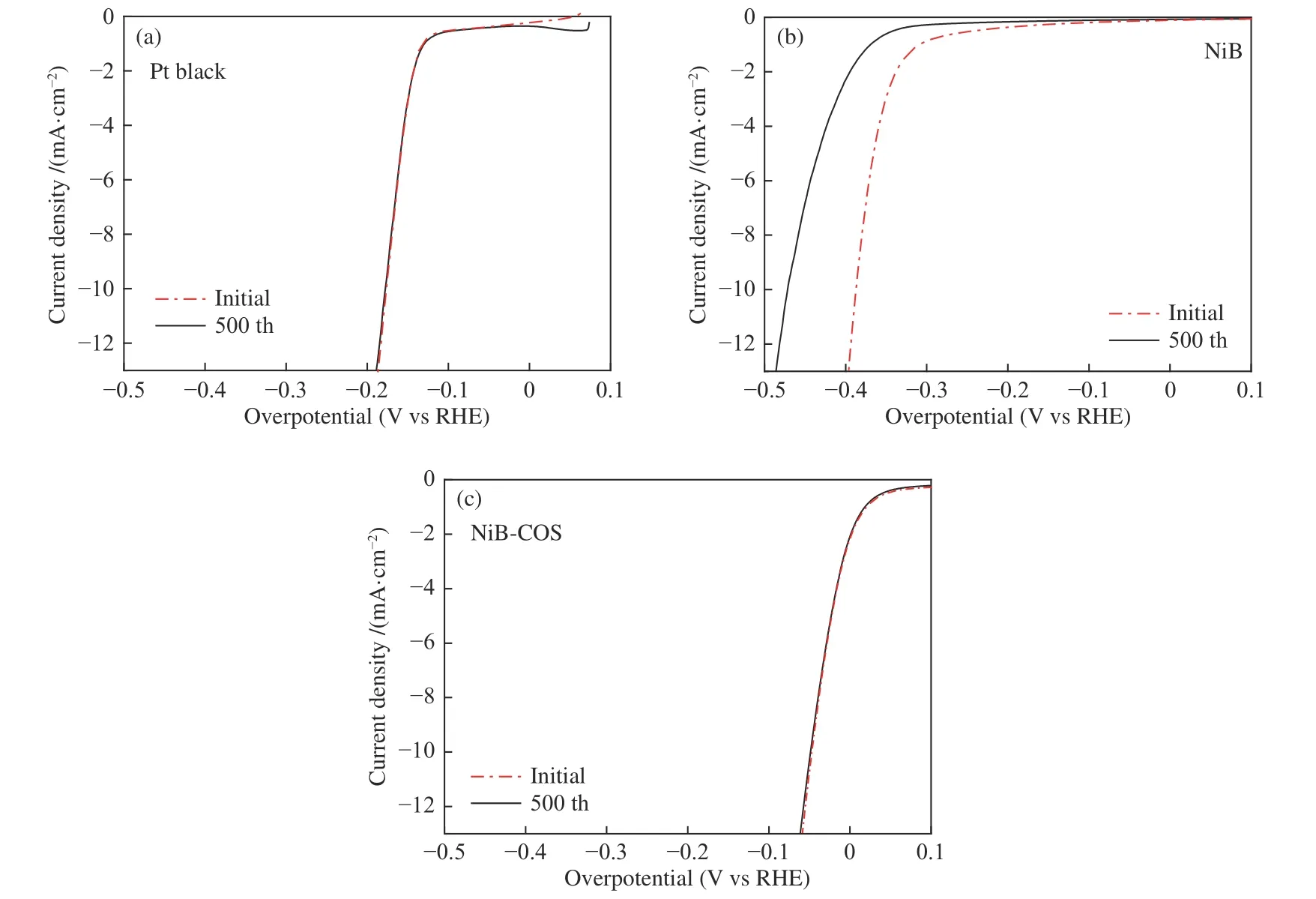
Figure 6 Electrochemical stability of the electrocatalysts in 1 mol/L NaOH solution(a): Pt black;(b): NiB;(c): NiB-COS
3 Conclusions
In summary,we reported a facile approach for the synthesis of nanosized NiB-COS using chitosan oligosaccharides as a protective agent.It is also worthwhile to emphasize that the above strategy could be applicable to a large number of materials that possess net-like shapes.Our study suggests that the introduction of chitosan oligosaccharides can turn a low active material into a highly efficient electrocatalyst toward HER. The electrocatalytic performance of this material is competitive with that of the best alkaline HER electrocatalysts reported so far.Detailed characterizations reveal that amorphous structure is formed with average particle size from 3 to 5 nm,which endows abundant exposed active sites on the surface of NiB-COS,and therefore significantly enhances the alkaline HER. Impressively,the overpotential is 49.4 mV at 10 mA/cm2,which is much lower than those of commercial Pt black (137 mV).This work not only provides a strategy for the fabrication of nanosized NiB-COS,but also promotes the fundamental researches on enhancing catalysis in transition-metal electrocatalytic materials.

