Stem cell therapy in retinal diseases
Audrey Voisin ,Amaury Pénaguin ,Afsaneh Gaillard ,Nicolas Leveziel
Abstract Alteration of the outer retina leads to various diseases such as age-related macular degeneration or retinitis pigmentosa characterized by decreased visual acuity and ultimately blindness.Despite intensive research in the field of retinal disorders,there is currently no curative treatment.Several therapeutic approaches such as cell-based replacement and gene therapies are currently in development.In the context of cell-based therapies,different cell sources such as embryonic stem cells,induced pluripotent stem cells,or multipotent stem cells can be used for transplantation.In the vast majority of human clinical trials,retinal pigment epithelial cells and photoreceptors are the cell types considered for replacement cell therapies.In this review,we summarize the progress made in stem cell therapies ranging from the pre-clinical studies to clinical trials for retinal disease.
Key Words:age-related macular degeneration;cell transplantation;clinical trial;retinal disease;retinal dystrophy;stem cell
Introduction
The progressive degeneration of photoreceptors characterizes various retinal diseases such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP),leading to progressive vision and visual field loss and ultimately to blindness (Verbakel et al.,2018;Voisin et al.,2022).In AMD,there is geographic atrophy (called atrophic AMD form) and abnormal and excessive growth of blood vessels in the eye called choroidal neovascularization (the exudative AMD form).Anti-vascular endothelial growth factor intravitreal therapy,which is the only effective treatment for exudative AMD,targets choroidal neovascularization but not the disease itself.To date,aside from some positive advances in gene therapy for very few particular forms of retinal dystrophy (i.e.,voreti gene neparvovec),there is no curative treatment for these retinal diseases (Stingl et al.,2022).In this context,over the past two decades,the development of cell-based therapy has been investigated as a means of replacing apoptotic or dystrophic cells and restoring visual function.Mammalian retinas have limited regenerative capacity,and stem cells in regenerative strategy seem to be a promoting approach (Salas et al.,2021).Despite encouraging results,stem cell therapy needs to be optimized to improve its effectiveness in the treatment of retinal diseases.This review focuses on the retinal dystrophies and the different stem cell populations and their use for transplantation in retinal diseases.
Search Strategy and Selection Criteria
Studies cited in this review were published between 2000 and 2022 and searched from PubMed or Google Scholar databases.The following keywords were used for this research: stem cell transplantation,retinal disease,retinal degeneration,retinal dystrophy,stem cell therapy,age-related macular degeneration,retina,retinitis pigmentosa,Stargardt disease,bone marrowderived stem cells,retinal precursor cells,clinical trials,retinal pigment epithelial cells,mesenchymal stem cells,embryonic stem cells,and induced pluripotent stem cells.
Retinal Degeneration
The human adult retina is organized in ten histological layers with one glial cell type,the Müller glial cells,and six major types of neurons: two main cell types of photoreceptors (PRs;rods and cones),bipolar cells,amacrine cells,horizontal cells,and ganglion cells.Retinal cell bodies are organized into three layers from apical to basal;the outer nuclear layer,the inner nuclear layer,and the ganglion cell layer.Aside from these layers,the outer and inner plexiform layers contain axons and dendrites of neuronal cells.The outer part of the retina is limited by the retinal pigment epithelium (RPE) layer.These cells fulfill many functions such as epithelial transport,visual cycle,phagocytosis,secretion,and immune modulation (Voisin et al.,2019).
Recessive Stargardt macular degeneration
Recessive Stargardt macular degeneration (STGD1) is common retinal dystrophy of varying severity,the most common and severe form during childhood,and the less common form during adulthood (Tanna et al.,2017).STGD1 is an autosomal recessive disease with an incidence of 1:8000 to 1:10,000,caused by mutations in the ATP binding cassette subfamily A member 4 (ABCA4) gene (Spiteri Cornish et al.,2017;Cicinelli et al.,2019).The protein encoded byABCA4is involved in the visual cycle and localized in the photoreceptor outer segments.The disease is characterized by choriocapillaris atrophy and by accumulation of lipofuscin,an age-related pigment,in RPE,leading to PR degeneration (Figure 1A).Patients affected by STGD1 usually experience a rapid bilateral central visual loss with dyschomatopsia and central scotoma.Disease-causing variants ofABCA4are also associated with cone,rod,and rod-cone dystrophies (Cremers et al.,2020).
RP
RP is the most frequent hereditary (30–40% autosomal dominant,40–60%autosomal recessive,and 5–15% X-linked) retinal disease with an incidence of approximately 1:4000 (Verbakel et al.,2018).Patients suffering from RP frequently report night blindness and progressive visual field loss beginning around the age of 20–30 years,and finally are complete blindness at the late stage.The disease is characterized by primary degeneration of PR rods(eventually secondary degeneration of cones in case of complete blindness)(Ali et al.,2017).At fundus examination,the disease is clinically characterized by dark bone-spicule pigmentation and attenuated blood vessels (Figure 1B;Verbakel et al.,2018).
AMD
AMD is a complex multi factorial disease characterized by degeneration of the macula usually preceded by drusen formation made up of the extracellular accumulation of proteins and lipids (Voisin et al.,2022).The disease usually affects older adult individuals and is the leading cause of blindness in industrialized countries.The pathogenesis of this retinal disease is associated with both genetic (CFH,ARMS2,etc.) and environmental factors (smoking and light exposure).AMD is classified in an atrophic form (Figure 1C) with the progressive development of macular atrophy and an exudative form (Figure 1D) with the development of choroidal neovascularization (Nashine,2021).Both forms are clinically characterized by visual acuity decrease,central scotoma,and metamorphopsia.
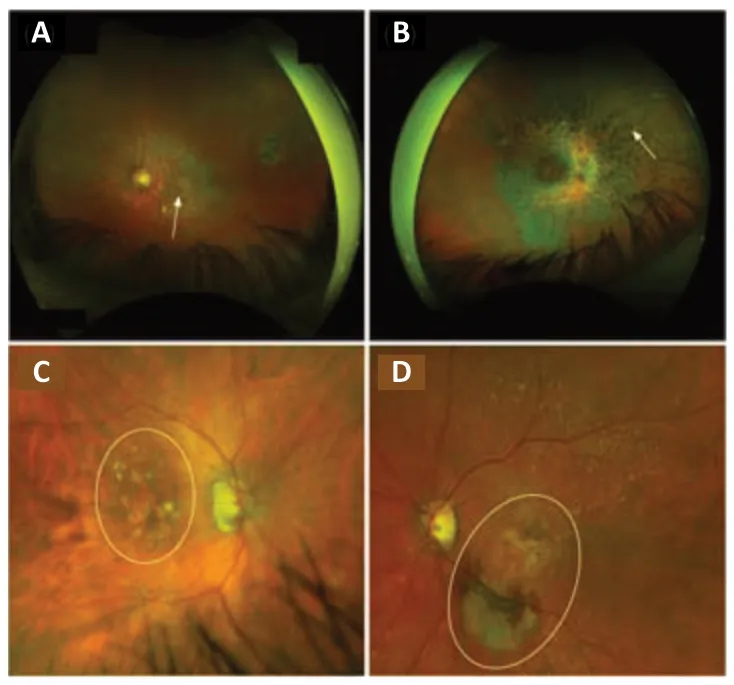
Figure 1|Fundus examination of patients affected by retinal diseases.
Cell-Based Therapy
One approach for vision restoration in AMD,RP,or STGD1 is cell-based therapy,which has two objectives: (1) to replace dysfunctional cells with new retinal cells derived from stem cells that could integrate the host tissue and restore retina function,and (2) to release trophic factors that contribute to rescue effect (Figure 2).To be functional,transplanted cells must integrate,demonstrate long-term survival and form new synaptic connections with the host retina.Moreover,the secretion of neurotrophic factors by grafted cells can protect the retina from degeneration and contribute to vision restoration.For example,the brain-derived neurotrophic factor is expressed in Müller cells and retinal ganglion cells and is essential for the development of neurons,cell survival,and synaptic activity (Jindal et al.,2017).Mainly expressed in RPE end PR,ciliary neurotrophic factor and glial cell-derived neurotrophic factor also have neuroprotective effects.Ciliary neurotrophic factor enhances the survival of PR and promotes the axonal regeneration of retinal ganglion cells (Li et al.,2011).Glial cell-derived neurotrophic factor mediates retinal neuroprotection through Müller cell activation involved in PR survival,retinal structural stabilization,and inflammatory modulation (Tackenberg et al.,2009;Read et al.,2010).Moreover,it has been shown that intravitreal injection of ciliary neurotrophic factor inhibits the progression of retinal degeneration and preserves the retina function in animal models of retinal degeneration (Dulz et al.,2020).Based on these neuroprotective effects,ciliary neurotrophic factor-based therapy has been tested in several clinical trials,but therapeutic effects are not sufficient for AMD or RP (Zhang et al.,2011).
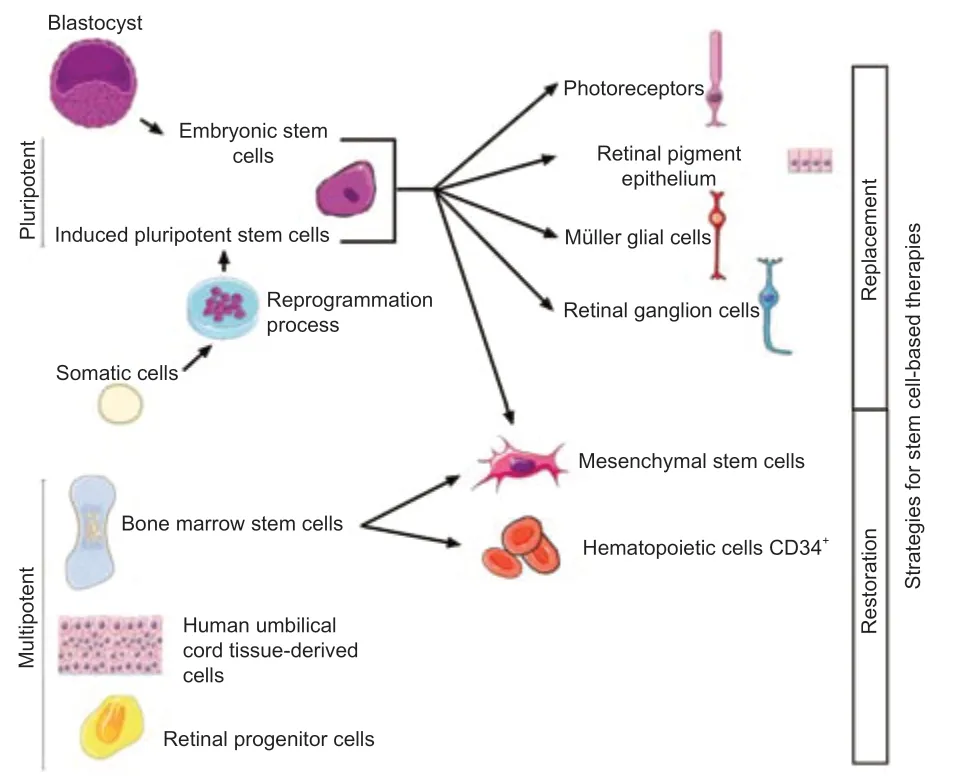
Figure 2|Two strategies for stem cell-based therapies: replacement or restoration of dysfunctional cells.
In cases of cell transplantation aimed at releasing trophic factors,different types of cells have been tested in clinical trials: bone marrow-derived mesenchymal stem cells (BMSCs),human umbilical tissue-derived cells(hUTCs),and retinal progenitor cells (RPCs) (Liu et al.,2017;Wiącek et al.,2021).For replacement of dysfunctional cells,candidate cells are PRs,RPEs,Müller glial cells,and retinal ganglion cell-derived from human embryonic stem cells (hESCs)/human induced pluripotent stem cells (hiPSCs),even if at present only hESC/hiPSC-RPE cells are used in clinical trials.
Cell Source
Stem cells have the ability to differenti ate into other cell types from all three germ layers: ectoderm,mesoderm,and endoderm.Thanks to their potenti al,much research has been done to use stem cells to replace or repair damaged cells in many diseases.
Embryonic and pluripotent stem cells
Embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass of blastocysts (Mackinlay et al.,2021).Since their derivation from human blastocysts,the potential application of ESCs was greatly studiedin vitro,andin vivofor cell-based therapy.One major problem associated with human ESC-derived cell transplantation is an uncontrolled immune reaction that can lead to the rejection of grafted cells,requiring the use of immunosuppressors (Zhao et al.,2017).A potential alternative to avoid immunological rejection is the use of induced pluripotent stem cells (iPSCs),which could be derived from patient-specific somatic cells.
The first derivation of hiPSC occurred in 2007 (Takahashi et al.,2007).Like ESCs,iPSCs are pluripotent stem cells that can be differentiated into all lineages of the body.They are similar to ESC in terms of proliferation and differentiation capacities,and morphology (Logan et al.,2019).iPSCs are obtained by reprogramming various somatic cells,such as fibroblasts,blood cells,and urine cells,by different methods including episomal DNA plasmid,Sendai virus,adenovirus,mRNAs,and proteins (Gerami-Naini et al.,2016).At the beginning,the reprogramming method consisted in introduction into somatic cells of four transcription factors (c-Myc,Oct4,Sox2,and Flf4) by retroviral vectors (Takahashi et al.,2007).The use of c-Myc,a pro-oncogene,and retroviral vectors,which can cause genomic mutation,made this method inapplicable in human clinical trials (Nakagawa et al.,2010).Several studies have raised concerns over the genetic and epigenetic abnormalities of iPSC induced by the reprogramming process.At present,many protocols exist to obtain suitable iPSCs for clinical trials from somatic cells,with L-Myc instead of c-Myc,in non-integrative methods for reprogramming.
The generation of iPSCs from somatic cells enables us to obtain stem cells without using human embryos,which raises ethical issues (Takahashi et al.,2007).Cells for transplantation derived from iPSCs could overcome ethical concerns.In fact,the isolation and use of ESCs from preimplantation embryos produced byin vitroferti lization raise ethical issues.One approach to overcome this issue is the use of hiPSCs.One advantage of iPSC is the possibility to obtain cells from various human leucocyte antigen (HLA)types (Tu et al.,2019).HLA typing is used to match patients and donors for transplantation.In this context,the creation of HLA-iPSC obtained from donors of blood group O (compatible with all blood groups) bank is considered as a future clinical strategy for HLA-matched cell transplantation to minimize the risk of graft rejection (Taylor et al.,2005;Morizane et al.,2017).
However,the utilization of iPSCs for cell therapy also has some drawbacks such as epigenetic memory.Indeed,it has been shown that RPE cell derived from hiPSC (obtained after fibroblast reprogramming) retains a “memory”of gene expression patterns of the cell of origin (Hu et al.,2010).These lineage-specific imprints in hiPSC could affect certain cell properti es such as proliferation and senescence.Moreover,due to their unlimited proliferative capacity,many questions have been raised about serious safety issues.Indeed,it is well-established that undifferentiated cells can generate teratomas or teratocarcinomas and induce potenti al immune reactions when transplanted(Schwartz et al.,2015).According to the literature,teratoma formation is expected to begin within the first few months after transplantation (Schwartz et al.,2015).Therefore,before clinical application of pluripotent stem cell materials,it is mandatory to develop robust differentiation protocols in order to eliminate pluripotent cells and minimize the risk of abnormal cell proliferation.
BMSCs and hUTCs
BMSCs are located in the bone marrow,which has the highest proportion of adult stem cells.Two types of BMSCs are defined: mesenchymal stem cells(MSCs) and hematopoietic stem cells,also called CD34+cells (Enzmann et al.,2017;Aboutaleb Kadkhodaeian et al.,2019).These cells are multi potent,and they have a capacity for differenti ation but it is more limited than pluripotent stem cells,and show paracrine trophic effects with secretion of neurotrophic factors or anti -inflammatory modulators.MSCs represent less than 0.1% of the cells in bone marrow but can be expanded easilyin vitro.They are also found in many tissues such as teeth or liver.
BMSCs have many advantages: they are able to migrate toward lesion sites and they have the capacity for trans-differentiation (ability to differentiate into cells of other organs in specific environment) (Hong and Xu,2011;Enzmann et al.,2017;Aboutaleb Kadkhodaeian et al.,2019).When RPE cells are damaged,they express specific chemoattractive cytokines/chemokines that induce migration of BMSCs to the injured site (Park et al.,2021).Once on the site,they can transdifferenti ate into retinal cells [RPE cells (Enzmann et al.,2017) and PRs (Hong and Xu,2011)] to repair the damaged tissue.BMSCs can also produce neurotrophic factors to promote cell survival and anti-inflammatory effects.Another advantage is that CD34+cells are easily obtained from patients and require minimal manipulation before use during autologous procedures for transplantation.
hUTCs are derived from extra-embryonic mesoderm: cord tissue or cord blood component (Ho et al.,2017).The factors secreted by hUTCs include growth factors (hepatocyte growth factor,glial cell-derived neurotrophic factor,etc.),multiple receptor tyrosine kinase ligands,bridge molecules,and cytokines.These cells also secrete the thrombospondin family proteins involved in synaptic connecti vity and neuronal growth (Koh et al.,2018).
RPCs
RPCs are the cells at the origin of retina formation during embryonic development and represent a highly interesting source of cells in retinal therapies.They can be obtained from the retina of human fetuses between 16 and 20 weeks of gestation (Wang et al.,2020).They are able to migrate and differenti ate along the PR lineage and may have the potenti al to replace rods and cones in degenerative retinal disease.Transplantation of RPCs has two advantages: promoting neuroprotection by secretion of trophic factors that enhance retinal survival (insulin-like growth factor-1,hemodiafiltration,etc.),and photoreceptor replacement (Stern et al.,2018).
Which type of cells for transplantation?
Restoration of vision using a single type of retinal cell seems simplistic and opti mistic especially in case of progressive retinal diseases.Indeed,the retina is composed of a highly complex layered structure where more than one type of cell is generally affected in retinal disease,due to the high degree of cellular interconnection.For example,during AMD,progressive RPE cell death is followed by underlying PR degeneration.Multiple studies have shown that hiPSCs/hESCs can evolve toward a three-dimensional (3D) retinal tissue with major retinal cell populations organized in different layers (Zhong et al.,2014;Hallam et al.,2018).The ability of ESCs/iPSCs to form retina in a dish is currently being developed to investigate retinal disease progression and as atissue for cell transplantation.
Currently,clinical trials are focused on transplantation of RPE cells derived from hESCs or hiPSCs to cure retinal degeneration.
Preclinical Model
In retinal disease,despite robust genetic animal models,the lack of macula in rodents is a serious of limitations.The distribution of rods and cones differs among rodent,primate and human (Shirai et al.,2016).Despite these limitations,studies of integration,survival and visual improvement induced by cell-based transplantation in rodents have led to major advances in stem cell therapy.
hESC/hiPSC-derived cell transplantations
Since 2004,many reports have shown that mammalian retinas have a regenerative potency and can regain visual function after incorporating retinal cells derived from ESC/iPSC by transplantation (Table 1).Inrho(rhodopsin)knockdown mice,embryonic retinal precursor cells differentiate into cells of retinal lineage after transplantation in eye (Klassen et al.,2004).Many studies have also shown that transplantation of mouse ESC/iPSC-derived cells can integrate and improve visual performance in a rat and mouse model of retinal degeneration (Sun et al.,2015;Tu et al.,2019;Salas et al.,2021).The adaptation of differenti ation protocol to human stem cells has demonstrated the long-term safety of hESC-RPE cells grafted as a cell suspension into the retina in both rat and mice models with rescue PR and improvement of visual functions (Zhu et al.,2020;Salas et al.,2021).It has been shown that hESC/hiPSC-RPE cells grafted as monolayer can integrate the host retina and establish ti ght junctions (Riera et al.,2016).The Royal College Surgeons rat is an animal model with inherited retinal degeneration (mutation in theMertkgene),widely used in preclinical studies for cell transplantations (Rajendran Nair et al.,2021).In this rat model,which is characterized by a phagocytosis defect in their RPE cells,hESC/hiPSC-RPE cell transplantation restored phagocytosis of photoreceptor,and improved retinal function up to 12 weeks post-transplantation (Riera et al.,2016;Salas et al.,2021).A neuroprotective effect of hESC/hiPSC-RPE cells has also been observed.Indeed,hESC/hiPSCRPE cell transplantation protects retina from both cell degeneration and glial stress (Sun et al.,2015;Riera et al.,2016).
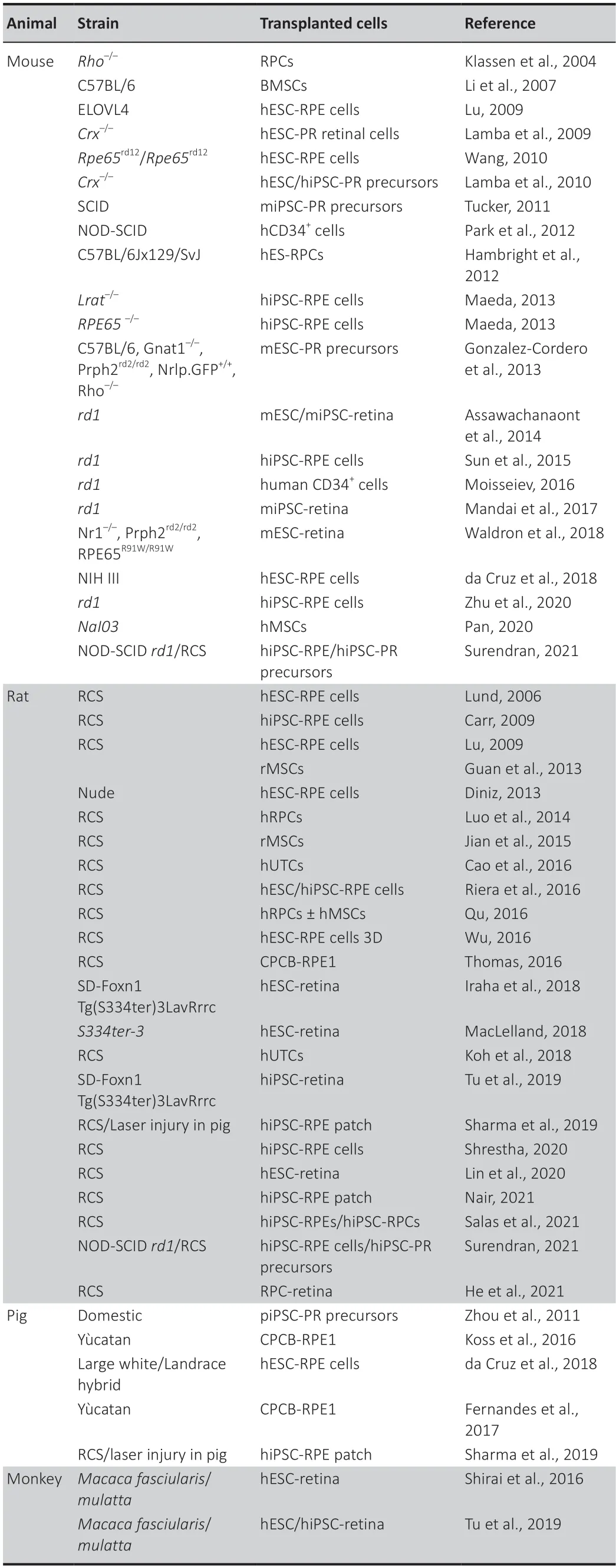
Table 1|Summary of stem cell-based transplantation in the animal model for retinal disease therapy from 2004 to 2022
hESC/hiPSC-PR precursors transplanted into the retina in animal models of retina degeneration have been robustly integrated into hosts and expressed typical markers of PR (Lamba et al.,2009,2010;Zhou et al.,2011).Since 2011,numerous works have described protocols to obtain appropriate stages of development PR from 3D embryoid body (Eiraku et al.,2011;Gonzalez-Cordero et al.,2013).Rescue of visual function following transplantation of hESC/hiPSC-PR precursors is associated with cytoplasmic material transfer between transplanted cells and host cells (Waldron et al.,2018).This protein exchange is made by cytoplasmic fusion and is exclusively restricted to PR grafted-PR endogenous interaction and contributes to the rescue of dysfunctional PRs.For this phenomenon to take place,it is necessary that PR be present and functional in the host retina,highlighting the importance of the host environment for transplantation outcomes.
hESC/hiPSC-retinal sheet transplantations
Diniz et al.(2013) evaluated cell survival and tumorigenicity of suspension or patch hESC-RPE cell transplantation in rats.hESC-RPE patches were plated as a polarized monolayer on a parylene membrane.Parylene is an arti ficial Bruch membrane that allows adherence of epithelial monolayer RPE cells.This substrate is non-degradable,permeable,and stable in the subretinal space after transplantation.They showed that a hESC-RPE patch improved the survival of transplanted cells compared to suspension cells.In another study,the safety,survival,and functionality of the hiPSC-RPE patch transplantation were confirmed in rats (Rajendran Nair et al.,2021).In parallel,it has been shown that hESC-RPE patch transplantation in pig eyes could also survive(Fernandes et al.,2017;Sharma et al.,2019).The authors demonstrated the surgical feasibility and reliability of transplantation,and also a lack of systemic distribution of grafted cells in comparison with cell suspension transplantation(Koss et al.,2016;da Cruz et al.,2018;Sharma et al.,2019).
Development of 3D differentiation yields self-formation of the stratified retinal tissue at any development stage for transplantation (Eiraku et al.,2011).Numerous protocols have been established to obtain large populations of postnatal-stage hESCs/hiPSC-PRs from 3D culture that can be used in transplantation (Assawachananont et al.,2014;Reichman et al.,2014;Zhong et al.,2014).Assawachanaont et al.(2014) evaluated the ability of grafted 3D-differentiated mouse ESC-or mouse iPSC-derived retinal sheets to integrate the host retina.They generated efficient and reproducible mouse ESC/iPSC optic vesicles with retinal neuroepithelial-like layers,and showed that subretinal transplantation in a mouse model could lead to a well-developed stratified retinal layer.Moreover,transplanted mouse ESCor iPSC-derived retinal sheets could form an integrated outer nuclear layer with mature PR able to respond to light (Shirai et al.,2016;Mandai et al.,2017;Iraha et al.,2018;McLelland et al.,2018;Lin et al.,2020).Shirai et al.(2016) showed that retinal sheets derived from hESCs could survive,mature(with the development of structured PR),and integrate after transplantation in monkey retina.Another study demonstrated that the transplantation of retinal cells derived from hiPSCs has the same potency as retinal cells derived from hESCs (Tu et al.,2019).They observed long-term survival of a transplanted retinal sheet derived from hiPSCs for more than 2 years in one monkey.
BMSC and hUTC transplantation
In rats,transplantation of hUTCs has been associated with the preservation of PR and visual function (Cao et al.,2016).In vitroanalysis explained that hUTCs rescue the phagocytic defect function of RPE by secretion of neurotrophic factors such as brain-derived neurotrophic factor,hepatocyte growth factor,and glial cell-derived neurotrophic factor (Cao et al.,2016).Subretinal injection of hUTCs before PR loss in Royal College Surgeons rat could also preserve retinal synaptic connectivity and attenuates Müller glial reactivity by the secretion of thrombospondin family protein (Koh et al.,2018).Indeed,synaptic defects are associated with changes in Müller glial morphology and reactivity.By secretion of thrombospondin,hUTCs improve the retinal environment by decreasing the activation of Müller glial cells and protecting the synaptic connecti vity.
Transplantation of BMSCs in a rat model of retinal degeneration can initi ate regenerative mechanism (Guan et al.,2013;Jian et al.,2015).Moreover,transplanted BMSCs secreted factors such as nerve growth factor that could stimulate Müller cells to transdifferenti ate into new retinal neurons and slow generation (Jian et al.,2015).
For intravitreal injection of CD34+cells in NOD-SCID mice,no major safety concerns were observed in different studies (Park et al.,2012).Even if no recovery of retinal function was observed after transplantation of CD34+cells,potenti al trophic regenerative effects have been observed with the change in gene expression involved in PR maintenance and apoptosis (Park et al.,2021).
RPC transplantation
Transplantation of RPCs has also been associated with the preservation of PRs and visual function in rats (Luo et al.,2014).Hambright et al.(2012)highlighted that transplantation of RPCs derived from hESCs can integrate the retina and differenti ate into PRs in a mouse model.Moreover,a recent study showed that transplanted organoid-derived RPCs in rats are able to establish synaptic connections with the host retina (He et al.,2021).In 2016,co-transplantation of RPCs and MSCs in a rat model of degeneration showed better results than RPC or MSC alone (Qu et al.,2017).In this study,the Qu et al.(2017) showed that combined grafted cells migrated better and differentiated into PRs more often after transplantation compared to a single-cell strategy,thanks to the secretion of neurotrophic factors that improve the microenvironment.They also concluded that combined cell transplantation highlights a higher improvement of vision in this animal model.
Clinical Trials
Currently,the most important limitation in transplantation therapy is the lack of suitable donor organs,tissue or cells.In humans,only allogenic,or HLAmatched donor-derived stem cells can be used in cell-graft ing therapy.Due to evidence that transplantation of cells in a preclinical model can rescue PR and prevent visual loss,in 2010 the US Food and Drug Administration approved the launch of phase I/II stem cell clinical trials for retinal diseases in humans(Schwartz et al.,2015) (Table 2).Currently,hESC/hiPSC-RPE is the only cell type suitable for the clinical grade.
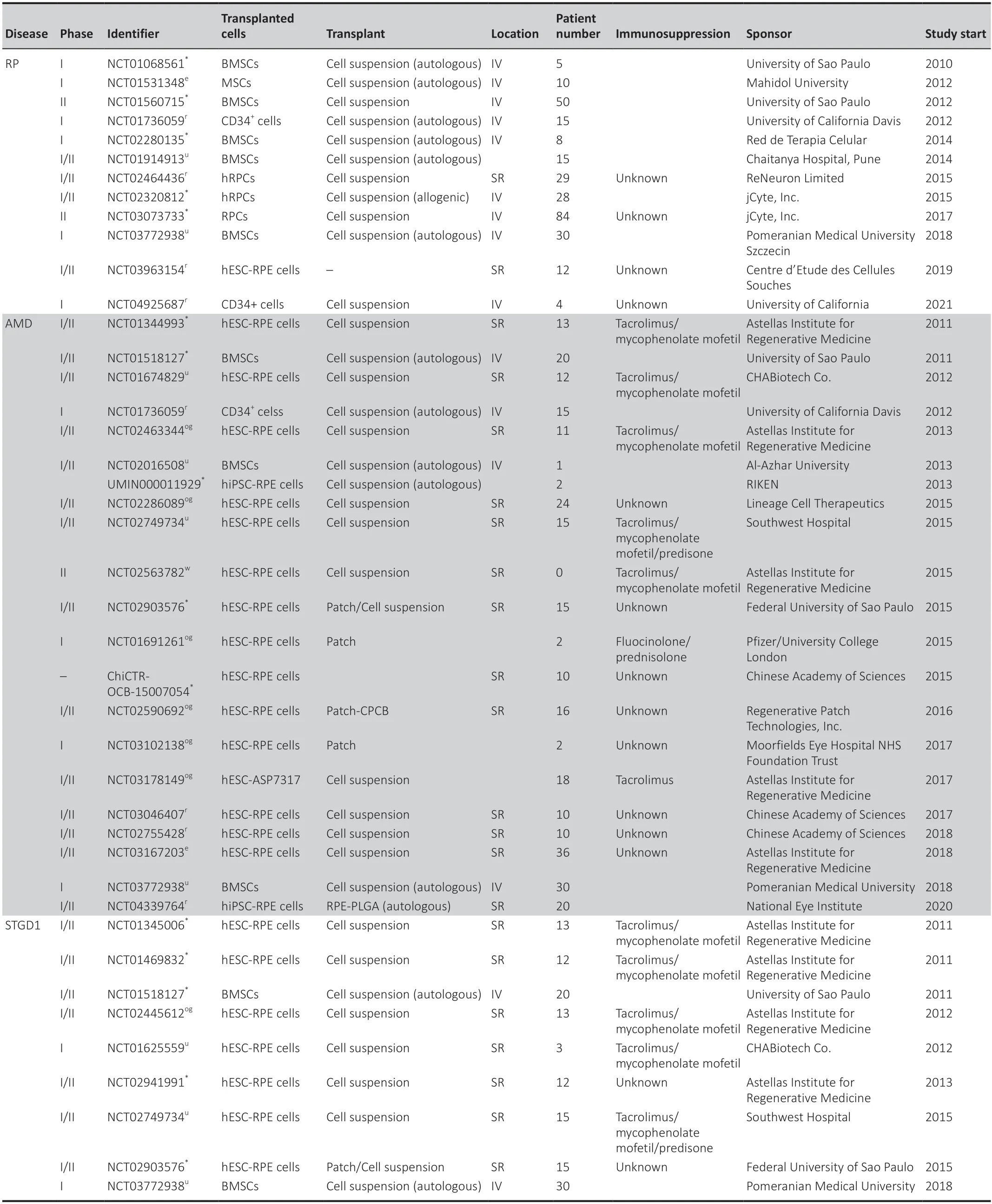
Table 2|Summary of hESC,hiPSC or multipotent cell transplantation trials in the world for retinal disease therapy from 2010 to 2022
RPE cell suspension or patch?
Up to now,clinical trials focused on the transplantation of RPE cells derived from hESCs or hiPSCs to prevent or slow down the retinal degeneration.Two different approaches to transplantation were evaluated: RPE cell suspension and RPE patch (Song et al.,2015;Kashani et al.,2018).Even if transplantation of cell suspension seems safe,without any serious adverse effect,it is not clear today whether RPE-transplanted cells can integrate or establish a monolayer organization after transplantation.For long-term efficacy,RPE cell-transplanted cells must be polarized into the host retina and establish connections with neighboring RPE cells to be able to play their role as phagocytosis of photoreceptor outer segment,maintenance of homeostasis and transport of nutriment into the new host retina.Moreover,RPE in suspension can be lost during the surgical procedure,due to reflux through the retinal hole created for the subretinal injection of donor cells (da Cruz et al.,2018).
Because they already form a monolayer epithelium,transplantation of a patch of RPE seems to be the ideal therapeutic substrate to treat retinal disease in stem cell therapy.Cells are fully differentiated,polarized withti ght junctions,and a configuration close to their native ones (da Cruz et al.,2018).In addition,delivery of patches could decrease the risk of vitreoretinal proliferation (cellular migration and proliferation in vitreous cavity resulting in retinal detachment),which is the most severe clinical complication (da Cruz et al.,2018).Nevertheless,RPE sheet may also increase the risk of retinal detachment insofar as a larger retinotomy may be required to inject the RPE patch,compared to subretinal injection of RPE cell suspension (MacLaren et al.,2016).A recent study showed that absence of scaffold for the RPE patch led to aggregation of RPE in the region of atrophy rather than uniform distribution (Kashani et al.,2018).RPE with a supportive scaffold appears to show improved integration in the host retina.Bruch membrane (the basement membrane of RPE) changes are observed with aging and affect RPE metabolism and attachment.In this context,RPE monolayer cells adherent to a substrate as ultrathin parylene substrate (California Project to Cure Blindness Retinal Pigment Epithelium 1,CPCB-RPE1) that mimics properti es of Bruch membrane (Kashani et al.,2018) or with a human vitronectincoated polyester membrane (da Cruz et al.,2018) could be a good option for therapeutic purposes.
Transplantation approach
Successful treatment for retinal dystrophies also depends on effective methods of cell administration.Clinical trials have used two major approaches in stem cell-based therapy: subretinal injection of a cell suspension,and subretinal positioning of a patch after retinotomy.Intravitreal transplantation requires a vitrectomy followed by a limited retinotomy,usually in the upper part of the macula,and the introduction of the patch.For subretinal injection of cells,no retinotomy is required because the cell suspension can be delivered through a subretinal cannula (Wilson et al.,2017;da Cruz et al.,2018).
The main complications of these surgical procedures are retinal detachment,vitreoretinal proliferation,endophthalmitis,and graft rejection (Hartman and Kompella,2018).Moreover,cells in suspension are not polarized and cells on patch can be lost or damaged in part,when delivered through the cannula (da Cruz et al.,2018).
Clinical trials using hESCs
Results of the first human trial using hESC-RPE cells were published by Schwartz et al.(2012) on 13 dry AMD patients and 13 STGD1 patients.Immunosuppressive systemic therapy was required for the first 3 months after cell transplantation to minimize cell rejection.However,immunosuppression was responsible for adverse effects in 28% of patients in this study (Schwartz et al.,2015).Indeed,the use of systemic immunosuppressants is related to complications such as urinary tract infection,gastrointestinal symptoms,or nonmelanoma skin cancers(Moutinho et al.,2020).In this first human trial with hESC-RPE cells,no signs of hyperproliferation could be observed after a 4-month follow-up.Furthermore,the safety and tolerability of the transplants in 18 patients with AMD (n=9) or STGD1 (n=9) were demonstrated during a longer follow-up between 12 and 36 months (Schwartz et al.,2015).In fact,the graft was present in 72% of patients after 1 year.They also observed an increase in best-corrected visual acuity in ten patients,while it remained stable in seven and deteriorated in one patient.No improvement was found in the patients’ untreated fellow eyes.Vision-related quality of life scoring increased by 25 points in cases of AMD and by 20 points in cases of Stargardt macular dystrophy.This study is the first to report the medium/long-term outcomes of stem cell application in AMD and STGD1 (Schwartz et al.,2015).However,there are sti ll concerns considering the lack of a masked control group to control for the placebo effect and examiner bias in the subjective measure of visual acuity,the very limited sample size,and poor initi al visual acuity.In another study,the long-term safety and tolerability of transplantation of hESC-RPE cells in seven patients with STGD1 were confirmed 5 years after transplantation (Li et al.,2021).
In parallel,Song et al.(2015) followed up four patients (two with dry AMD and two with STGD1) for 1 year after hESC-RPE transplantation and observed no evidence of adverse proliferation or tumorigenicity.Like Schwartz et al.(2012),hESC-RPE transplantation led to improvement in visual acuity in three patients (one remained stable).Subretinal injection of hESC-RPE cells has been shown to parti ally restore the structure and functionality of RPE cells(Kashani et al.,2018).
Since the initial report of hESC-RPE cell transplantation,many other stem cell treatment strategies have been proposed with the inclusion of arti ficial material with hiPSC-RPE sheets from HLA-matched with the host.The first transplantation of hESC-RPE cells plated on CPCB-RPE1 showed no evidence of safety concerns for patient and none of the implanted eyes showed progression of vision loss (Kashani et al.,2018).Moreover,one patient had improved vision,and two others improved fixation (ability to visually fixate in a specific location).hESC-RPE cells can also be plated onto a human vitronecti n-coated polyester membrane to be transplanted into the subretinalspace (da Cruz et al.,2018).In this first report,the authors suggested the efficacy and safety of RPE patch after 12 months of follow-up in two patients with severe wet AMD.In one patient,improvement of PR function was observed by electro-oculography.In parallel,12 patients with severe STGD1 transplanted with hESC-RPE cells showed the safety and potenti al efficacy of the graft (Mehat et al.,2018).
Clinical trials using hiPSCs
The first human clinical trial using autologous hiPSC was investigated by RIKEN in 2014,a research institute in Japan,to treat one patient affected by AMD (Mandai et al.,2017).Because this cell transplantation is autologous,no systemic immunosuppression was needed.The trial was interrupted because of a new regulatory framework required for regenerative medicine adopted in 2014,even if the patient showed no serious adverse effects(Garber,2015).There was no visual acuity gain either.The main concern was the formation of tumors but it was not observed during this trial.The second patient was not transplanted because three single nucleotide variations and three copy-variant numbers were observed in hiPSCs but not in the original patient’s somatic cells (Garber,2015).Later,Mandai et al.(2017)demonstrated the feasibility of transplanting a sheet of autologous RPE cells derived from hiPSCs,obtained from skin fibroblasts,in one patient with wet AMD.No adverse events were observed within 25 months of follow-up and no improvement in visual acuity neither.As in RIKEN clinical trial,the second patient could not be transplanted due to the presence of mutations in his iPSCs.
One major issue with the utilization of hESC/hiPSC-derived cells for transplantation is the rejection by the host immune system.Autologous iPSCbased cell transplantation seems to be an appropriate alternative but has yet to become a standard treatment because of the costs and the amount oftime required.In this context,RIKEN grafted five patients with HLA-matched allogenic hiPSC-RPE cells in 2017 (Sugita et al.,2020).During 1-year follow-up,no abnormal growth was observed but some adverse events occurred,such as corneal erosion (1/5),epiretinal membrane (1/5),elevated intralocular pressure (3/5),endophthalmitis (1/5),and mild immune rejection in eye(1/5).Despite these adverse events,this was the first proof of concept that it is possible to have the safety and survival of allogenic hiPSC-RPE cell graft s.A new clinical trial was initi ated in 2020 by the National Eye Institute on 20 AMD patients to evaluate the safety of transplantation of hiPSC-RPE cells on poly(lactic-glycolic acid support.Poly(lactic-glycolic acid) is a biocompati ble and biodegradable scaffold for RPE cell transplantation that has been shown to form a Bruch membrane-equivalent structure.
Clinical trials using BMSCs
The first trial preliminary reports after CD34+cells intravitreal transplantation showed the safety and the feasibility of the procedure (Puertas-Neyra et al.,2020).Patients included in this trial presented AMD,retinal occlusion,STGD1,or RP.No intraocular inflammation or hyperproliferation was observed but there was no stati stically significant visual acuity improvement after 6 months and the authors were not able to show the intraretinal incorporation of these CD34+cells during this trial.In another clinical trial,they confirmed that BMSC transplantation is safe (no adverse event) and effective (improvement of visual acuity) for a long period of 12 months (Wiącek et al.,2021).
Clinical trials using RPCs
In 1999,when patients with RP received a suspension of hRPCs injected into the subretinal space (Das et al.,1999),no clinical appearance of adverse effects (inflammation,infection,or rejection) was observed.In another study,Liu et al.(2017) confirmed the safety and the feasibility of vision improvement through transplantation with RPCs in RP patients after a 24-month follow-up study.They observed a significant but transient improvement in visual acuity in five patients.However,this improvement was not maintained 12 months after transplantation.Another clinical trial using human RPCs began in 2015 for an enrollment of 28 patients with RPs (NCT02320812).These patients received a single intravitreal injection (0.5–1–2 or 3 million cells).Patients were followed for 2 years to evaluate the safety and tolerability of the transplantion.Preliminary results showed that intravitreal injection of RPCs was safe and seems to positively impact visual acuity at high dose cell level(Kuppermann et al.,2018).
Conclusion
Stem cell therapy is considered as a very promising therapeutic approach for many pathologies such as Parkinson’s disease,traumatic brain injury,and AMD.Recently,the US Food and Drug Administration provided a warning about stem cell therapies,recalling that any stem cell treatment must be Food and Drug Administration-approved in the US or must be under a clinical investigation plan submitted and allowed to proceed by the US Food and Drug Administration,because unscrupulous providers offered stem cell products that were both unapproved and unproven (US Food and Drug Administration,2019).
In the case of retinal dystrophies,most clinical studies established the safety and tolerability of transplantation of RPE cells derived from pluripotent stem cells as a curative treatment (Song et al.,2015).Despite the major role of RPE cells for retina homeostasis and maintenance of the blood-retinal barrier,it could be better to co-transplant RPE cells with PRs to ensure the beneficial effect on vision.Indeed,in eye diseases such as AMD,multiple retinal cell subtypes are affected (RPE cells,PRs,choriocapillaris) and transplantation of one type of cells could not be sufficient (McLelland et al.,2018).In the same way,even if transplantation of other retinal cells would be needed in RPs,replacing only PRs without RPE cells seems not to be a good option for long-term efficacity and remains at a preclinical stage (Barnea-Cramer et al.,2016).In this context,the development of co-transplantation of organized layer cells for clinical trials is essenti al.In this way,optimization of protocols to obtain more complex multi layer retinal cells used for transplantation need to be developed to improve the integration and the survival of transplanted cells.Since the last decade,the emergence of retinal organoids,in vitrominiaturized and simplified model systems of organs,allow access to more physiologically relevant model systems for clinical transplantation (McLelland et al.,2018).Development of more complex systems could provide more functional cells,an essenti al criterion for graft cell survival (Qiu,2019).If it has been shown that RPE-grafted cells are able to establish ti ght junctions between each other and with host cells,transplantation of epithelium RPE cells could have a better benefit compared to suspension-grafted cells that do not form confluent monolayer RPE.This question is essenti al because (i) RPE epithelium organization is essenti al for retina morphological integrity and for maintenance of blood-retinal barrier (ii) proper connections among different retinal cell types are needed to transmit the electrical signal.In addition,in vitrostudies have shown that embryonic RPE cells can adhere to normal but not aged Bruch membranes from post-mortem individuals and retinal detachment was the most severe clinical complication of AMD (da Cruz et al.,2018).
Another important question is which type of transplanted cells– allogenic or autologous– should be used in stem cell therapies.Autologous transplantation can be considered as opti mal condition but requires time to prepare cell suspension or graft and are not available rapidly (da Cruz et al.,2018).In comparison,allogenic subretinal transplantation requires prolonged systemic immunosuppression.However,ocular side effects can occur using long-term systemic immunosuppressive drugs as higher risk of mortality and fatal cancer (Kempen et al.,2008).Indeed,it has been hypothesized that systemic immunosuppressive therapy,by impairing immunity itself,may increase the risk of malignancies.In order to avoid side effects of immune suppressive drugs,HLA-matched allogenic transplantation without immune suppression could be used but the cost of iPSC preparation is high.
In parallel,we have to determine at which disease stage,the cell transplantation will have the most beneficial effect.It could be better to perform stem cell therapy at the beginning of the retinal disease,when transplanted cells could be integrated into existing layers and help to ensure the survival of cell host,but at this stage of the disease,visual acuity is usually maintained.
In conclusion,developments of stem cell technologies have allowed multiple clinical trials to be currently ongoing.Despite encouraging results,novel therapies based on stem cell sti lls need to be optimized,including the surgical procedure,the conditioning and the subtypes of the cells,and the postoperative protocols.
Author contributions:AV designed the manuscript.AV and AP wrote the manuscript.All authors reviewed and approved the final version of this manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

