Functional hyperconnecti vity related to brain disease: maladaptive process or element of resilience?
Markus Aswendt,Mathias Hoehn
Neuroimaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography provide uniquein vivodata to analyze structural and functional connectivity of the whole brain.Recent advances in small animal neuroimaging have opened new opportunities for the study of structure-function interactions in healthy and diseased brain networks,which are essential to develop therapies targeting network reorganization associated with functional improvement.Based on clinical studies,a common network response to acute neurological insult(e.g.,stroke) and neurodegeneration (e.g.,Alzheimer’s disease) is hyperconnectivity,i.e.,an increase of functional connectivity strength above (healthy) control levels,a process which is,however,not well understood.For example,in resting-state functional MRI (rs-fMRI),which measures the hemodynamic response to neuronal activity,hyperconnectivity would refer to a drastic increase in the correlation between two regional time series.In absence of an absolute threshold defining hyperconnectivity,it relates to a control group or baseline measurement before intervention.It was hypothesized that hyperconnecti vity follows a nonlinear distribution representing an interaction between actual demands,injury severity,and resource availability(Hillary and Grafman,2017).Hyperconnectivity is significantly larger than transient variability in functional networks.The increase in connecti vity follows a nonlinear distribution that reaches a maximum and transitions to a state of hypoconnectivity when a critical loss of structural resources is reached (Figure 1).As an extension of this hypothesis,we consider hyperconnecti vity as part of an understudied and not well understood compensatory mechanism,which is embedded in the framework of the brain’s resilience capacity to respond to (network) disturbances.
Recently,we were able to confirm hyperconnectivity in a translational comparison of human strokeversustwo experimental mouse models of cortical stroke (Blaschke et al.,2021).Hyperconnectivity in response to brain injury,which eventually leads to network disruption,seems to be surprising,also considering that most neurological disorders result in neuronal cell death,triggering a progressive response of axonal damage and structural disconnection.Indeed,we and others have observed an initial reduction of functional connectivity paralleled with structural disconnection in the acute phase after stroke (van Meer et al.,2012;Harris et al.,2016;Aswendt et al.,2022).However,there is no linear relationship between structural and functional connectivity,and the adaption of functional connecti vity during disease progression and (spontaneous) functional recovery appears to be much more complex.
In stroke,alteration of functional connectivity strength seems to depend on lesion size: larger strokes,characterized by hypoconnectivity,covering cortical or cortical and subcortical areas show no or much less recovery of functional connecti vity (van Meer et al.,2012;Green et al.,2018) compared to small cortical strokes in which hyperconnectivity appears over a time course of 4–8 weeks (Blaschke et al.,2021).In amyloidosis and tau mouse models representing forms of dementia such as Alzheimer’s disease,the picture is less clear,however,with a tendency of hyperconnecti vity in the early stage (pre-behavior symptoms) and hypoconnectivity at the late stage (in fully expressed pathology as confirmed by histology) (Van der Linden and Hoehn,2021).These results mirror the clinical literature with a mixed and dynamical distribution of both hypoand hyperconnectivity in patients with traumatic brain injury,multiple sclerosis,mild cognitive impairment,and Alzheimer’s disease (Hillary and Grafman,2017).Few so far existing experimental studies including therapeutic treatments have all shown (re-)normalization of functional connecti vity strength.
Thus,many experimental data appear to support the hypothesis of compensatory mechanism of functional networks,reflected as hyperconnecti vity in early-stage disease or mild lesion severity,while an aggravated,late-stage disease and high severity results in hypoconnectivity (Figure 1).While additional experimental (and clinical)data are needed for further support,it must be emphasized that this alteration of functional connectivity may only be seen as a diagnostic/prognostic “fingerprint”,which,however,is still lacking mechanistic understanding.
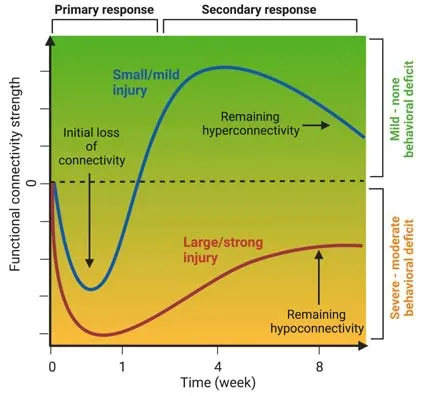
Figure 1|Illustration of the time course of functional connecti vity strength representing small/mild (blue) and large/strong (red) brain injury.
Comparing global connecti vity or specific regionby-region changes over time neglects the intrinsic property of the brain to respond to a disease with network-wide changes,which are best represented using graph theory.In this mathematical approach,anatomic regions are considered nodes and the connections between these nodes are expressed as edges,thus providing a wealth of possibilities to mathematically describe network (re-)organization in human and animal MRI (Scharwachter et al.,2022).Interestingly,several studies reported an increase in the network parameter small worldness (van Meer et al.,2012;Blaschke et al.,2021;Aswendt et al.,2022),which should not be misunderstood as an increased network efficiency.For rather small cortical strokes,an increase at 14 days is driven by elevated levels of global clustering (more segregation between local clusters) and lower characteristic path length(smaller number of steps or more efficient way to reach the destination).Similar to the connecti vity comparisons,here,the size of the initi al damage and the timing drive the development of small worldness differently.At 70 days post stroke,smaller strokes show normalized small worldness,in larger strokes small worldness remained increased driven by high characteristic path length(van Meer et al.,2012).Interestingly,in traumatic brain injury,small worldness was initially (at 7 days post-injury) decreased mainly because of decreased clustering coefficient (indicating a more random network) (Harris et al.,2016).Dynamic changes in network parameters might reflect neuronal plasticity leading to structural changes,i.e.,new local and long-range synaptic connections(Scharwachter et al.,2022).The initi al overshoot in connectivity might reflect random integration which is refined and selectively stabilized during the recovery process (van Meer et al.,2012).
Recent studies have focused on investigating the role of selective cell types.Astrocytes play an important role in guiding these plasticity mechanisms.The knockout of essential intermediate filaments glial fibrillary acidic protein and Vimentin in astrocytes leads to attenuated astrogliosis,impaired recovery of sensorimotor function and aberrant restoration of global neuronal connectivity.These mice show higher number of lost and newly formed functional connections between primary and secondary targets of cortical stroke regions compared to wildtype control and increased peri-infarct expression of the axonal plasticity marker Gap43 paralleled by the loss of small worldness network property at 4 weeks post stroke (Aswendt et al.,2022).Similarly,elimination of blood-borne macrophages and microglia,the brain resident immune cells,results in increased connectivity (Aswendt et al.,2021).At a neuronal level,the increase of measured connectivity might be directly related to spiking output during neuronal firing and phase locking across nodes (Hillary and Grafman,2017).However,increased connectivity was also shown in a recent study using chronic chemogenetic inhibition.Electrophysiological recordings confirmed that the inhibition promotes intra-and interareal slow oscillatory coherence in a frequency range measured with rs-fMRI (0.1–4 Hz) (Rocchi et al.,2022).Comparably,stroke patients with larger infarct volume show higher delta band (0.5–4 Hz)power in bilateral hemispheres and higher delta band coherence between ipsilateral motor cortex and bilateral regions.In subacute but not chronic stroke,higher delta coherence correlates with poorer motor status (Cassidy et al.,2020).
To further characterize the clinical relevance of hyperconnectivity,longitudinal imaging studies are necessary,which carefully dissect the temporal profile of connectivity changes in response to distinct levels of severity achieved with different animal models of acute and neurodegenerative diseases.Finally,structural network alterations,reflecting primary and secondary tissue damage,but also spontaneous regeneration processes,may be expected to have direct influence on the functional network behavior during certain time windows.Thus,the direct reciprocal interaction between structural and functional network changes must be included in future detailed analysis.Furthermore,effective connectivity analysis of fMRI data,i.e.,based on a mechanistic model of the causal effects,would be an additional way of better understanding the dynamics of neural coupling during phases of hyperconnectivity.In this way,the positive or negative impact of a localized increase on neighboring regions can be estimated.We are confident that future increasingly complex analysis with graph theory will help to unravel distinct network patterns and their dynamics during disease progression and/or upon therapeutic treatment.
MA gratefully acknowledges financial support by the Friebe Foundation: project ID T0498/28960/16 and the Deutsche Forschungsgemeinschaft (DFG,German Research Foundation): project ID 431549029–SFB 1451.
Markus Aswendt,Mathias Hoehn*
Department of Neurology,Faculty of Medicine and University Hospital Cologne,University of Cologne,Cologne,Germany (Aswendt M)
Cognitive Neuroscience,Institute of Neuroscience and Medicine (INM-3),Research Center Juelich,Juelich,Germany (Aswendt M,Hoehn M)
*Correspondence to:Mathias Hoehn,MD,m.hoehn@fz-juelich.de.
https://orcid.org/0000-0001-5996-7572(Mathias Hoehn)
https://orcid.org/0000-0003-1423-0934(Markus Aswendt)
Date of submission:August 12,2022
Date of decision:October 27,2022
Date of acceptance:November 3,2022
Date of web publication:November 25,2022
https://doi.org/10.4103/1673-5374.361541
How to cite this article:Aswendt M,Hoehn M(2023) Functional hyperconnecti vity related to brain disease: maladaptive process or element of resilience? Neural Regen Res 18(7):1489-1490.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

