Inhibiting phosphatase and actin regulator 1 expression is neuroprotective in the context of traumatic brain injury
Yao Jing ,Lin Zhang ,Shi-Wen Chen ,Yan Guo,Shi-Ming Ju,Fang Yuan,Hao Chen,Dian-Xu Yang,Heng-Li Tian ,Zhi-Ming Xu ,Jun Ding
Abstract Studies have found that the phosphatase actin regulatory factor 1 expression can be related to stroke,but it remains unclear whether changes in phosphatase actin regulatory factor 1 expression also play a role in traumatic brain injury.In this study we found that,in a mouse model of traumatic brain injury induced by controlled cortical impact,phosphatase actin regulatory factor 1 expression is increased in endothelial cells,neurons,astrocytes,and microglia.When we overexpressed phosphatase actin regulatory factor 1 by injection an adeno-associated virus vector into the contused area in the traumatic brain injury mice,the water content of the brain tissue increased.However,when phosphatase actin regulatory factor 1 was knocked down,the water content decreased.We also found that inhibiting phosphatase actin regulatory factor 1 expression regulated the nuclear factor kappa B signaling pathway,decreased blood-brain barrier permeability,reduced aquaporin 4 and intercellular adhesion molecule 1 expression,inhibited neuroinflammation,and neuronal apoptosis,thereby improving neurological function.The findings from this study indicate that phosphatase actin regulatory factor 1 may be a potential therapeutic target for traumatic brain injury.
Key Words:apoptosis;aquaporin 4;blood brain barrier;intercellular adhesion molecule 1;neuroinflammation;nuclear factor kappa B;occludin;phosphatase and actin regulator-1;traumatic brain injury;zonula occludens 1
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability among young people worldwide.The incidence ranges from 108 to 332 hospital admissions per 100,000 individuals every year (Lasry et al.,2017).On average,about 39% of cases of severe TBI are fatal,and about 60% have unfavorable outcomes according to the Glasgow Outcome Scale (Rosenfeld et al.,2012;Lasry et al.,2017).Although progress has been made in understanding the mechanisms and pathophysiology of TBI,this has not led to clear improvements in outcomes.Neuroinflammation following TBI is an important secondary injury process that can trigger ongoing neuronal damage,which has garnered significant attention over the past 20 years (Corrigan et al.,2016).Many pre-clinical studies have implied that targeting the inflammatory response improves outcomes after TBI,but clinical trials have shown that alleviating the inflammatory response has either no effect or harmful effects on patients with TBI (Roberts et al.,2004;Edwards et al.,2005).Thus,more research is needed to elucidate the mechanisms of inflammation after TBI,and to identi fy novel therapeutic targets for this disease.
Phosphatase and actin regulator-1 (PHACTR-1) is highly expressed in endothelial cells and neurons (Allen et al.,2004).PHACTR-1 is thought to regulate synaptic plasticity by modulating protein phosphatase 1 (PP1)activity,and it is important for neuronal activity (Ceulemans and Bollen,2004).In endothelial cells,PHACTR-1 regulates F-actin expression and PP1 acti vity during vascular remodeling,possibly through binding to actin (Sagara et al.,2009;Allain et al.,2012).We previously showed that PHACTR-1 plays a very important role in endothelial cell function (Jing et al.,2019).At the same time,in a previous study,PHACTR-1 mutation was associated with the formation of caroti d plaque and promoted stroke (Kuveljic et al.,2019).
Apoptosis is an important type of cell death,and its role in TBI is well known.Apoptosis is characterized by morphological changes in cytoplasm and nucleus,chromatin fragmentation,and cleavage of genomic DNA.Nuclear factor kappa B (NF-κB) is a transcription factor whose main functions are to regulate the expression of genes involved in cell proliferation and apoptosis and to regulate inflammation (Jimi et al.,2019).NF-κB activation is associated with a wide range of central nervous system pathologies,including TBI (Zhang et al.,2017a),ischemic stroke (Howell and Bidwell,2020),and glioblastoma(Soubannier and Stifani,2017).Inhibition of the transcriptional activator subunit of NF-κB,p65,promotes neuronal survival in TBI (Yuan et al.,2016;Jing et al.,2020).Activating NF-κB can also trigger neural tissue inflammation and scar formation (Kaur and Sharma,2018),but inhibiting NF-κB can markedly promote neurofunctional recovery (Jing et al.,2020).We previously showed that increasing NF-κB/p65 nuclear translocation enhances NF-κB activation,upregulates target genes encoding pro-inflammatory cytokines and aquaporin (AQP)-4,and increases endothelial cell-associated bloodbrain barrier (BBB) disruption (Jing et al.,2020).These results suggest that activating the NF-κB/p65 pathway aggravates TBI (Yuan et al.,2016).
Because NF-κB plays an important role in the pathological processes that occur after TBI,in this study we sought to verify the relationship between PHACTR-1 and the NF-κB pathway and the effects of PHACTR-1 in a mouse model of TBI.We used molecular biotechnology to determine whether PHACTR-1 inhibition attenuates neuroinflammation and cell apoptosis after TBI and to investigate the underlying mechanisms.We hope that PHACTR-1 may be a promising therapeutic target for TBI.
Methods
Animal models
The use of all animals was approved by the Institutional Animal Care and Use Committee of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (approval No.SYXK (Hu) 2011-0128) on February 26,2015.The animal experiments were performed according to the Guidelines for Laboratory Animal Research of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Because of the protective effects of estrogen on post-TBI in female mice,only male mice were used for this study to avoid data misinterpretation (Gong et al.,2022).C57BL/6J male mice (n=140,approximately 8–10 weeks old,20–25 g in weight) were purchased from Shanghai Sipul -Bikai Laboratory Animal Company (license No.SCXK 2013-0016).Green fluorescent protein-adenoassociated virus (AAV)9,which was purchased from Shanghai Genechem Co.,Ltd.(Shanghai,China),was used to overexpress PHACTR-1 or knock down PHACTR-1 expression in the brain tissues of experimental animals.After animals were anestheti zed by intraperitoneal injection of xylazine (10 mg/kg)and ketamine (75 mg/kg) (Sinopharm Chemical Reagent Co.,Ltd.,Shanghai,China),they were randomly divided into overexpression,overexpression control,knockdown,and knockdown control groups (n=5/group).The mice were immobilized by inserting the head into a stereotaxic frame (PinPoint Precision Cortical Impactor 3000,Hatteras Instruments Inc.,Cary,NC,USA).A median incision,about 1.2 cm in length,was then made in the skin and subcutaneous tissue.The right side of the skull was exposed,and holes were drilled at the far right,middle,and far left of the diameter of the longest transverse axis of the bone window (between the lower margin of the coronal suture and the sagittal suture).All three cranial holes were 2 mm apart.Next,for overexpression and knockdown,3 µL virus (about 1.67–3.69 × 1012–13vg/mL) was delivered using a Milton microsyringe (Beijing Nvidia Technology Co.,LTD.,Beijing,China),as follows.First,the flat needle head was placed on the surface of the dura mater through the rightmost cranial foramen and parallel to it.Then,the needle was slowly advanced at a rate of 0.1 µL/min for 10 minutes to achieve a depth of 2 mm.After the injection was complete,the needle was left in place for 10 minutes,then slowly withdrawn.Virus was then similarly injected in the remaining two holes.When all three injections had been administered,a sterile cotton ball was used to cover the holes in the skull.When blood and transparent liquid stopped flowing out of the holes,the subcutaneous tissue and skin were intermittently sutured as the whole layer.After the mice had completely recovered consciousness,they were replaced in their cages and fed routinely for 4 weeks for subsequent detection experiments and CCI model establishment.
After anesthesia,fixation,and scalp incision,virus-transfected mice and normal mice were used to establish the controlled cortical impact (CCI) model.A bone window with a 4-mm diameter (the central area surrounding the virus injection hole) was removed from the right parietal center.Mice with intact dura were treated as the sham operation group.An impactor with a diameter of 3 mm was inserted into the CCI device (Hatteras Instruments Inc.) and placed on the intact dura mater.The surface of the dura mater surface was vertically impacted with an impact velocity of 1.5 m/s,a deformation depth of 1.5 mm,and a residence time of 100 ms to establish a CCI model simulating TBI.We have used this model in previous studies (Yuan et al.,2016;Jing et al.,2020).
Western blot analysis
The injured tissue and surrounding brain tissues were collected at 6,12 hours,1,3,and 7 days after delivery of the cortical impact,and the samples were placed in lysis buffer.Next,the proteins in the lysed samples were denatured and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis,loading equal amounts of proteins into each well.The proteins were then transferred onto a polyvinylidene fluoride membrane,which was blocked with 5% milk and incubated at 4°C overnight with primary antibodies against PHACTR-1 (mouse,1:200,Santa Cruz Technology,Santa Cruz,CA,USA,Cat# sc-514800),β-actin (mouse,1:200,Cell Signaling Technology,Boston,MA,USA,Cat# 3700S),zonula occludens 1 (ZO-1;rabbit,1:200,Invitrogen,Carlsbad,CA,USA,Cat# 61-7300,RRID: AB_138452),occludin(mouse,1:200,Invitrogen,Cat# 33-1500,RRID:AB_87033),glyceraldehyde-3-phosphate dehydrogenase (GAPDH;rabbit,1:200,Cell Signaling Technology,Cat# 5174S),AQP-4 (mouse,1:200,Santa Cruz Technology,Cat# sc-32739),β-tubulin (rabbit,1:200,Cell Signaling Technology,Cat# 2128S),intercellular cell adhesion molecule (ICAM)-1 (mouse,1:200,Abcam,Cambridge,UK,Cat#ab2213,RRID: AB_302892),NF-κB p65 (rabbit,1:200,Abcam,Cat# ab16502,RRID: AB_443394),and histone H3 (rabbit,1:200,Abcam,Cat# ab176842,RRID: AB_2493104).After washing,the membrane was incubated at 20°C for 1 hour with the appropriate secondary antibody (goat anti-mouse IgG,Cat# ab6789,RRID: AB_955439,and goat anti -rabbit IgG,Cat# ab6721,RRID:AB_955447,Abcam,Cambridge,UK).After removing the secondary anti body,chemiluminescence developer (Shanghai Yase Biotechnology Co.,Ltd.,Shanghai,China) was dropped onto the membrane,and a gel imaging system(Tannon 4600,Shanghai Tianeng Technology Co.,LTD.,Shanghai,China) was used to capture images.Optical density was analyzed using ImageJ soft ware(v1.43;National Institutes of Health,Bethesda,MD,USA) (Schneider et al.,2012).
Immunostaining
Brain samples were collected 3 and 7 days after surgery,fixed with 4%paraformaldehyde,permeabilized in 0.1% Triton X-100,and then blocked in 5% bovine serum albumin (Shanghai Yisheng Biotechnology Co.,LTD.,Shanghai,China).The tissues were incubated at 4°C overnight with primary anti bodies against PHACTR-1,CD31 (goat,1:200,R&D Systems,Minneapolis,MN,USA,Cat# AF3628,RRID: AB_2161028),glial fibrillary acidic protein (GFAP;rabbit,1:200,Merck Millipore,Darmstadt,Germany,Cat# AB5804,RRID:AB_2109645),ionized calcium-binding adapter molecule 1 (Iba1;rabbit,1:200,Wako,Tokyo,Japan,Cat# 019-19741,RRID: AB_839504),NeuN (rabbit,1:200,Merck Millipore,Cat# ABN78,RRID: AB_10807945),ZO-1,occludin,AQP-4,ICAM-1,CD16/32 (rat,1:200,Invitrogen,Cat# 14-0161,RRID: AB_467132),CD206 (goat,1:200,R&D Systems,Cat# AF2535,RRID: AB_2063012),and NFκB p65.Subsequently,the tissues were incubated with appropriate secondary anti bodies (all 1:400,donkey anti -mouse IgG Alexa Fluor 488,Invitrogen,Cat#A21202,RRID: AB_141607,donkey anti -goat IgG Alexa FluorTM555,Invitrogen,Cat# A21432,RRID: AB_2535853,donkey anti -rabbit IgG Alexa Fluor™ 594,Invitrogen,Cat# A21207,RRID: AB_141637,donkey anti-mouse IgG Alexa FluorTM647,Cat# A32787,RRID: AB_2762830,donkey anti -rabbit IgG Alexa FluorTM647,Invitrogen,A32795,RRID: AB_2762835,donkey anti -rabbit IgG Alexa FluorTMPlus 594,Invitrogen,Cat# A32754,RRID: AB_2762827,chicken anti -rat IgG Alexa FluorTM647,Invitrogen,Cat# A21472,RRID: AB_2535875,donkey anti -goat IgG Alexa FluorTMPlus 647,Invitrogen,Cat# A32849,RRID:AB_2762840) for about 1–2 hours and then stained with 4′,6-diamidino-2-phenylindole (Shanghai Uning Bio-tech Co.,Ltd.,Shanghai,China) for about 10 minutes in the dark.TdT-mediated dUTP nick-end labeling (TUNEL) staining was performed using the TUNEL Apoptosis Assay Kit (Cat# C1086;Beyotime Technology,Beijing,China) according to the manufacturer’s instructions.The TUNEL solution was added to mounted brain slices that had been prestained for NeuN and incubated in a humidified box at 37°C for 1 hour in the dark.Images were obtained using fluorescence microscope (Leica TCS SP8,Solms,Germany),and the TUNEL-positive cells were counted.
Measurement of brain edema and Evans blue extravasation
Wet-dry methods (Jing et al.,2020) were used to determine the water content of mouse brain tissue approximately 3 mm in front of and behind the impact center 3 days after surgery.The sections were weighed immediately to determine the wet weight,and then oven-dried (101-2BS,Shanghai Lichen Instrument Technology Co.,Ltd.,Shanghai,China) at 100°C for 24 hours,after which the dry weight was measured.The water content of brain tissue was calculated as follows: (wet weight– dry weight)/wet weight × 100%.
Extravasation of Evans Blue (EB) was analyzed to assess BBB damage 3 days after TBI.Mice were injected intravenously with 2% EB dye (Sigma-Aldrich,St.Louis,MO,USA),and 2 hours later perfused via the heart with 0.9%sodium chloride solution to completely wash the intravascular dye.The two hemispheres of the brain were then divided and weighed immediately.Each sample was homogenized in 50% trichloroacetic acid and centrifuged at 10,000 ×gfor 15 minutes.The resulting supernatant was transferred to three volumes of ethanol.The EB content of the cerebral hemispheres was measured using a spectrophotometer (BioTek,Winooski,VT,USA) at 610 nm and is expressed in micrograms per gram of brain tissue.
Real-time polymerase chain reaction
Total RNA was extracted from brain tissues 3 days after TBI.Realtime polymerase chain reaction was performed to assess expression of inflammatory factors.GAPDH was used as an endogenous control.The sequences of the primers targeting tumor necrosis factor alpha (TNF)-α,interleukin 1 beta (IL-1β),IL-6,IL-4,IL-10,and GADPH were as follows: TNF-α:5′-CCC TCA CAC TCA GAT CAT CTT CT-3′ (forward),5′-GCT ACG AGT GGG CTA CAG-3′ (reverse);IL-1β: 5′-GC AAC TGT TCC TGA ACT CAA CT-3′ (forward),5′-ATC TTT TGG GGT CCG TCA ACT-3′ (reverse);IL-6: 5′-TCT ATA CCA CTT CAC AAG TCG GA-3′ (forward),5’-GAA TTG CCA TTG CAC AAC TCT TT-3′ (reverse);IL-4: 5′-GGT CTC AAC CCC CAG CTA GT-3′ (forward),5′-GCC GAT GAT CTC TCT CAA GTG AT-3′ (reverse);IL-10: 5′-GCT CTT ACT GAC TGG CAT GAG-3′ (forward),5′-CGC AGC TCT AGG AGC ATG TG-3′ (reverse);GADPH: 5′-AGG TCG GTG TGA ACG GAT TTG-3′ (forward),5′-TGT AGA CCA TGT AGT TGA GGT CA-3′ (reverse).The cycler (7900HT,ABI,Foster City,CA,USA) was programmed as follows:reverse transcription reaction 42°C,5 minutes,95°C,10 seconds;PCR reaction 95°C,5 seconds,60°C,30 seconds,40 cycles;dissolution curve collection 95°C,15 seconds,60°C,1 minute,95°C,15 seconds.The 2–∆∆Ctcalculation method (Jing et al.,2019) was used to stati stically analyze the results.
Nissl staining
Nissl staining was used to measure volume loss in the injured brain tissues after TBI.Tissue from the same brain site was collected 7 days after TBI from the different groups and then soaked in Nissl staining solution for 10 minutes.After washing,images were recorded,and loss of volume was analyzed using ImageJ.
Cytosolic and nuclear fractionation to examine p65 expression
Brain tissue samples were collected 3 days post-TBI,and pulping protein extract was prepared using a kit according to the manufacturer’s instructions(P0028,Beyotime Biotechnology).Protein extraction solution was then added,and the samples were centrifuged at 12,000 ×gat 4°C for 15 minutes to yield supernatants containing tissue plasma proteins.To precipitate 1 µL of tissue,10 µL of plasma protein extract A solution containing phenylmethyl sulfonyl fluoride was added,and the tissue precipitate was completely dispersed by vigorous shaking for 15 seconds.After allowing the samples to stand on ice for 15 minutes,an appropriate volume of plasma protein extract B solution(to a ratio 1: 0.5) was added,and the tissue plasma protein mixture was vigorously shaken for 15 seconds then centrifuged at 4°C,12,000 ×gfor 15 minutes to remove any remaining tissue plasma proteins.After removing the supernatant,50 µL nuclear protein extract solution containing phenylmethyl sulfonyl fluoride was added to the pellet,and the tubes were oscillated vigorously for 15 seconds to completely disperse the precipitates.After allowing the samples to stand on ice for 2 minutes,the mixture was again oscillated vigorously for 15 seconds,and this process was repeated for a total of 30 minutes.Finally,the samples were centrifuged at 12,000 ×gat 4°C for 15 minutes to yield supernatants containing nuclear proteins.
Behavioral test
The modified neurological severity score (mNSS) was applied to assess neurological function 3 days after TBI.Scores were compared between the TBI+NC-RNAi and TBI+PHACTR-1-RNAi groups.The mNSS assessed motor,sensory,balance,and reflex tests;a normal score is 0,and a maximal deficit score is 14,with a high score indicating more severe neurological damage(Bieber et al.,2019).In addition,a rotarod test was performed to assess motor coordination (Shiotsuki et al.,2010).Briefly,all mice were trained in balancing in the rod rotating at a speed of 4 to 40 r/min for 5 minutes each day for 3 days.The value was calculated from three trials performed before TBI was used as the baseline value for each mouse.After TBI,the rotarod test was performed every day for 14 days.
Stati stical analysis
No stati stical methods were used to predetermine sample sizes;however,our sample sizes were similar to those reported in previous publications (Yuan et al.,2016;Jing et al.,2020).No animals or data points were excluded from the analysis.The evaluators were blinded to the group assignments.Data were analyzed using SPSS soft ware (version 19;IBM,Armonk,NY,USA).Histogram images were generated using GraphPad Prism 6.00 software (GraphPad Soft ware Inc.,San Diego,CA,USA,www.graphpad.com).The data are shown as the mean ± standard deviation (SD) from at least 3–5 independent trials for each group.Statistically significant differences were calculated by oneway analysis of variance (three groups or more) (including Tukey’s honestly significant differencepost hoctest if necessary) or Student’st-test (two groups) with independent samples.P<0.05 was considered statistically significant.
Results
PHACTR-1 is overexpressed after TBI
To explore the expression changes of PHACTR-1,western blotting was used to determine the overall expression level of PHACTR-1 after TBI.The PHACTR-1 protein level was higher in the TBI group than in the sham group (P<0.05,P<0.01),and this higher expression level was maintained for 7 days (Figure 1AandB).Immunofluorescence showed that the cellular localization of PHACTR-1 was altered after TBI: it was expressed only in endothelial cells and neurons in the sham group but was expressed in astrocytes and microglia in the TBI group (Figure 1C).This may explain the increased overall expression of PHACTR-1 after TBI and suggests a potenti al correlation between increased PHACTR-1 expression and function after TBI.
Verification of the effects of AAV brain tissue transfection
To overexpress and knockdown the PHACTR-1,we transfected mouse braintissue with AAV by subcortical three-point injection.After 4 weeks of normal feeding,none of the mice exhibited abnormal vital signs or had died.To determine whether transfection was successful,frozen sections of brain tissue from a randomly selected mouse were observed under a microscope.Many green fluorescent points representing transfected virus were seen at the junction between the cortex and the hippocampus (Figure 2A).
PHACTR-1 expression in the different AAV transfection groups was detected by western blotting 3 days after establishment of the TBI model by CCI.There was no significant difference in PHACTR-1 expression between the TBI,TBI +NC-OE,and TBI+NC-RNAi groups (P>0.05).However,PHACTR-1 expression was significantly higher in brain tissue from the TBI+PHACTR-1-OE group than that from the TBI group (P<0.01),and PHACTR-1 expression in the TBI+PHACTR-1-RNAi group was significantly lower than that seen in the TBI group (P<0.01;Figure 2BandC).These findings suggest that the transfected mice could sti ll achieve the expected expression effect of PHACTR-1 after the establishment of CCI model.
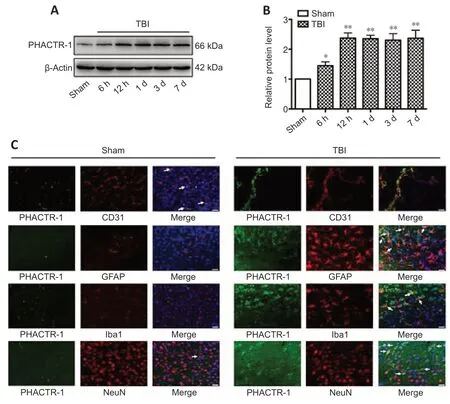
Figure 1|PHACTR-1 expression in the brain after TBI.
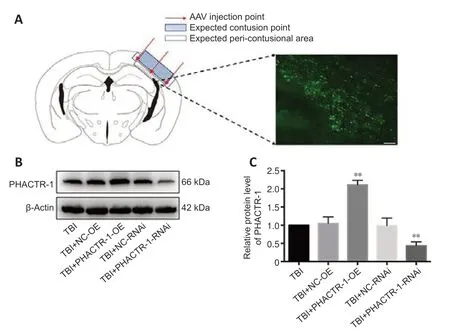
Figure 2|Effect of AAV targeting PHACTR-1 on the contusion point after TBI.
Inhibition of PHACTR-1 expression alleviates brain edema and alters the expression of related proteins after TBI
We calculated brain tissue water content using a wet-dry method.Compared with the TBI group,the water content was similar in the TBI+NC-RNAi group(P>0.05);however,it was decreased in the TBI+PHACTR-1-RNAi group (P<0.05) (Figure 3A).Thus,the TBI+NC-RNAi group was used in place of the TBI group for subsequent tests.Less EB dye extravasation from the contusion lesion was seen in the TBI+PHACTR-1-RNAi group compared with in the TBI+NC-RNAi group (Figure 3B),as confirmed by spectrophotometry (P<0.01;Figure 3C).Tight junctions (TJs) act as paracellular barriers to diffusion,and TJ disruption leads to an increase in BBB permeability and vasogenic edema.ZO-1 and occludin are important components of TJs and play an important role in maintaining BBB integrity (Jing et al.,2020).We used immunofluorescence and western blotting to show that ZO-1 (P<0.05) and occludin (P<0.01) expression levels were significantly increased in the TBI+PHACTR-1-RNAi group compared with the TBI+NC-RNAi group (Figure 3D–F).AQPs are a group of proteins related to transmembrane transport of fluid,and AQP-4 is the most abundant AQP isoform (Yuan et al.,2016).ICAM-1 binds to some antigens in leukocytes to regulate the infiltration of leukocytes into the brain parenchyma (Michinaga and Koyama,2019).Immunofluorescence and western blotting revealed that the increased AQP-4 and ICAM-1 immunopositivity after TBI was significantly reduced by PHACTR-1 knockdown (bothP<0.01;Figure 4).Together,these results show that inhibition of PHACTR-1 expression promotes BBB integrity and reduces brain water content,thereby maintaining normal physiological function in the brain.
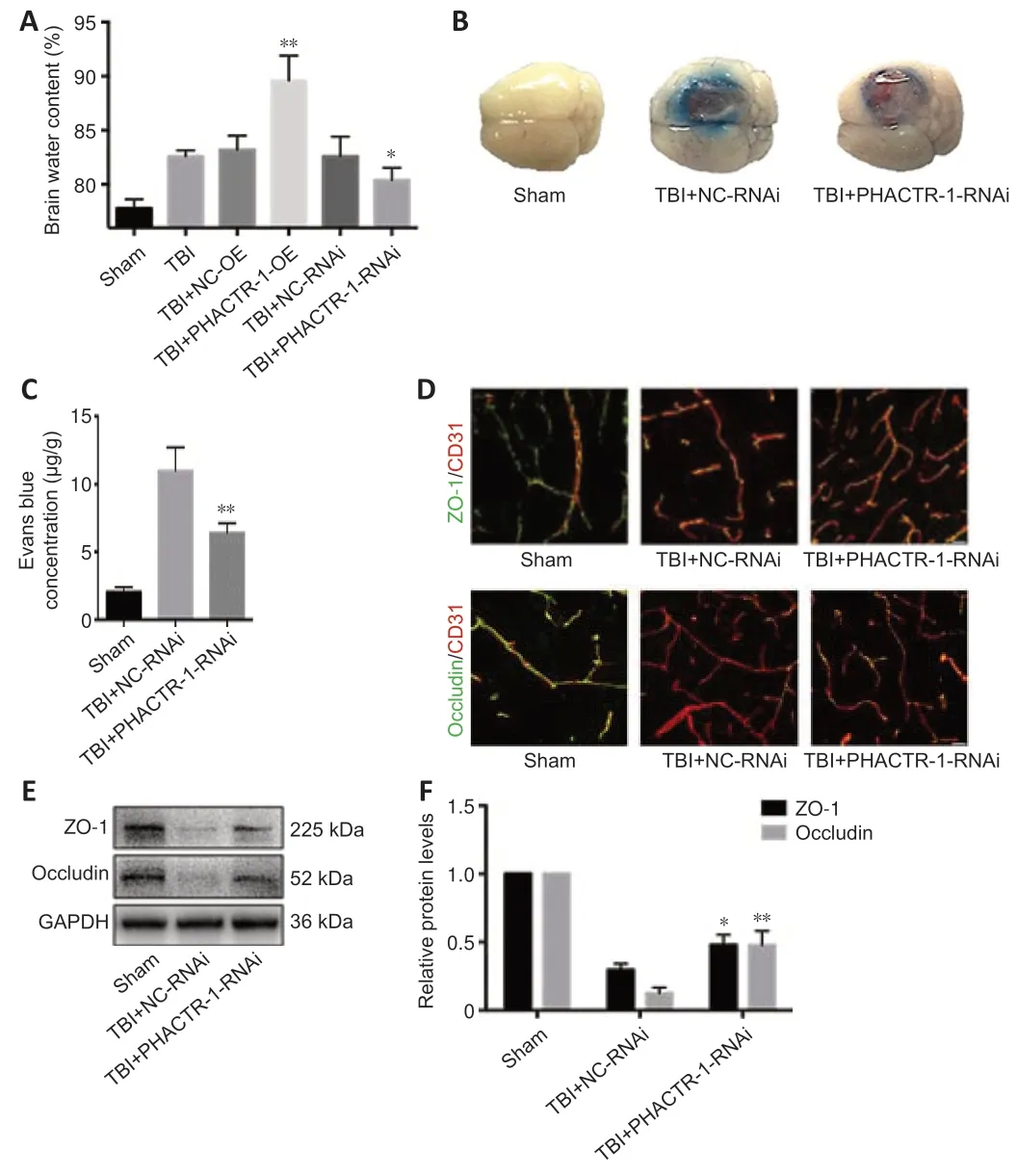
Figure 3|Effect of PHACTR-1 on brain edema and BBB integrity 3 days after TBI.
Inhibition of PHACTR-1 expression reduces neuroinflammation after TBI
We examined astrocyte and microglial activation and the mRNA levels of inflammatory cytokines to assess the degree of neuroinflammation after TBI.Immunofluorescence staining for GFAP and CD16/32/Iba1 indicated significantly reduced astrocyte and microglial activation in the TBI+PHACTR-1-RNAi group compared with the TBI+NC-RNAi group,while CD206/Iba1 expression was significantly increased (Figure 5A).In addition,the mRNA levels of the inflammatory cytokines TNF-α,IL-1β,and IL-6,were decreased (allP<0.01;Figure 5B),whereas those of the anti -inflammatory cytokines IL-4 (P<0.01) and IL-10 (P<0.05) were increased (Figure 5C).These findings clearly show that the neuroinflammation induced by TBI decreased when PHACTR-1 expression was inhibited.
Inhibition of PHACTR-1 expression reduces neuronal apoptosis after TBI
We used TUNEL/NeuN staining to detect nerve cell apoptosis in the three groups.After staining,we found no apoptotic cells in the sham group.However,in the TBI+NC-RNAi group,large numbers of apoptotic neurons and other nerve cells were observed.After PHACTR-1 knockdown,the number of apoptotic neurons and other nerve cells was significantly reduced (bothP<0.01;Figure 6A–C).Nissl staining was used to determine the loss of volume in injured brain tissues after TBI.The percentage of volume loss was about 10%in the TBI+NC-RNAi group,compared with about 6% in the TBI+PHACTR-1-RNAi group (P<0.05),indicating that PHACTR-1 knockdown reduced brain volume loss after TBI (Figure 6DandE).
Inhibiting PHACTR-1 expression regulates NF-κB signaling after TBI
The NF-κB signaling pathway is activated after TBI,and p65,the core protein of the NF-κB complex,is translocated from the cytoplasm into the nucleus,promoting the initi ation of apoptosis (Teng et al.,2017;Mettang et al.,2018;Kong et al.,2019).Immunofluorescence staining and western blotting were used to assess p65 expression levels.p65 protein expression levels were low in the sham group,and both p65 expression and translocation to the nucleus were significantly increased in the TBI+NC-RNAi group.PHACTR-1 knockdown inhibited p65 expression and translocation to the nucleus (Figure 7A).Western blotting analysis of p65 localization to the cytoplasm and nucleus showed that PHACTR-1 knockdown reduced activation of the NF-κB signaling pathway after TBI and reduced p65 expression in both the cytoplasm and the nucleus (P<0.05 andP<0.01,vs.TBI+NC-RNAi group,respectively,Figure 7BandC).
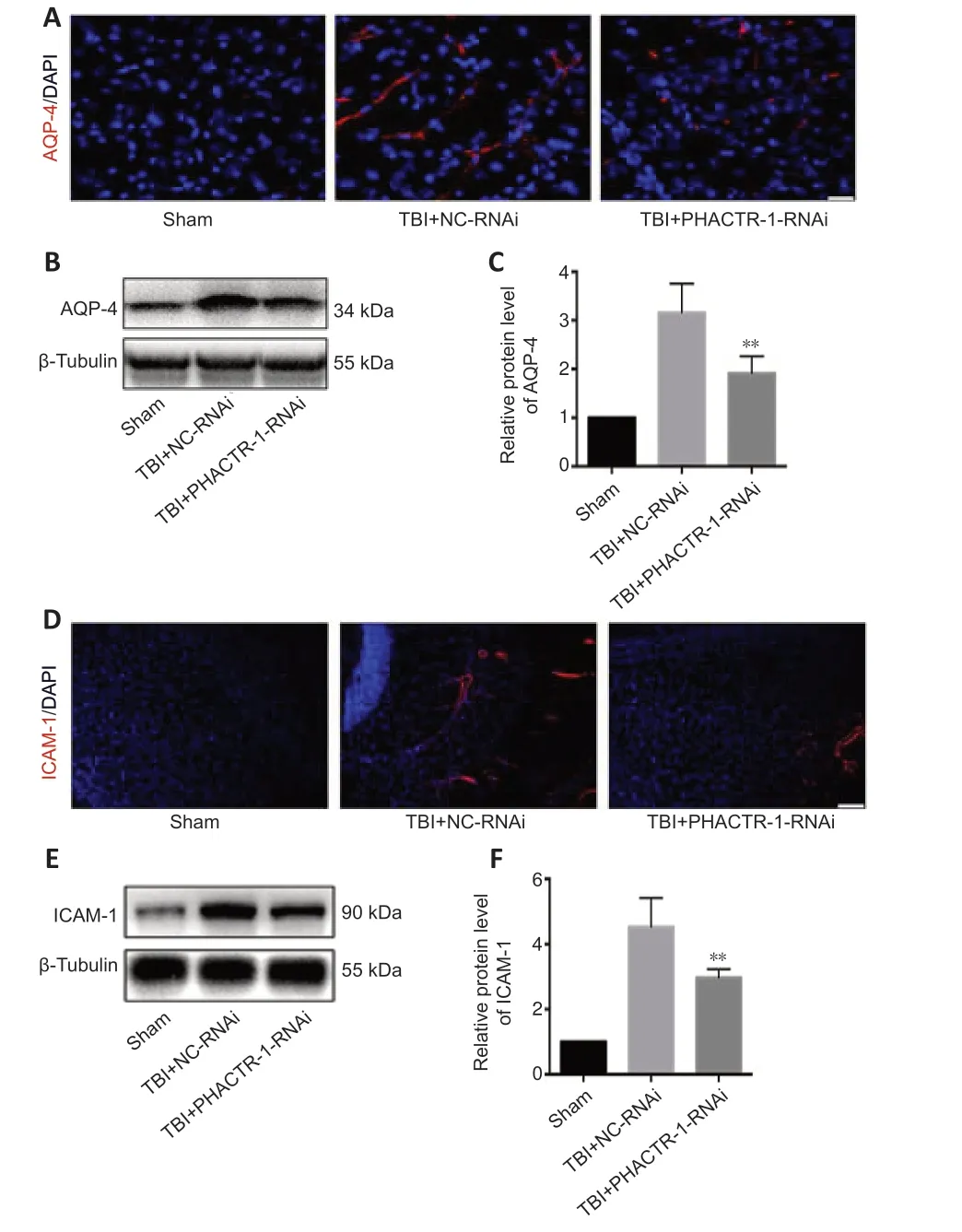
Figure 4|Effect of PHACTR-1 on AQP-4 and ICAM-1 expression in injured braintissues 3 days after TBI.
Inhibiting PHACTR-1 expression improves motor,balance,sensory,and reflex function after TBI
The mNSS and the fatigue rotarod test were used to evaluate neurological function in each group.PHACTR-1 knockdown attenuated the motor,balance,sensory,and reflex neurological deficits induced by TBI in mice (P<0.01 andP<0.05;Figure 8AandB).
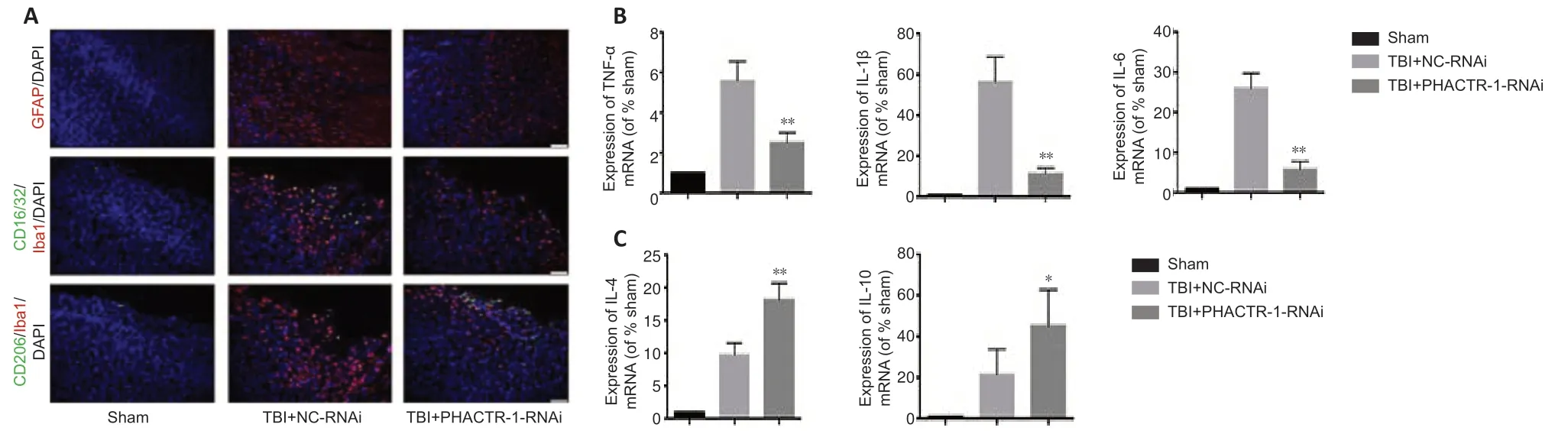
Figure 5|Effect of PHACTR-1 on neuroinflammation in injured brain tissues 3 days after TBI.
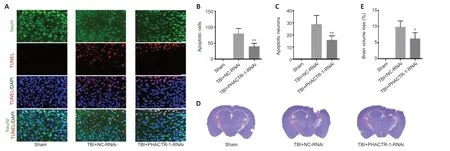
Figure 6|Effect of PHACTR-1 on neuronal apoptosis and brain volume loss 7 days after TBI.
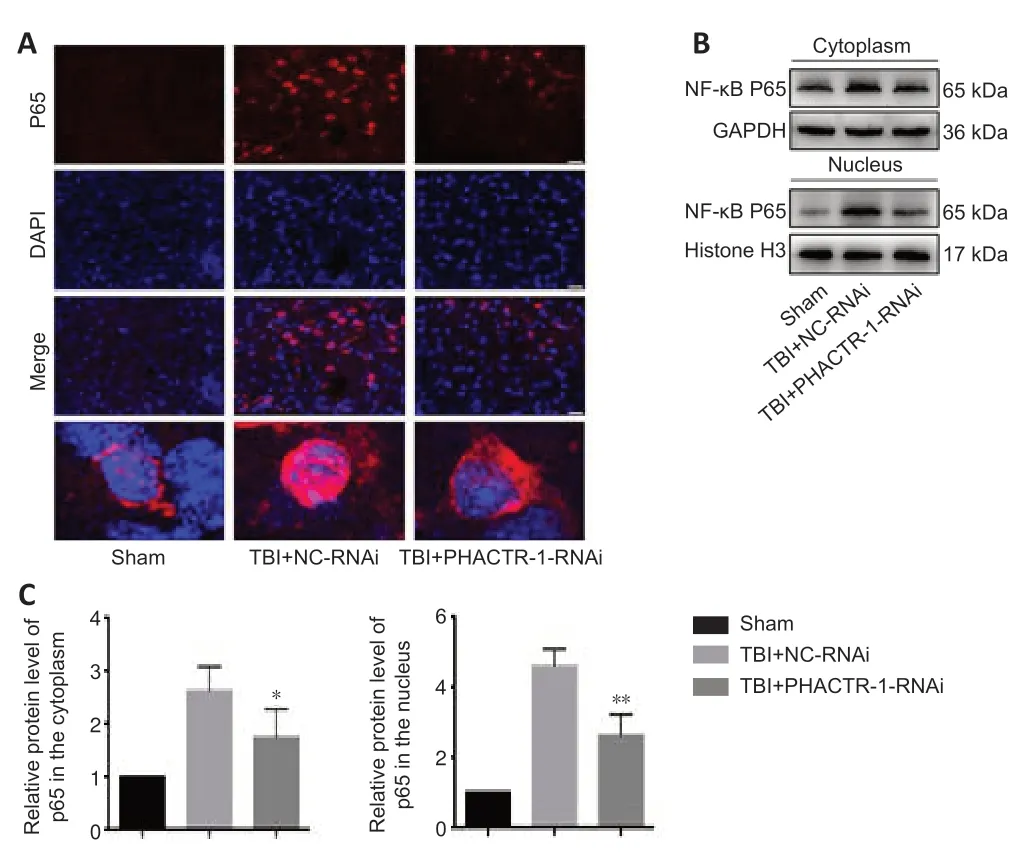
Figure 7|Effect of PHACTR-1 on NF-κB/p65 expression in injured brain tissue 3 days after TBI.
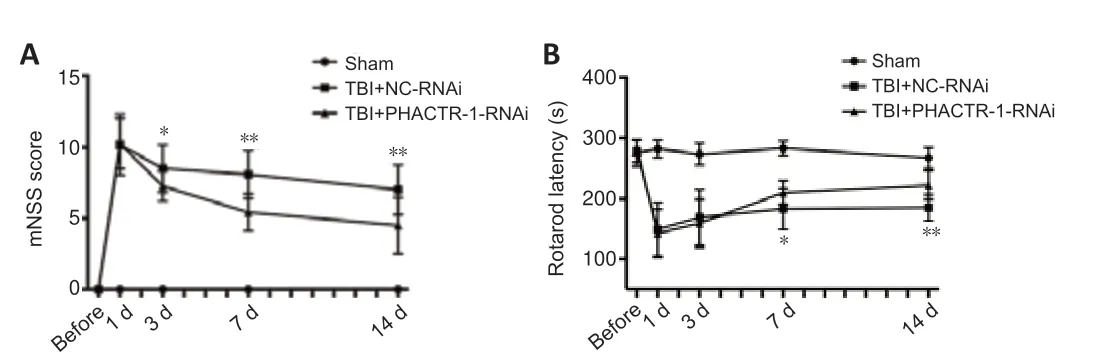
Figure 8|Effect of PHACTR-1 on neurological function after TBI.
Discussion
TBI is a major cause of death and disability in young adults worldwide and creates a huge economic burden for the patients’ families and society.In recent decades,a substanti al amount of research has been conducted on the pathophysiological mechanisms of and potenti al therapeutic targets for TBI.In this study,we investigated the potenti al role of PHACTR-1 in TBI using a CCIinduced mouse model.
We first examined PHACTR-1 expression after TBI.We found that PHACTR-1 expression was increased,and that it was expressed in astrocytes and microglial cells in addition to neurons and endothelial cells.Based on this,we speculated that PHACTR-1 plays an important role in the pathophysiology of TBI,and that higher PHACTR-1 expression in astrocytes and microglial cells resulted in higher overall PHACTR-1 expression levels in mice with TBI.As astrocytes and microglial cells are associated with neuroinflammation,we next evaluated the relationship between PHACTR-1 and neuroinflammation in TBI.
To confirm the relationship between PHACTR-1 and brain edema,we determined the brain water content using a wet-dry method and evaluated BBB integrity by assessing EB extravasation.Water content and EB extravasation were significantly decreased after PHACTR-1 knockdown.Brain edema after TBI is related to BBB disruption.BBB is an actively selective,functional barrier between the central nervous system and peripheral circulation that is crucial for maintaining biological homeostasis in the brain (Alluri et al.,2016).The BBB is composed mainly of endothelial cells,the basement membrane,pericytes,and astrocytes (Abbott et al.,2010;Daneman and Prat,2015).Endothelial cells are the core components responsible for maintaining BBB permeability,and they express low levels of cell adhesion molecules,including ICAM-1,which binds to leukocyte anti gens and promotes the leucocyte infiltration into the brain parenchyma (Michinaga and Koyama,2019).This process results in aggravated parenchymal damage.AQPs are related to the transmembrane transport of fluid,with AQP-4 being the most abundant isoform (Yuan et al.,2016).In this study,we found that AQP-4 and ICAM-1 expression levels were reduced by PHACTR-1 knockdown;thus,we speculated that low PHACTR-1 expression levels are associated with mild edema after TBI.TJs act as paracellular diffusion barriers,and fluid extravasation from the intravascular to extravascular space increases upon TJ disruption.After TBI,loss of TJ proteins in endothelial cells alters BBB permeability (Blixt et al.,2018;Li et al.,2018;Lu et al.,2018).We found that expression levels of the TJ proteins ZO-1 and occludin were significantly increased by PHACTR-1 knockdown,thereby maintaining BBB integrity.Taken together,these results suggest that the decreased AQP-4 and ICAM-1 expression and increased ZO-1 and occludin expression induced by PHACTR-1 knockdown alleviated brain edema after TBI.
Neuroinflammation is a common pathophysiological process after TBI (Chiu et al.,2016;Simon et al.,2017),and the number of activated astrocytes and microglial cells is believed to reflect the extent of neuroinflammation(Karve et al.,2016).Increased astrocyte acti vity in response to injury,known as astrocyte hyperplasia (Sofroniew and Vinters,2010),can increase the secretion of inflammatory factors and growth factors,resulting in a persistent inflammatory response that leads to formation of glial scars (Gorina et al.,2011;Paintlia et al.,2013).In this study,we used immunofluorescence to examine astrocyte and microglial cell activation.We found that the inflammation of both types of cells was decreased and anti -inflammation was increased by PHACTR-1 knockdown.The mRNA levels of the inflammatory cytokines TNF-α,IL-1β,and IL-6 decreased,whereas those of the antiinflammatory cytokines IL-4 and IL-10 increased in response to PHACTR-1 knockdown,indicating that neuro-inflammation was alleviated when PHACTR-1 expression was inhibited.
The direct consequence of BBB destruction and neuroinflammation after TBI is cell injury,including apoptosis of neurons and endothelial cells.Apoptosis is a reversible form of programmed cell death (Zhang et al.,2017b).TUNEL staining revealed a decrease in the number of apoptotic cells after PHACTR-1 knockdown,and brain volume loss was also significantly reduced.Apoptosis can be regulated by a variety of signaling pathways including the NF-κB pathway (He et al.,2018;Liu et al.,2018).After TBI,the NF-κB signaling pathway is activated,and its core protein p65 is translocated from the cytoplasm into the nucleus,promoting the initi ation of apoptosis (Teng et al.,2017;Mettang et al.,2018;Kong et al.,2019).Immunofluorescence staining and western blot analysis showed that PHACTR-1 knockdown reduced the nuclear translocation of p65 in damaged cells,thereby playing an antiapoptotic role after TBI.
To determine whether PHACTR-1 inhibition leads to functional improvement after TBI,we used the mNSS and the fatigue rotarod test to evaluate neurological function.Our results showed that PHACTR-1 knockdown increased ZO-1 and occludin expression and reduced AQP-4 and ICAM-1 expression,astrocyte and microglial cell activation,expression of inflammatory factors,and neuronal apoptosis,indicating recovery of neurological function.However,in vitrocell experiments to verify the mechanism have not been carried out and should be performed in subsequent studies.
In conclusion,inhibition of PHACTR-1 expression promotes recovery of neurological function by protecting BBB integrity,reducing neuronal apoptosis,and alleviating neuroinflammation via activation of the NF-κB pathway.Our results indicate that PHACTR-1 may be a potenti al therapeutic target for TBI.
Acknowledgments:We thank Prof.Guo-Yuan Yang and Yao-Hui Tang (School of Biomedical Engineering and Med-X Research Institute Shanghai Jiao Tong University,Shanghai,China) for their technology guidance.
Author contributions:Study design: JD,ZMX,HLT;experiment implementation:YJ,LZ,SWC;data analysis: YJ;manuscript draft ing and editing: YG,SMJ,FY,HC,DXY.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Luis B.Tovar-y-Romo,Universidad Nacional Autonoma de Mexico,Mexico;Dinesh Kumar Verma,University of Minnesota,USA.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

