Post-functionalization of graphitic carbon nitride for highly efficient photocatalytichydrogen evolution
YANG Yi-long,LI Shan-ying,MAO Yan-li,DANG Li-yun,JIAO Zhuo-fan,XU Kai-dong
(1.School of Materials and Chemical Engineering, Henan University of Urban Construction, Pingdingshan 467036, China;2.Henan Province Key Laboratory of Water Pollution Control and Rehabilitation Technology, Henan University of Urban Construction, Pingdingshan 467036, China)
Abstract: In this work we report the feasible modification of graphitic carbon nitride (g-C3N4) polymer through a postfunctionalization progress.The resultant photocatalyst exhibits boron doping and mesoporous structure with a high surface area of 125 m2/g,leading in an increased surface activity for photocatalytic water splitting reaction.X-ray diffraction,X-ray photoelectron spectroscopy,PL emission spectra and UV-Vis spectra were used to detect the properties of as-prepared samples.Based on X-ray photoelectron spectroscopy analysis,boron is proposed to dope in the g-C3N4 lattice.Optical studies indicated that boron doped g-C3N4 exhibits enhanced and extended light absorbance in the visible-light region and a much lower intensity of PL emission spectra compared to pure g-C3N4.As a result,boron doped g-C3N4 shows activity of 10.2 times higher than the pristine g-C3N4 for photocatalytic hydrogen evolution.This work may provide a way to design efficient and mesoporous photocatalysts through post modification.
Key words: boron-doping;mesoporous structure;post-functionalization;photocatalytic hydrogen evolution
The awareness of energy conservation and environmental protection has been roused due to the worldwide energy shortage and environmental issues,while numerous research focus on the exploration of renewable and clean energy[1].Since the first discovery of photoelectrochemical (PEC) water splitting processing with titanium dioxide (TiO2),semiconductor-based photocatalysis for solar hydrogen production has attracted global interests[2-4].However,it is still challenging to achieve high solar-to-hydrogen(STH) conversion efficiency due to the restricted utilization of sunlight.To make good use of solar energy,the development of visible light responsive photocatalysts is highly desirable.In 2009,Wang et al.[5,6]developed graphitic carbon nitride (g-C3N4) for visible-light-driven photocatalytic water splitting,making it a hot research topic in the last decade.
Unlike TiO2,g-C3N4affords a narrow bandgap of ca.2.7 eV with the conduction band (CB) position at ca.-1.1 eV and valance band (VB) position at ca.+1.6 eV vs.normal hydrogen electrode (NHE),respectively[5]. Furthermore,g-C3N4photocatalyst possesses the advantages of stability,non-toxicity,abundant source,absorption in visible-light region and easy to control and modify.As a novel visible-lightdriven photocatalyst,its remarkable property has been demonstrated in various photocatalytic applications such as H2and O2evolution[7-10],photodegradation of pollutants[11],CO2reduction[12],organic synthesis[13]and photoelectrocatalysis[14].However,the three pivotal issues of light absorption efficiency,charge separation efficiency and surface reaction efficiency still limit the performance of g-C3N4-based photocatalysis.Therefore,it is highly desirable to modify g-C3N4for efficient solar water splitting.Doping is considered as a very convenient and promising strategy to tune the band structure,enhance the lighting adsorption and improve the performance of photocatalyst[15].Various doping strategies are employed to modify the g-C3N4to improve the light harvesting,suppress the photogenerated charge recombination and enhance the photocatalytic activity. Combining g-C3N4with strongly electronegative dopants such as fluorine to form an F-doped material not only raised the valence band,but also effected the thermodynamic driving force for H2reduction[16,17].Fluorinated g-C3N4solids was also reported with excellent visible-light photocatalytic activity[18].Besides fluorine,I doping also lead positive effect like enlarged specific surface area,enhanced optical absorption,narrowed bandgap and accelerated charge carriers transfer rate as well as increased H2evolution rate[19].Moreover,potassiummodified g-C3N4(K-g-C3N4) nanosheets were synthesized[20].Photocatalytic H2evolution experiments under visible light irradiation showed that K-g-C3N4nanosheets have high photocatalytic activities (up to about thirteen times higher than that of pure g-C3N4) as well as good stability (no reduction in activity within 16 h).However,the doping methods have been reported mainly via calcination of precursors and doping source,which results in the bulk structure and heterogeneous distribution of doped ions and limited enhanced activity of photocatalysis.Therefore,it is of great importance to develop a feasible one-pot route for forming the porous structure and homogenizing the doping ions,which induces more reaction sites and optimizes the electronic structure.Furthermore,the selection of boron source may show significant effect on the doping sites and Lewis acidity on the surface of g-C3N4,which may also act as specific reaction sites for reactant molecules[21].In particular,the textural,electronic,and structural properties of g-C3N4can be modulated to boost the electron transfer and form a stablep-conjugation system.Thereby the doping and porous structure of g-C3N4through a postfunctionalization method may be feasible to achieve superior photocatalytic activity.
Herein,we report a facile strategy to synthesize porous B-doped g-C3N4nanosheets by postfunctionalization with PEI and Ph4BNa.It is interesting to see that the resulted samples achieve boron doping and show porous structure simultaneously,while induce enhanced photocatalytic activity in photocatalytic hydrogen generation.As a result,an impressive H2production rate as high as 4280 μmol/(h·g) is achieved,which is 10.2 folds higher than that of pristine g-C3N4.
1 Experimental
1.1 Materials
Urea (CH4N2O),Tetraphenylboron sodium(Ph4BNa,(C6H5)4BNa),and triethanolamine(C6H15NO3) were purchased from Sinopharm Chemical Reagent Beijing Co. Ltd. Polyethyleneimine((CH2CH2NH)n,M.W.70000,99%) were purchased from Alfa Aesar (China) Chemicals Co.Ltd.All chemicals were analytical grade and used as received without further purification.
1.2 Preparation of g-C3N4
The g-C3N4powders were synthesized via the thermal polycondensation of urea according to the procedures described in literature[22].In a typical synthesis,urea (10.0 g) sealed in a crucible was heated at a heating rate of 10 °C/min in a muffle furnace from room temperature (RT) to 550 °C and kept at this temperature for 4 h.The resulting yellow product was collected for further use.
1.3 Preparation of boron-doped g-C3N4
2 g g-C3N4powders were immersed in 200 mL of DI water.Subsequent ultrasonication and vigorous stirring were applied to the prepared solutions for 0.5 and 12 h,respectively.After that,0.2 g PEI was added to the above mixture.After continuously stirring for 1 h,suspension was separated through centrifugation at 10000 r/min,and the supernatants were poured out.The precursor solution of unmodified g-C3N4was prepared by diluting the reserved solid into 200 mL of DI water.Then,20 mg Ph4BNa solution (1 mg/mL solution in DI water) was dropwise added to the above precursor solution of 20 mL while applying stirring for 1 h.The precursor precipitate was obtained by filtering and dried at 60 °C overnight for further synthesis.Boron-doped g-C3N4was prepared by calcining the precursor precipitate,which was heated to 500 °C at a rate of 5 °C/min and maintained at 500 °C for 4 h in air.The B-doped g-C3N4samples were denoted as BPCNX,in which X was the Ph4BNa solution of 20,50,70,and 110 mg,respectively.For comparison purposes,another B-doped g-C3N4(denoted as BCN)was also prepared by mixing the urea and Ph4BNa directly without Ph4BNa added according to the above procedures.
1.4 Characterization of B-doped g-C3N4
The samples were characterized using X-ray diffraction (Shimadzu XRD-7000 with CuKα radiation,λ=0.15418 nm),scanning electron microscopy (Hitachi S-8020U) and transmission electron microscopy (JEOL JEM-2010,operated at 200 kV).Nitrogen adsorptiondesorption isotherms and pore-size distributions were measured on a Micromeritics ASAP 2020 at -196 °C.Specific surface areas and mean pore sizes were calculated according to the BET and Barrett-Joyner-Halenda (BJH) methods,respectively.A Shimadzu UV-3100 PLUS spectrophotometer was used to measure the UV-visible (UV-Vis) absorption spectra of the samples.X-ray photoelectron spectroscopic (XPS) analysis was performed on a PHI Quantera SXM X-ray photoelectron spectrometer using the AlKα radiation. The photoluminescence (PL) spectra of g-C3N4and B-doped g-C3N4were recorded using the Edinburgh Instruments Xe900 equipped with a xenon (Xe) lamp with an excitation wavelength of 380 nm.The FT-IR spectrum was collected using a FT-IR-8400S spectrometer.Electrochemical and photoelectrochemical activities of samples were recorded on a CHI 660B electrochemical workstation in three-electrode quartz cells.A platinum electrode was used as the counter electrode,a saturated calomel electrode (SCE) was used as the reference electrodes,and g-C3N4and B-doped g-C3N4electrodes on FTO served as the working electrodes in 0.1 mol/L Na2SO4aqueous solution.A 300 W Xe lamp (CHF-XW-300W) with a 420 nm cutoff filter was employed as the visible light source.The amperometrici-tcurves of the samples were measured at 0 V with light on and light off.The electrochemical impedance spectra (EIS) were determined at 0 V.A sinusoidal ac signal of 5 mV was applied to the electrode in the frequency range of 1-100 kHz.
1.5 Photocatalytic experiments
Photocatalytic water splitting was carried out in a top-irradiation vessel connected to a LabSolar H2photocatalytic hydrogen evolution system (Perfectlight,Beijing) including a 300 W Xe lamp with a 420 nm cutoff filter. In a typical reaction,50 mg of photocatalyst powder was dispersed in 100 mL aqueous solution containing 10% triethanolamine scavenger and 1% (respect to Pt,acting as co-catalysts) H2PtCl6·6H2O.The temperature of the reaction solution was carefully maintained at (5 ± 1) °C during the whole experiment.The reactor was then sealed and evacuated 40 min to remove air before irradiated.The amount of evolved H2was analyzed by gas chromatography(Agilent,7890A GC system) equipped with a thermal conductive detector (TCD) and a 0.5 nm molecular sieve column,using high-purity nitrogen as the carrier gas.
2 Results and discussion
The crystal structure of g-C3N4and B-doped g-C3N4photocatalysts were examined by XRD,as illustrated in Figure 1(a).The diffractogram of the samples featured two distinct (100) and (002)reflections at 2θ=13.08° and 27.58°,corresponding to an in-plane structural packing motif and the long-range interplanar stacking of the conjugated aromatic system,respectively[23].The XRD patterns of BPCN samples were similar to that of pristine g-C3N4,however,as for the doped samples,the intensity of the (002) crystal plane reduced substantially with the increase of boron amount,and the (002) diffraction of BPCN-110 (the heaviest doped g-C3N4) was significantly weakened,which can be attributed to the presence of excessive B atoms doping in the graphitic structure.This can be an evidence for the successful doping in g-C3N4.The morphology of the as-prepared g-C3N4samples was examined by field-emission SEM (FE-SEM).The SEM image of BPCN-70 in Figure 1(b) showed a clearly wrinkle 2D structure.As shown in inset of Figure 1(b),the nanosheet was composed of uniform distributed C,N,B and O elements,indicating that B elements were homogeneously doped in g-C3N4.The nanostructures of g-C3N4and BPCN-70 were characterized by transmission electron microscopy (TEM).BPCN-70 showed thin nanosheet structure with mesoporous structure (Figure 1(c)),indicating that the postfunctionalization process developed in this work can achieve the delamination and formation of mesopore.This thin nanosheet and uniform mesoporous structure was highly desirable for photocatalytic reaction because the thin layer reduced the distance of charge transfer and pore structure can provide the photocatalytic activity sites.To further confirm the mesopore structure of B-doped g-C3N4,textural properties of g-C3N4and BPCN-70 were investigated by the nitrogen adsorption-desorption measurement.The obtained absorption isotherms and Barrett-Joyner-Halenda (BJH) pore-size distributions were shown in Figure 1(d).The adsorption-desorption isotherm of these two samples have been classified as type IV with a H3 hysteresis loop,which was characteristic of mesoporous materials with slit-shaped pores[24].The surface area (SBET) and pore volume of BPCN-70 was calculated as to be 125 m2/g and 0.35 cm3/g,whereas that of g-C3N4was 101 m2/g and 0.49 cm3/g.It was reasonable to state that the increased BET surface area was mainly derived from post-functional treatment,which would provide more active sites for photocatalytic hydrogen evolution. The pore-size distribution curves calculated using the BJH model clearly showed that g-C3N4material had two pore-size families located at 3.9 and 30-50 nm,while the primary pore size of BPCN-70 was centered at 13 nm(inset of Figure 1(d)),which agreed well with that estimated from TEM images.This pore structure was attributed to the formation of uniform mesoporous structure. These results indicated that the postfunctionalization of g-C3N4yielded a large surface area and mesoporosity.B-doped g-C3N4prepared by other doping amount presented regularSBETvalues,which were 116,110,99 m2/g for the BPCN-20,BPCN-50 and BPCN-110 samples,respectively.
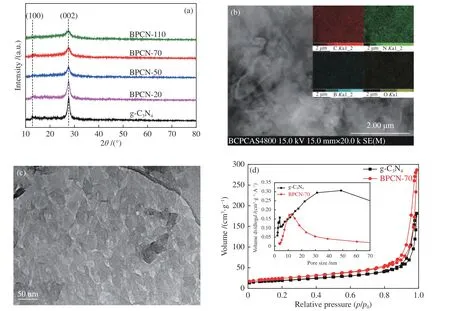
Figure 1 (a) XRD patterns of g-C3N4 and BPCN samples;(b) SEM and (c) TEM image of BPCN-70 nanosheet,inset in (b): the element mapping of C,N,B and O for BPCN-70,respectively;(d) Nitrogen adsorption-desorption isotherms,inset: pore size distributions of g-C3N4 and BPCN-70
Figure 2(a) depicted the absorbance spectra of g-C3N4and B-doped g-C3N4,which all featured a notable absorption extending from UV part to the visible light region.In comparison with that of g-C3N4,there was a little red shift of the absorption in BCN and BPCN samples,which may due to the existence of Boron species.The band gaps of g-C3N4,BCN-70 and BPCN-70 materials were 2.92,2.87 and 2.72 eV.BPCN-70 exhibited typical VB characteristics of g-C3N4,with the VBM energy at about 1.51 eV versus RHE[25].Since the band gap of BPCN-70 was 2.72 eV from the optical measurement,the CBM can be estimated at about-1.21 eV.As a result,the material gap can be narrowed and the ability of BPCN-70 to harvest visible light can be improved with this post-functional modification.The improved photoabsorption property demonstrated that more electrons generated and participated in the photocatalytic reaction,which can result in enhanced photocatalytic activity.
The chemical structure of g-C3N4nanosheets was confirmed by the FT-IR spectra shown in Figure 2(b).The broad peaks between 3500 and 3000 cm-1originating from the N-H stretches can be clearly observed,suggesting the partial hydrogenation of some nitrogen atoms in the nanosheets.Characteristic bands of aromatic CN heterocycles are at 1200 to 1600 cm-1and 802 cm-1is characteristic of s-triazine derivatives[24,26].Specifically,the vibrations of the Brelated group (N-B-N) were observed at 1370 cm-1in the doped CN matrix,despite overlapped of the band with that of the C-N vibrations[27].Clearly,the FT-IR spectrum of B doped g-C3N4nanosheets was similar to that of original g-C3N4,indicating that the B doped g-C3N4nanosheets keep the same chemical structure as their parent g-C3N4.Furthermore,the thermogravimetric analysis (TGA-DSC) has been performed to analyze the stability of BPCN-70,as shown in Figure 2(c).The first stage with 8.75% mass loss corresponds to the release of water molecules adsorbed and oxygen-containing groups[28].When the temperature was higher than 500 °C,the structure of BPCN-70 began to break up and BPCN-70 sample can be completely decomposed into small molecules such as C2N2and NH3[29].In addition,the temperature for the maximum endothermic peak of BPCN-70 was at 684 °C,resulting in a strong thermal stability.
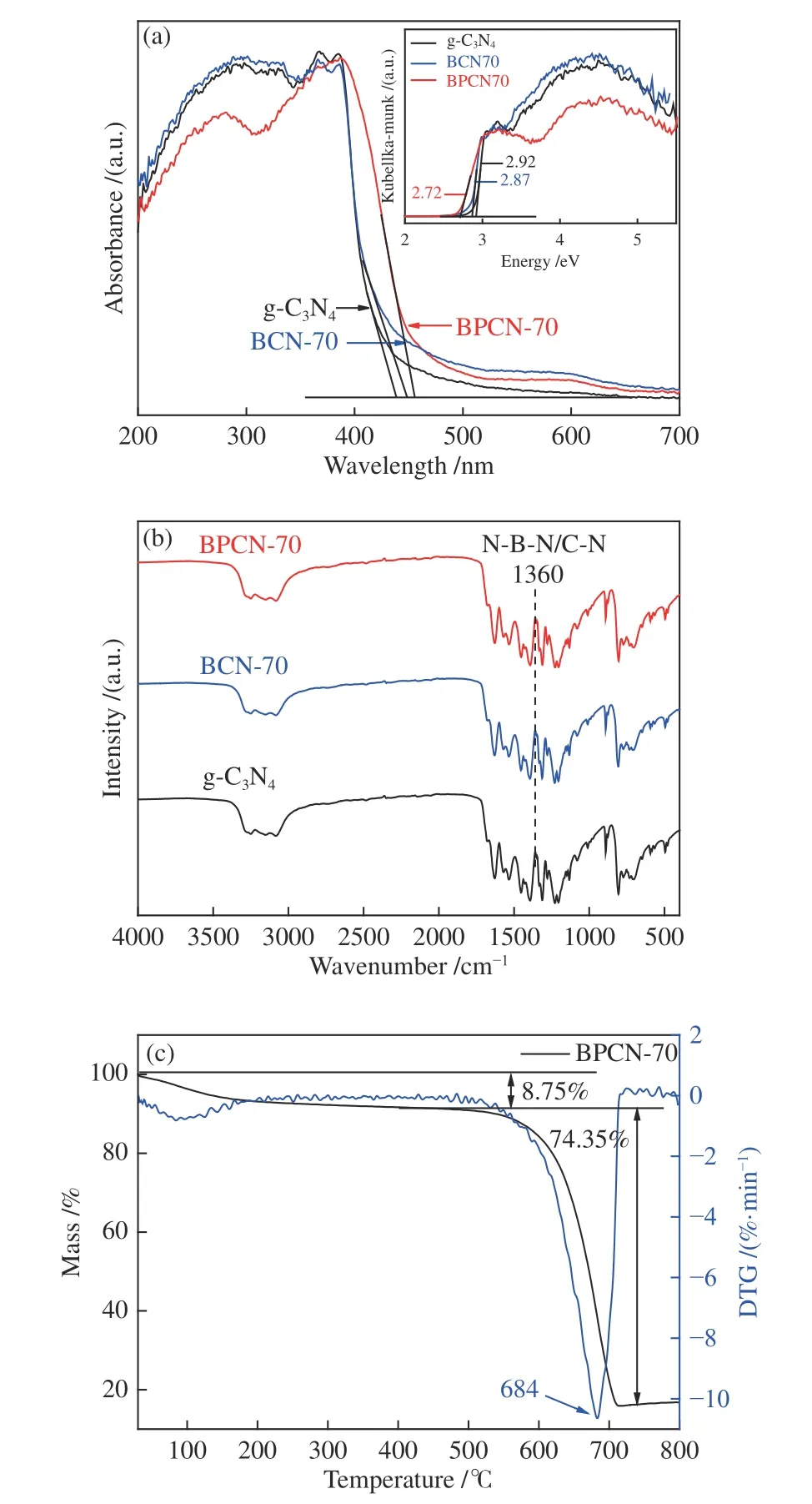
Figure 2 (a) UV-Visible diffuse reflectance spectra of g-C3N4,BCN-70 and BPCN-70 samples;inset: transformed Kubelka-Munk function versus the energy spectra of g-C3N4,BCN-70 and BPCN-70 samples;(b) FT-IR spectra of g-C3N4,BCN-70 and BPCN-70 samples;(c) TG spectra of BPCN-70
To further determine the incorporation of B atoms and their oxidation state in the CN framework,The XPS analysis was performed and the results are shown in Figure 3.The XPS survey spectrum in Figure 3(a)showed that the sample contains only C,N and O species without other impurities,while peak of B cannot be noted in the survey spectrum due to the lower intensity compared to that of N specie.A clear B 1sXPS peak with a binding energy (EB) of 191.88 eV was observed for the BPCN-70 sample (Figure 3(b)),corresponding to N-B-N coordination in the unit of heptazine[30].This indicated that some of the B atoms were introduced into the carbon sites of CN matrix.The C 1sspectrum (Figure 3(c)) of the BPCN-70 sample displayed two peaks atEB=285.48 and 288.88 eV,which was attributed to contaminated carbon and surface carbon,and the C-(N)3 groups of g-C3N4,respectively[31].Figure 3(d) showed the N 1sXPS spectrum of the BPCN-70.This spectrum has been fitted to four peaks,which are attributed to thesp2-hybridized nitrogen (C-N=C) species atEB=399.08 eV,the N-(C)3 species atEB=400.08 eV,the quaternary N bonded to three C atoms in the aromatic cycles atEB=401.02 eV and charging effects atEB=404.58 eV[32].According to the XPS and FT-IR analyses,it was concluded that the boron element was indeed doped in g-C3N4nanosheets.
The photocatalytic H2production activity of B doped g-C3N4was evaluated and compared with g-C3N4.As shown from H2production rates over B doped g-C3N4samples with different B contents (Figure 4(a)),the photocatalytic H2production rate increased dramatically with B doping.It reached maximum 4280 μmol/(h·g) over BPCN-70 that showed 10.2-fold improvement in comparison with pure g-C3N4(420 μmol/(h·g)).The H2-evolution rate achieved in this work was one of the best for boron doped g-C3N4(Table 1).Moreover,the long-term ability of BPCN-70 was further evaluated (Figure 4(b)).The recycle kinetics curves demonstrated a slight deactivation with time in the first five runs.When an appropriate amount of triethanolamine (TEOA) was added to the reaction solution,BPCN-70 can maintain sustainably stable photocatalytic H2production rate over 5 cycles accumulated 20 h,which indicate that it has the good durability and reusability. The photocatalytic performance of g-C3N4,BPCN-70 and BCN-70 with no cocatalyst was shown in Figure 5(c).g-C3N4presented a H2-generation rate of 70 μmol/(h·g),which is much lower than that with Pt.Similarity,with no Pt as cocatalyst,the BPCN-70 sample still presented the fastest H2-production rate of 610 μmol/(h·g),which approaches 8.7 times that of pure g-C3N4.Additionally,this value is also higher than that of the BCN-70(220 μmol/(h·g)).
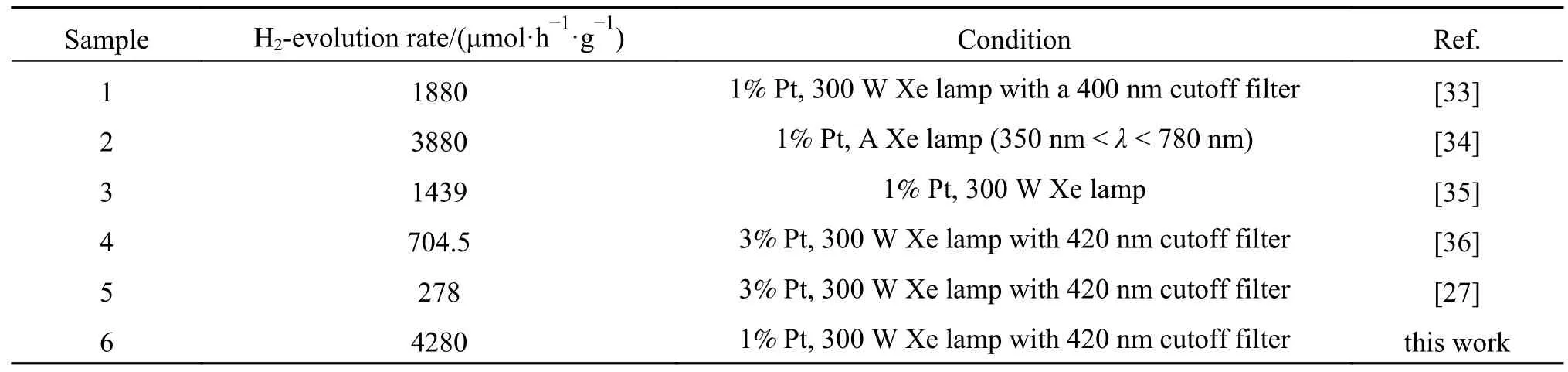
Table 1 Summary of recent result in boron doped g-C3N4 for solar water splitting
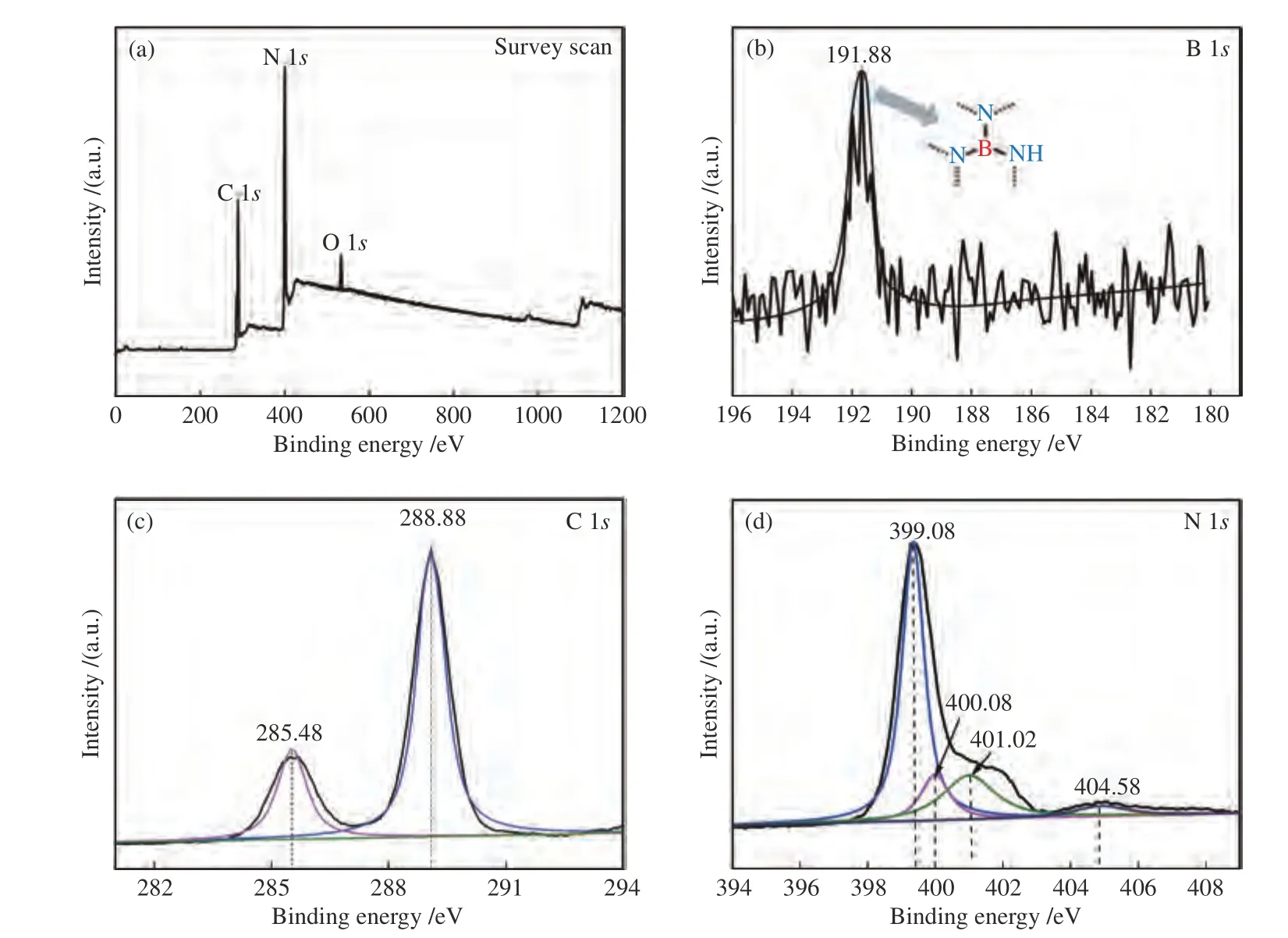
Figure 3 ((a),(b),(c) and (d)) XPS spectra of survey,B 1s,C 1s and N 1s,respectively,for BPCN-70 sample
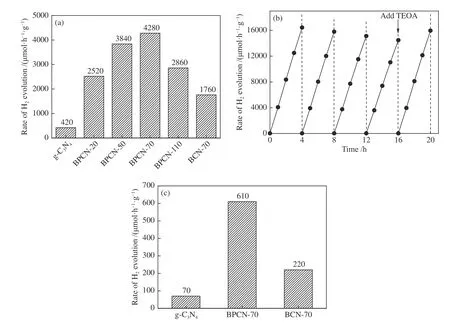
Figure 4 (a) Photocatalytic H2-generation activity over the typical samples with 1% Pt as cocatalyst;(b) cyclic running kinetics curves of H2 production over BPCN-70;(c) photocatalytic H2-generation activity over the typical samples with no cocatalyst
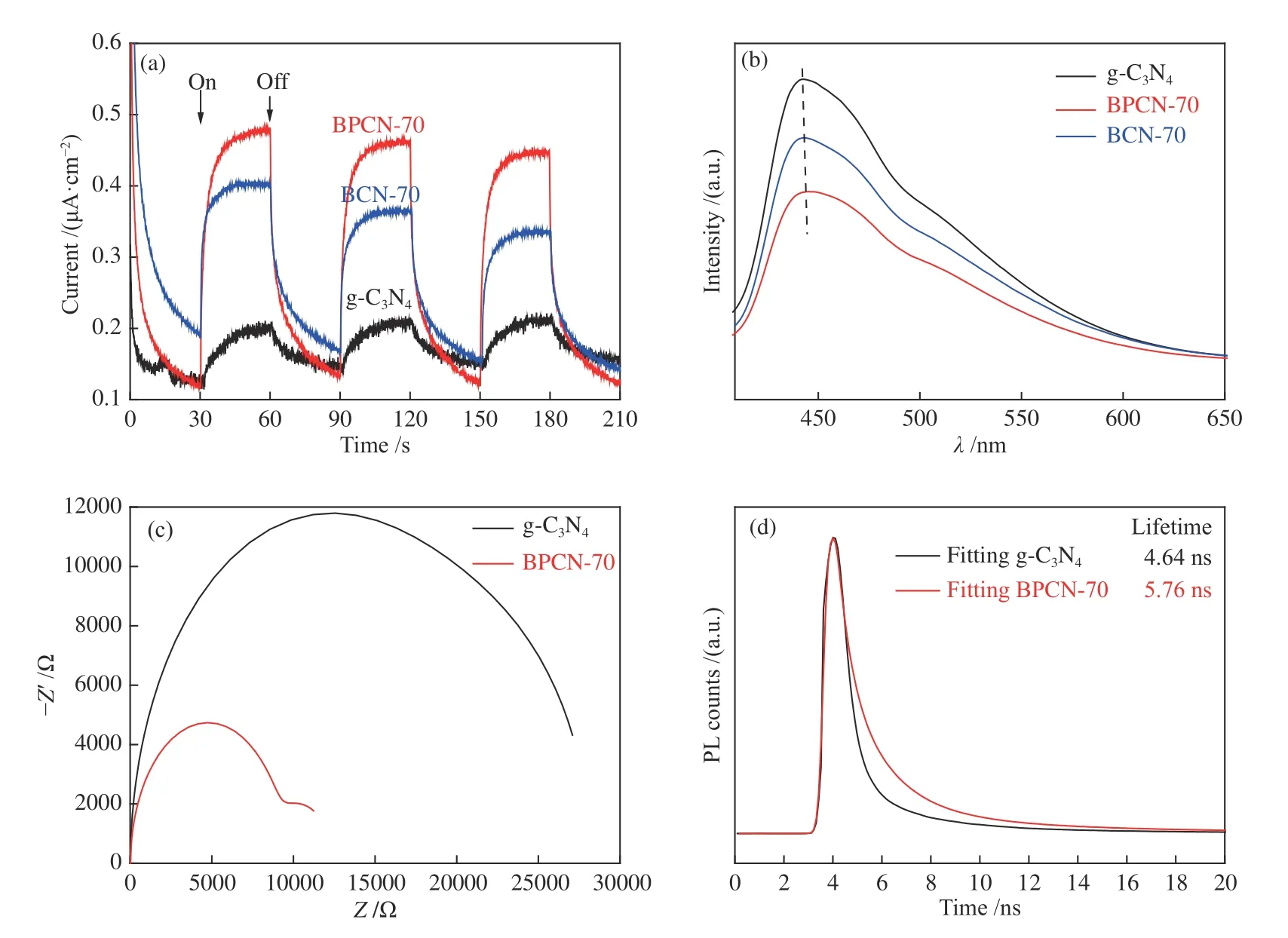
Figure 5 (a) transient photocurrent density versus time;(b) photoluminescence spectra;(c) EIS Nyquist plots;(d) time-resolved transient PL spectra of typical samples
To further investigate the underlying mechanism for improved photocatalytic activity,the effect of doping species on the charge carrier migration and separation behaviors were investigated by transient photocurrent responses,EIS and PL spectra.Seen from Figure 5(a),BPCN-70 produced the continually stable transient photocurrent response during the three on/off intermittent irradiation cycles over 210 s under the visible light.It can be seen that the photocurrent of BPCN-1 sample was about 2.4 and 1.2 times higher than that of g-C3N4and BCN-70,respectively,suggesting that B doping species by post-modification can efficiently promote the carrier separation and the enhanced photocatalytic activity can be ascribed to the significantly improved photogenerated charge separation efficiency.PL spectra of B doped g-C3N4and g-C3N4samples were recorded at an excitation wavelength of 380 nm to determine the transfer and separation efficiency of photogenerated electron-hole pairs[37].As shown in Figure 5(b),the main emission band was centered at about 460 nm for pure g-C3N4,which was due to the recombination process of selftrapped excitation[38].The emission peak positions of BCN-70 and BPCN-70 samples were similar to those of g-C3N4.However,the emission intensity of BPCN-70 composite sample was lower than that of pristine g-C3N4and BCN-70.This result clearly indicates that the recombination of photogenerated charge carriers was inhibited and B-doping into the g-C3N4lattice contributed to the separation of photoinduced charge carriers,which was in agreement with the photocatalytic H2evolution results.Moreover,the EIS spectra in Figure 5(c) presented that the lesser arc radius of Nyquist plot was produced on BPCN-70 electrode compared with g-C3N4,proving that the charge carrier recombination was suppressed and interfacial charge transfer ability was promoted effectively,leading to a higher photocatalytic hydrogen production performance.The time-resolved transient PL spectra in Figure 5(d) were further carried out to investigate charge separation behaviors.The fitted fluorescence lifetime increased from 4.64 ns of g-C3N4to 5.76 ns of BPCN-70,which further verified that the doped B significantly prolonged the PL lifetime and hindered the annihilation of charge carriers.Thus,more electrons can participate in the photocatalytic H2-evoultion reaction,which may enhance the performance of BPCN.
Based on the above results,the postfunctionalization process can achieve B-doping into the structure and induce formation of mesoporous structures.The synergistic effect of element doping and mesostructured controlling promotes charge carrier separation and transfer,leading to an enhanced photocatalytic activity.
To gain insight into the stability of BPCN-70,XRD and FT-IR are carried out on BPCN-70 after cycle runs.It can be noted that no obvious change in material structures was observed through XRD and FTIR examinations (Figure 6(a) and 6(b)) for the sample before and after reaction.This indicates that the decrease in the activity after the first run is mainly due to the decreased concentration of triethanolamine.Based on the above discussion,it is reasonable to conclude that the structure of g-C3N4with postfunctional modification is quite stable.
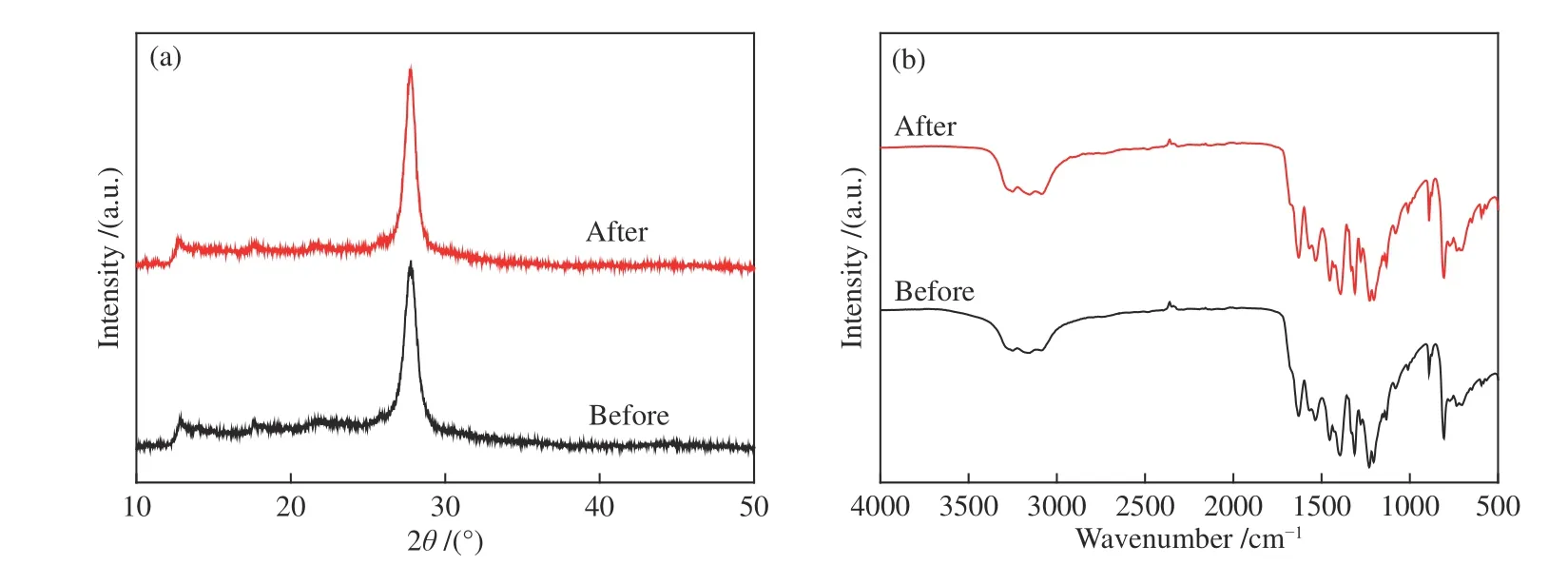
Figure 6 (a) XRD patterns of BPCN-70;(b) FT-IR spectra of BPCN-70 before and after hydrogen evolution reaction
3 Conclusions
In summary,a feasible post-functionalization method has been applied to modify g-C3N4to achieve B doping and mesoporous structure,which exhibited enhanced photocatalytic hydrogen production performance.The superior photocatalytic activity and stability were ascribed to the greatly improved charge migration and separation ability.The optimized BPCN-70 exited optimal H2production rate of 4280 μmol/(h·g)that was 10.2-fold improved in comparison with pristine g-C3N4(420.0 μmol/(h·g)).Meanwhile,the cycle running experiments indicated that BPCN-70 had good stability and reusability.The findings demonstrate a useful strategy to achieve element doping and structure controlling simultaneously for enhanced photocatalytic performance.

